Firing Parameters Effect on the Physical and Mechanical Properties of Scheelite Tailings-Containing Ceramic Masses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Incorporation of Scheelite Tailings in the Ceramic Mass and Preparation of Specimens
2.3. Characterizations
2.4. Carbonation Resistance
3. Results and Discussion
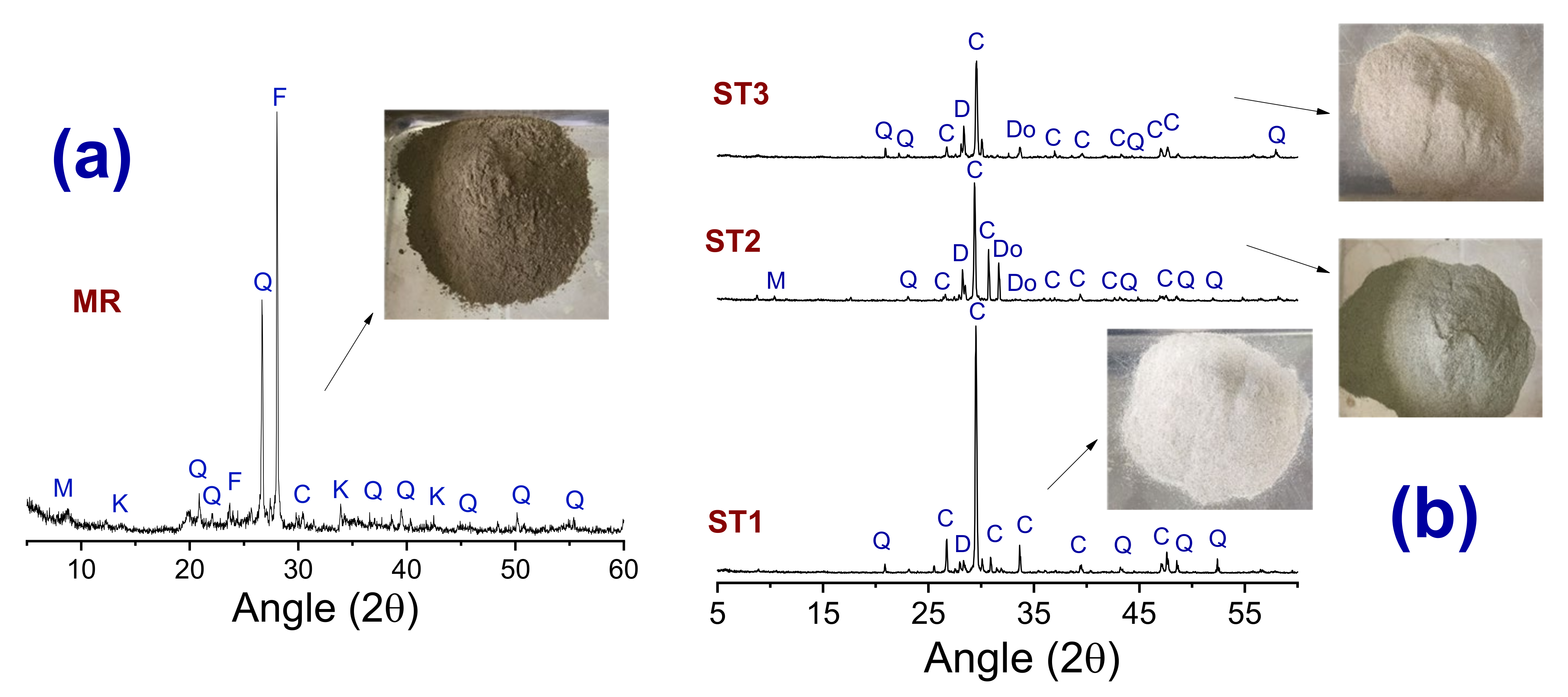
3.1. Chemical and Mineralogical Composition of the Scheelite Tailings and Ceramic Masses
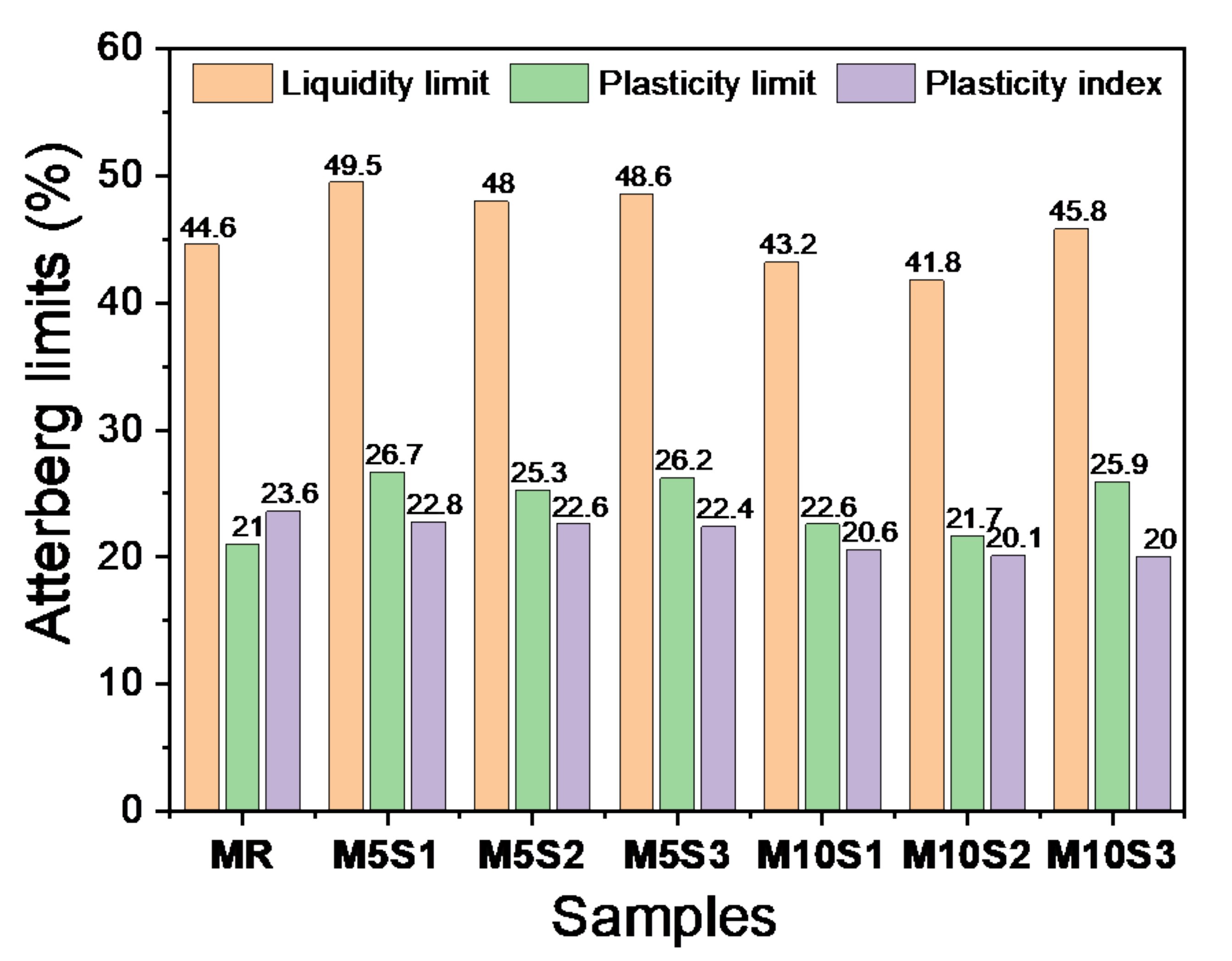
3.2. Granulometry and Consistency of Ceramic Masses
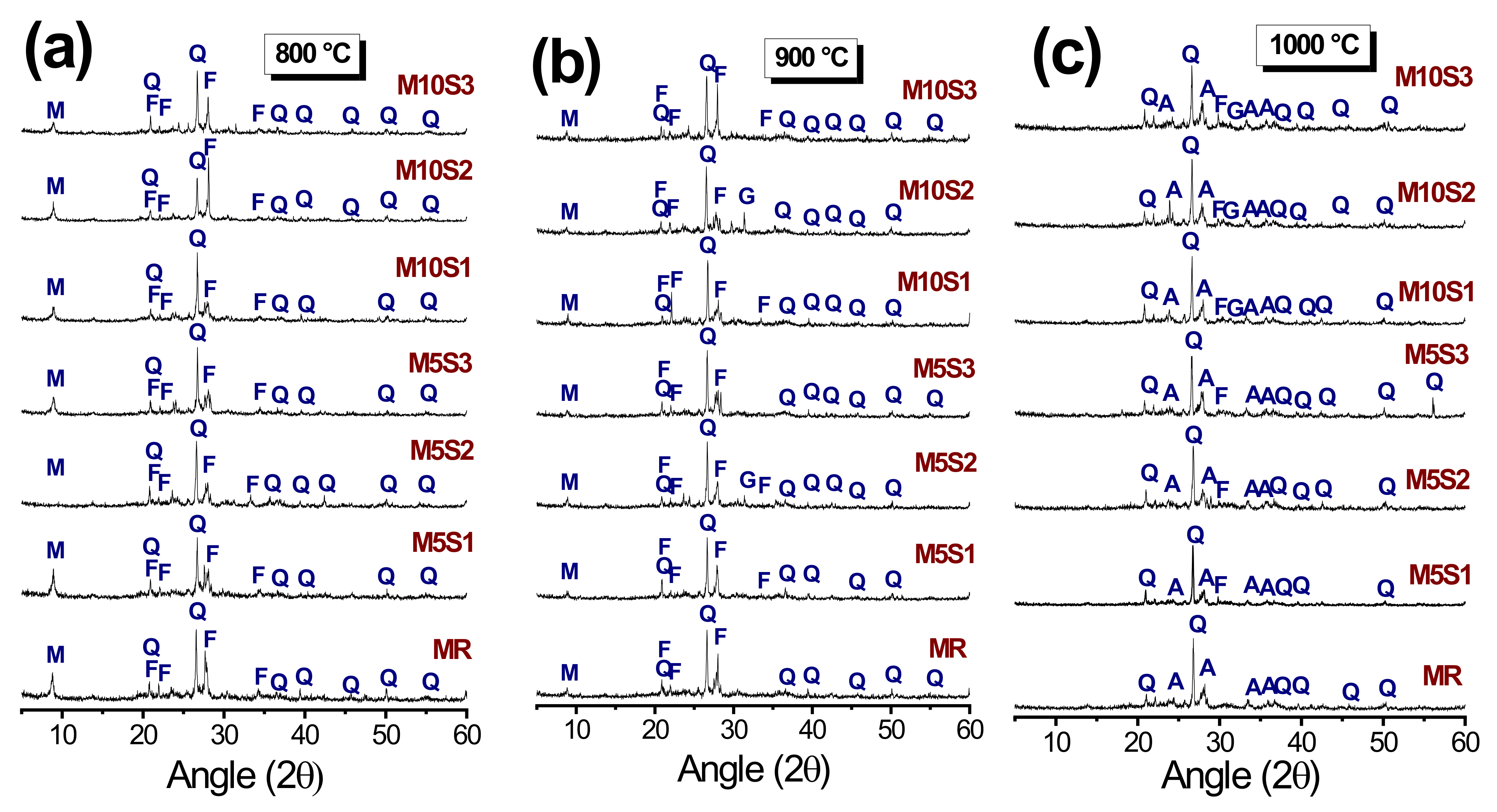
3.3. Mineralogical Phases of the Samples after Firing
3.4. Physical and Mechanical Properties of Sintered Samples
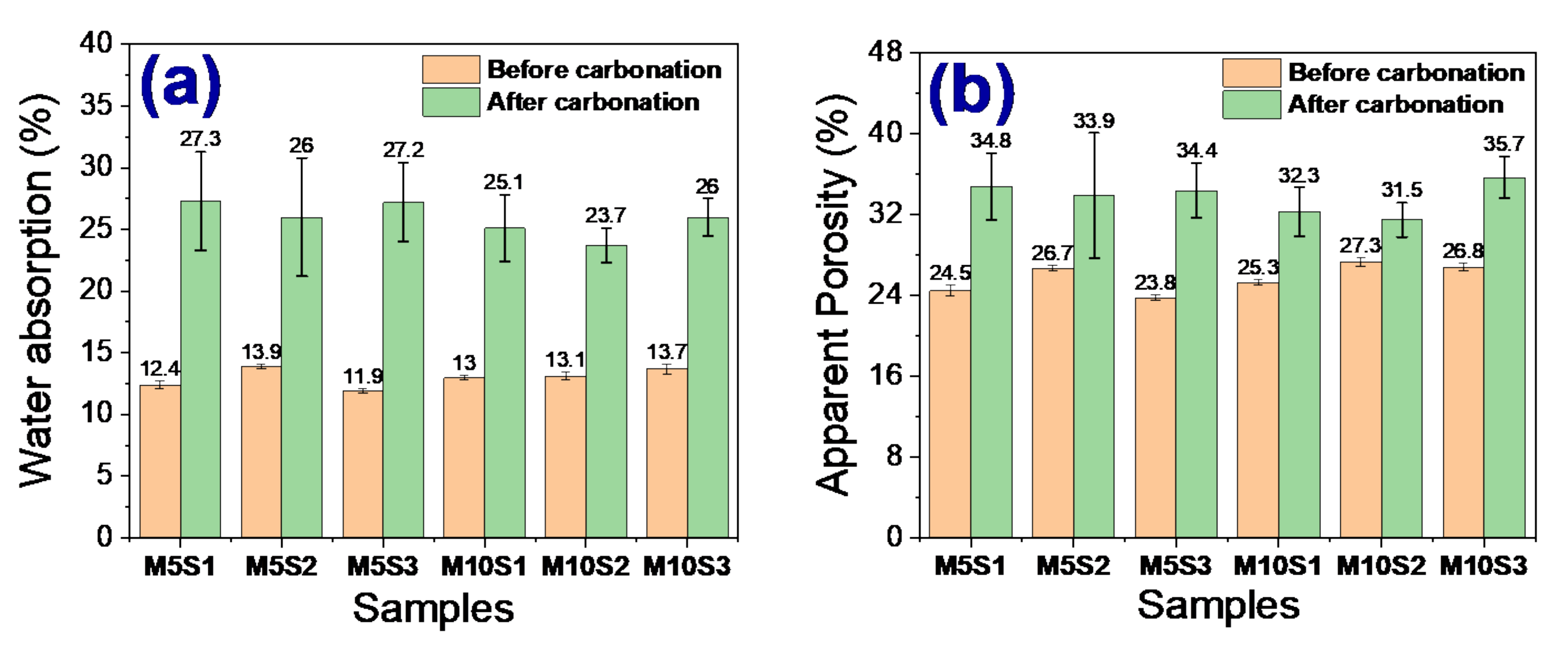
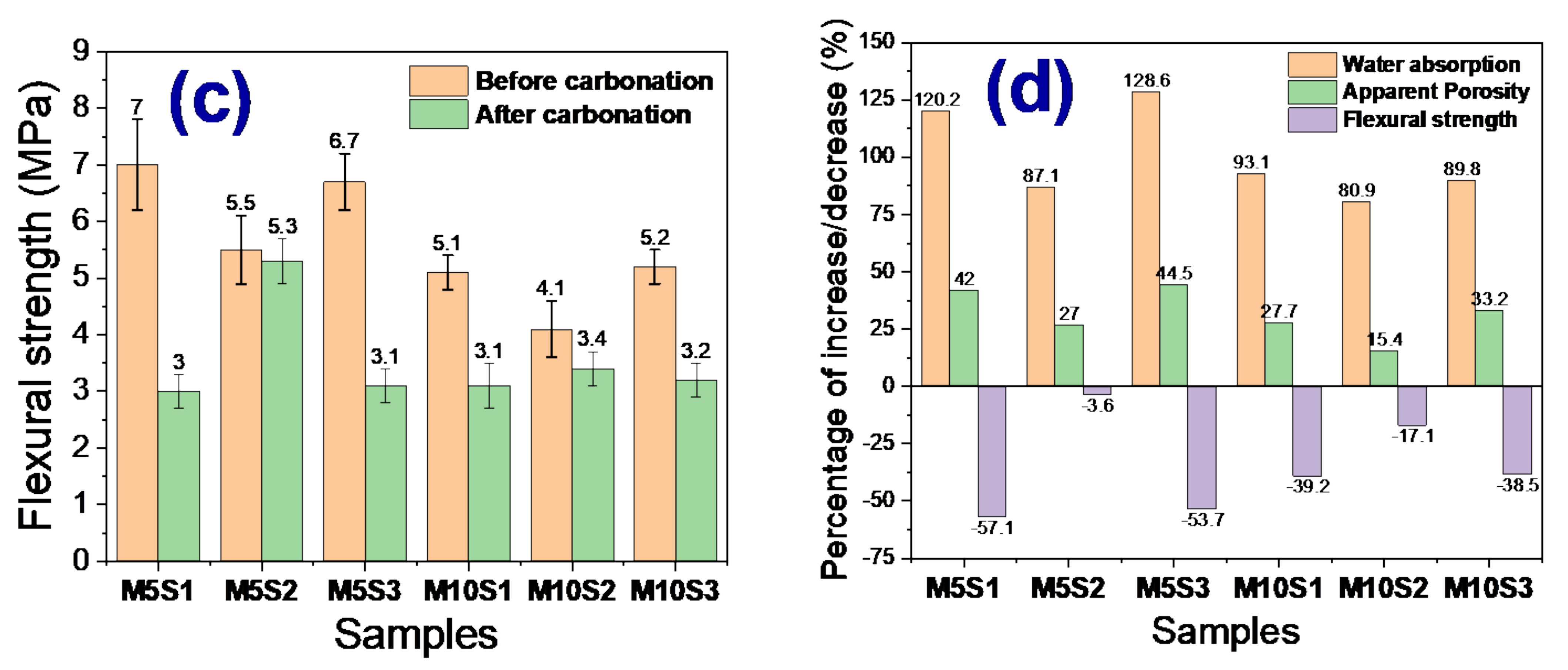
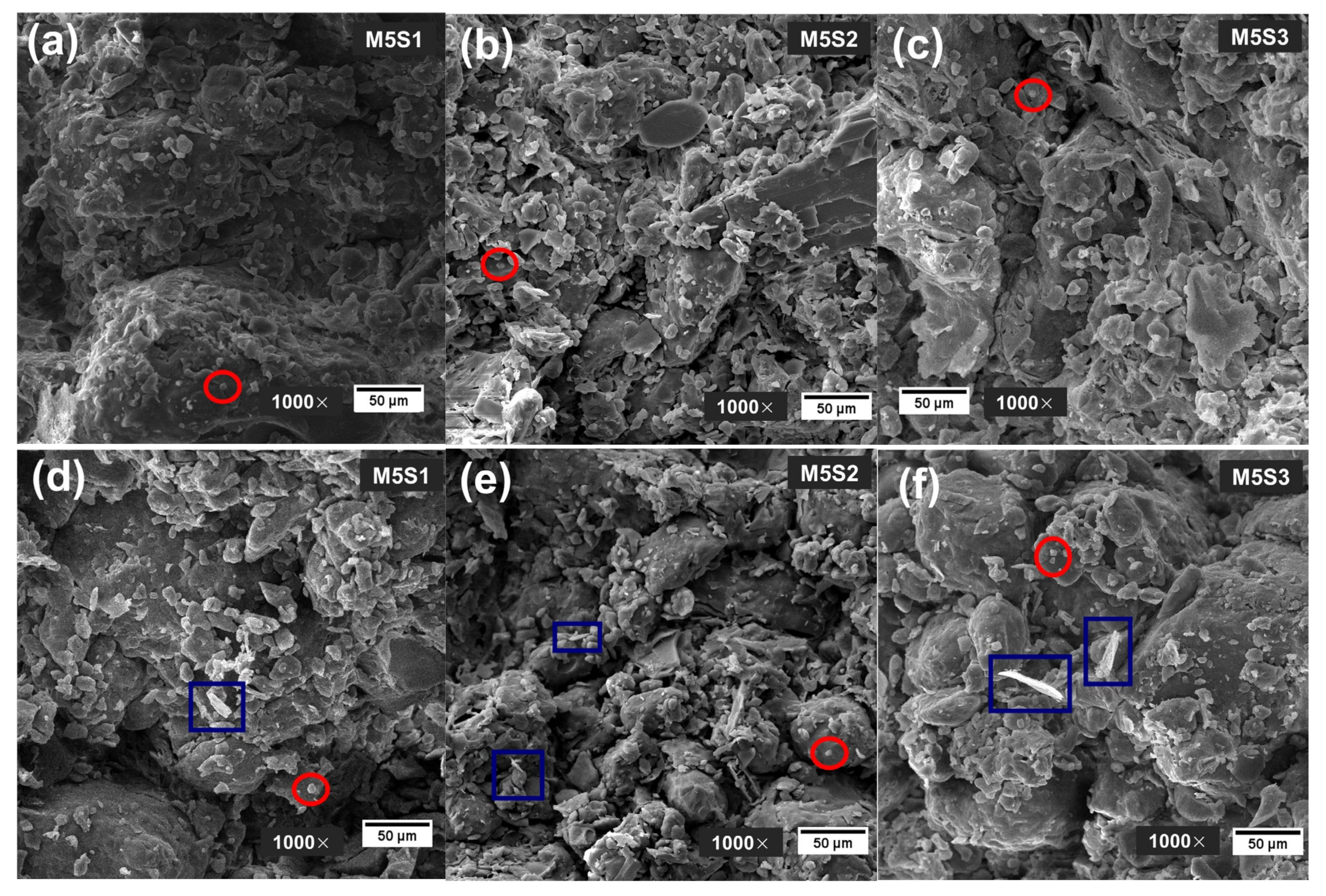
3.5. Carbonation Resistance
4. Conclusions
- The DRX standards of the 900 and 1000 °C sintered samples identified the gehlenite and anorthite phases, which contributed to the increase of the mechanical resistance of the materials.
- No significant differences were observed in the physical and mechanical properties as a function of the different types of scheelite tailings (ST1, ST2, and ST3) incorporated into the ceramic masses.
- The best physical and mechanical properties (lower water absorption and porosity values and higher flexural strength values) were obtained for samples with 5% scheelite tailings and heated at a rate of 5 °C min−1. For samples with 5% tailings and sintered at 1000 °C, the increase in the heating rate from 5 to 10 °C min−1 did not significantly compromise the properties.
- Samples exposed to ambient conditions for 3 months showed a loss of physical and mechanical properties, probably due to the onset of the carbonation phenomenon. The M5S2 and M10S2 samples were the ones that presented the lowest percentages of resistance loss.
- The incorporation of scheelite tailings presented the potential for application in red ceramics and an alternative to reduce environmental pollution and conserve mineral resources.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida, E.P.; Carreiro, M.E.A.; Rodrigues, A.M.; Ferreira, H.S.; Santana, L.N.L.; Menezes, R.R.; Neves, G.A. A new eco-friendly mass formulation based on industrial mining residues for the manufacture of ceramic tiles. Ceram. Int. 2021, 47, 11340–11348. [Google Scholar] [CrossRef]
- Da Costa, F.P.; da Morais, C.R.S.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Luna, C.B.B.; de Farias, A.F.F.; de Medeiros, S.A.S.L.; Meneghetti, S.M.P.; Rodrigues, A.M.; de Costa, A.C.F.M. From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst. Sustainability 2020, 12, 10159. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Farias, A.F.F.; Pontes, J.R.M.; Rodrigues, A.M.; de Costa, A.C.F.M. Synthesis of the ZnO-Ni0.5Zn0.5Fe2O4-Fe2O3 magnetic catalyst in pilot-scale by combustion reaction and its application on the biodiesel production process from oil residual. Arab. J. Chem. 2020, 13, 7665–7679. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Guedes, D.G.; da Costa, F.P.; Rodrigues, A.M.; de Neves, G.A.; Menezes, R.R.; de Santana, L.N.L. Sustainable Ceramic Materials Manufactured from Ceramic Formulations Containing Quartzite and Scheelite Tailings. Sustainability 2020, 12, 9417. [Google Scholar] [CrossRef]
- De Figueirêdo, J.M.R.; da Costa, F.P.; Fernandes, J.V.; Rodrigues, A.M.; de Neves, G.A.; Menezes, R.R.; de Santana, L.N.L. Development of Scheelite Tailings-Based Ceramic Formulations with the Potential to Manufacture Porcelain Tiles, Semi-Stoneware and Stoneware. Materials 2020, 13, 5122. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Ahmad, M.; Rashid, K. Influence of fluxing oxides from waste on the production and physico-mechanical properties of fired clay brick: A review. J. Build. Eng. 2020, 27, 100965. [Google Scholar] [CrossRef]
- Mymrin, V.; Alekseev, K.; Catai, R.; Nagalli, A.; Aibuldinov, Y.; Bekturganov, N.S.; Izzo, R.L. Red ceramics from composites of hazardous sludge with foundry sand, glass waste and acid neutralization salts. J. Environ. Chem. Eng. 2016, 4, 753–761. [Google Scholar] [CrossRef]
- Xu, Z.; Li, S.; Meng, Q.; Wang, X.; Zhu, Q.; Li, J.G. Generalized synthesis of NaLn(MoO4)2 nano/microcrystals (Ln = La–Lu and Y): The effects of lanthanide contraction, structure, and down-/up-conversion luminescence. J. Alloys Compd. 2020, 830, 154676. [Google Scholar] [CrossRef]
- De Brito, I.P.; de Almeida, E.P.; de Araújo Neves, G.; de Lucena Lira, H.; Menezes, R.R.; da Silva, V.J.; de Lima Santana, L.N. Development of cordierite/mullite composites using industrial wastes. Int. J. Appl. Ceram. Technol. 2021, 18, 253–261. [Google Scholar] [CrossRef]
- Loutou, M.; Misrar, W.; Koudad, M.; Mansori, M.; Grase, L.; Favotto, C.; Taha, Y.; Hakkou, R. Phosphate mine tailing recycling in membrane filter manufacturing: Microstructure and filtration suitability. Minerals 2019, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Fontes, W.C.; de Carvalho, J.M.F.; Andrade, L.C.R.; Segadães, A.M.; Peixoto, R.A.F. Assessment of the use potential of iron ore tailings in the manufacture of ceramic tiles: From tailings-dams to “brown porcelain”. Constr. Build. Mater. 2019, 206, 111–121. [Google Scholar] [CrossRef]
- Liu, S.; Guan, X.; Zhang, S.; Dou, Z.; Feng, C.; Zhang, H.; Luo, S. Sintered bayer red mud based ceramic bricks: Microstructure evolution and alkalis immobilization mechanism. Ceram. Int. 2017, 43, 13004–13008. [Google Scholar] [CrossRef]
- Xu, X.; Song, J.; Li, Y.; Wu, J.; Liu, X.; Zhang, C. The microstructure and properties of ceramic tiles from solid wastes of Bayer red muds. Constr. Build. Mater. 2019, 212, 266–274. [Google Scholar] [CrossRef]
- Drif, B.; Taha, Y.; Hakkou, R.; Benzaazoua, M. Integrated valorization of silver mine tailings through silver recovery and ceramic materials production. Miner. Eng. 2021, 170, 107060. [Google Scholar] [CrossRef]
- Karhu, M.; Lagerbom, J.; Solismaa, S.; Honkanen, M.; Ismailov, A.; Räisänen, M.L.; Huttunen-Saarivirta, E.; Levänen, E.; Kivikytö-Reponen, P. Mining tailings as raw materials for reaction-sintered aluminosilicate ceramics: Effect of mineralogical composition on microstructure and properties. Ceram. Int. 2019, 45, 4840–4848. [Google Scholar] [CrossRef]
- Silva, M.C.A.; Leão, V.A.; Reis, E.L. Incorporation of quartzite fines in the production of red ceramics. J. Clean. Prod. 2021, 288, 125098. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Peng, J.; Zhang, W. Separation of scheelite and calcite using calcium lignosulphonate as depressant. Sep. Purif. Technol. 2018, 199, 346–350. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Yeşilay, S.; Çakı, M.; Ergun, H. Usage of marble wastes in traditional artistic stoneware clay body. Ceram. Int. 2017, 43, 8912–8921. [Google Scholar] [CrossRef]
- Traore, K.; Kabre, T.; Blanchart, P. Gehlenite and anorthite crystallisation from kaolinite and calcite mix. Ceram. Int. 2003, 29, 377–383. [Google Scholar] [CrossRef]
- Reis, G.; Cazacliu, B.; Artoni, R.; Torrenti, J.M. Effect of the accelerated carbonation treatment on the recycled sand physicochemical characteristics through the rolling carbonation process. J. CO2 Util. 2020, 39, 101181. [Google Scholar] [CrossRef]
- De Santana, L.L.; da Silva, B.J.; Gonçalves, W.P.; Cartaxo, J.M.; Lira, B.S.; dos Santos, R.C.; Menezes, R.R. Influence of firing conditions on properties of red ceramic. Mater. Sci. Forum 2012, 727, 721–726. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Bennour, A.; Srasra, E.; Zargouni, F. Characterization, firing behavior and ceramic application of clays from the Gabes region in South Tunisia. Appl. Clay Sci. 2017, 135, 215–225. [Google Scholar] [CrossRef]
- Kong, L.; Han, M.; Yang, X. Evaluation on relationship between accelerated carbonation and deterioration of concrete subjected to a high-concentrated sewage environment. Constr. Build. Mater. 2020, 237, 117650. [Google Scholar] [CrossRef]
- Basu, P.; Gupta, R.C.; Agrawal, V. Effect of Carbonation on the Mechanical and Durability Properties of Sandstone Modified Self-Compacting Concrete. In Materials Today Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 32, pp. 686–691. [Google Scholar]
- Li, T.; Wang, S.; Xu, F.; Meng, X.; Li, B.; Zhan, M. Study of the basic mechanical properties and degradation mechanism of recycled concrete with tailings before and after carbonation. J. Clean. Prod. 2020, 259, 120923. [Google Scholar] [CrossRef]
- Mi, R.; Pan, G.; Liew, K.M. Predicting carbonation service life of reinforced concrete beams reflecting distribution of carbonation zones. Constr. Build. Mater. 2020, 255, 119367. [Google Scholar] [CrossRef]
- Martín, D.; Aparicio, P.; Galán, E. Time evolution of the mineral carbonation of ceramic bricks in a simulatedpilot plant using a common clay as sealing material at superficial conditions. Appl. Clay Sci. 2019, 180, 1–11. [Google Scholar] [CrossRef]
- Machado, T.G.; Gomes Uílame, U.; Monteiro, F.M.; Valcacer, S.M.; da Silva Gilson, G. Analysis of the incorporation of scheelite residue in kaolinitic clay of Boa Saúde -RN. Mater. Sci. Forum 2012, 727, 844–849. [Google Scholar] [CrossRef]
- Casagrande, A. Notes on the design of the liquid limit device. Geotechnique 1958, 8, 84–91. [Google Scholar] [CrossRef]
- Da Costa, F.P.; da Morais, C.R.S.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- Song, J.L.; Lee, G.Y.; Choi, J.P.; Lee, J.S. Compaction behavior of bimodal iron nanopowder agglomerate. Powder Technol. 2018, 338, 333–341. [Google Scholar] [CrossRef]
- Menezes, R.; Ferreira, H.; Neves, G.; Lira, H.D.L.; Ferreira, H.C. Use of granite sawing wastes in the production of ceramic bricks and tiles. J. Eur. Ceram. Soc. 2005, 25, 1149–1158. [Google Scholar] [CrossRef]
- McConville, C.J.; Lee, W.E. Microstructural development on firing illite and smectite clays compared with that in kaolinite. J. Am. Ceram. Soc. 2005, 88, 2267–2276. [Google Scholar] [CrossRef]
- Torres, P.; Manjate, R.S.; Quaresma, S.; Fernandes, H.R.; Ferreira, J.M.F. Development of ceramic floor tile compositions based on quartzite and granite sludges. J. Eur. Ceram. Soc. 2007, 27, 4649–4655. [Google Scholar] [CrossRef]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T. Influence of firing temperature on the ceramic properties of Triassic clays from Tunisia. J. Mater. Process. Technol. 2009, 209, 2812–2817. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Zhao, L.; Cang, D. Novel ceramics prepared from inferior clay rich in CaO and Fe2O3: Properties, crystalline phases evolution and densification process. Appl. Clay Sci. 2017, 143, 199–204. [Google Scholar] [CrossRef]
- Gonzáles-García, F.; Romero-Acosta, V.; García-Ramos, G.; Gonzáles-Rodríguez, M. Firing transformations of mixtures of clays containing illite, kaolinite and calcium carbonate used by ornamental tile industries. Appl. Clay Sci. 1990, 5, 361–375. [Google Scholar] [CrossRef]
- Ouahabi, M.; Daoudi, L.; Hatert, F.; Fagel, N. Modified mineral phases during clay ceramic firing. Clays Clay Miner. 2015, 65, 404–413. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F.; Havlica, J.; Holešinsk, R. Kinetics and mechanism of formation of gehlenite, Al–Si spinel and anorthite from the mixture of kaolinite and calcite. Solid State Sci. 2013, 26, 53–58. [Google Scholar] [CrossRef]
- ASTM. C20-00, Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water; ASTM Int.: West Conshohocken, PA, USA, 2015. [Google Scholar] [CrossRef]
- ASTM. C67/C67M-20, Standard Test Methods for Sampling and Testing Brick and Structural Clay Tile; ASTM Int.: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- De Faria, J.; Manhães, R.; da Luz, F.S.; Monteiro, S.N.; Vieira, C.M.F. Incorporation of unserviceable tire waste in red ceramic. J. Mater. Res. Technol. 2019, 8, 6041–6050. [Google Scholar] [CrossRef]
- Coletti, C.; Maritan, L.; Cultrone, G.; Hein, A.; Molina, E.; Mazzoli, C. Recycling trachyte waste from the quarry to the brick industry: Effects on physical and mechanical properties, and durability of new bricks. Constr. Build. Mater. 2018, 166, 792–807. [Google Scholar] [CrossRef]
- Martín, D.; Aparicio, P.; Galan, E. Accelerated carbonation of ceramic materials. Application to bricks from Andalusian factories (Spain). Constr. Build. Mater. 2018, 181, 598–608. [Google Scholar] [CrossRef]


| Ceramic Masses | Addition Content (%wt) | Scheelite Tailings Type |
|---|---|---|
| MR | 0 | - |
| M5S1 | 5 | ST1 |
| M5S2 | 5 | ST2 |
| M5S3 | 5 | ST3 |
| M10S1 | 10 | ST1 |
| M10S2 | 10 | ST2 |
| M10S3 | 10 | ST3 |
| Raw Materials/Samples | Oxides (%wt) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | K2O | TiO2 | CaO | MgO | WO3 | Others | LOI * | SiO2/Al2O3 | |
| ST1 | 18.1 | 8.7 | 7.2 | 0.4 | 0.6 | 37.6 | 2.7 | 0.4 | 1.3 | 23.0 | - |
| ST2 | 21.8 | 7.6 | 9.8 | 0.6 | 0.7 | 38.5 | 3.3 | 0.2 | 1.8 | 15.7 | - |
| ST3 | 22.9 | 10.8 | 9.6 | 0.6 | 0.4 | 40.2 | 3.3 | 0.7 | 1.7 | 9.8 | - |
| MR | 47.5 | 20.9 | 8.9 | 3.0 | 1.2 | 2.1 | 2.6 | - | 0.5 | 13.3 | 2.27 |
| M5S1 | 45.0 | 19.8 | 8.2 | 2.9 | 1.2 | 3.5 | 2.5 | - | 0.6 | 16.3 | 2.27 |
| M5S2 | 43.7 | 19.4 | 9.0 | 2.7 | 1.0 | 4.8 | 2.5 | - | 1.0 | 15.9 | 2.25 |
| M5S3 | 45.8 | 20.0 | 9.0 | 2.9 | 1.2 | 3.0 | 2.5 | - | 0.6 | 15.0 | 2.29 |
| M10S1 | 43.5 | 19.4 | 8.5 | 2.8 | 1.1 | 3.7 | 2.5 | - | 0.8 | 17.7 | 2.24 |
| M10S2 | 43.0 | 19.0 | 9.0 | 2.7 | 1.0 | 5.7 | 2.5 | - | 0.9 | 16.2 | 2.26 |
| M10S3 | 42.9 | 18.9 | 8.7 | 2.6 | 1.1 | 7.2 | 2.4 | - | 0.6 | 15.6 | 2.27 |
| Samples | Accumulated Mass (%) | Average Diameter (µm) | ||
|---|---|---|---|---|
| Fine (x * < 2 µm) | Medium (2 µm < x < 20 µm) | Gross (x > 20 µm) | ||
| MR | 22.1 | 55.5 | 22.4 | 12.5 |
| M5S1 | 20.4 | 58.8 | 20.8 | 12.2 |
| M5S2 | 21.5 | 57.3 | 21.2 | 12.9 |
| M5S3 | 20.9 | 56.2 | 22.9 | 12.6 |
| M10S1 | 20.6 | 57.6 | 21.8 | 12.1 |
| M10S2 | 20.5 | 57.6 | 21.9 | 12.6 |
| M10S3 | 20.6 | 57.8 | 21.6 | 12.4 |
| Rate (°C∙min−1) | Samples | Physical and Mechanical Properties of the Masses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Absorption (%) | Apparent Porosity (%) | Flexural Strength (MPa) | |||||||||
| 800 °C | 900 °C | 1000 °C | 800 °C | 900 °C | 1000 °C | 800 °C | 900 °C | 1000 °C | |||
| 5 | MR | 17.7 ± 0.3 | 17.5 ± 0.2 | 13.2 ± 0.1 | 32.2 ± 0.4 | 31.7 ± 0.3 | 26.0 ± 0.2 | 2.0 ± 0.2 | 3.2 ± 0.3 | 6.4 ± 0.4 | |
| M5S1 | 19.9 ± 0.3 | 16.0 ± 0.3 | 12.4 ± 0.3 | 35.1 ± 0.4 | 30.6 ± 0.6 | 24.5 ± 0.5 | 2.0 ± 0.4 | 5.6 ± 0.5 | 7.0 ± 0.8 | ||
| M5S2 | 17.6 ± 0.4 | 18.0 ± 0.2 | 13.9 ± 0.2 | 32.6 ± 0.6 | 32.9 ± 0.6 | 26.7 ± 0.3 | 3.6 ± 1.2 | 2.5 ± 0.2 | 5.5 ± 0.6 | ||
| M5S3 | 15.5 ± 0.1 | 14.7 ± 0.2 | 11.9 ± 0.2 | 29.8 ± 0.2 | 27.9 ± 0.3 | 23.8 ± 0.3 | 5.0 ± 0.3 | 4.7 ± 0.4 | 6.7 ± 0.5 | ||
| M10S1 | 18.9 ± 0.3 | 16.0 ± 0.2 | 13.0 ± 0.2 | 34.5 ± 0.4 | 29.9 ± 0.3 | 25.3 ± 0.3 | 2.3 ± 0.5 | 4.1 ± 0.2 | 5.1 ± 0.3 | ||
| M10S2 | 18.8 ± 0.5 | 16.9 ± 0.3 | 13.1 ± 0.3 | 33.7 ± 0.4 | 31.4 ± 0.4 | 27.3 ± 0.4 | 3.5 ± 0.5 | 2.3 ± 0.2 | 4.1 ± 0.5 | ||
| M10S3 | 18.6 ± 0.4 | 14.6 ± 0.3 | 13.7 ± 0.4 | 34.1 ± 0.5 | 27.9 ± 0.4 | 26.8 ± 0.4 | 4.5 ± 0.2 | 4.0 ± 0.2 | 5.2 ± 0.3 | ||
| 10 | MR | 17.2 ± 0.5 | 17.4 ± 0.3 | 13.4 ± 0.2 | 31.1 ± 0.5 | 31.6 ± 0.4 | 26.2 ± 0.3 | 1.4 ± 0.4 | 3.1 ± 0.2 | 6.4 ± 0.4 | |
| M5S1 | 19.5 ± 0.4 | 16.3 ± 0.3 | 12.8 ± 0.4 | 34.5 ± 0.6 | 30.8 ± 0.5 | 25.1 ± 0.5 | 1.8 ± 0.2 | 5.0 ± 0.4 | 6.6 ± 0.4 | ||
| M5S2 | 17.5 ± 0.3 | 18.3 ± 0.2 | 14.4 ± 0.4 | 32.3 ± 0.5 | 33.2 ± 0.5 | 27.5 ± 0.6 | 3.6 ± 1.6 | 2.1 ± 0.1 | 4.5 ± 0.5 | ||
| M5S3 | 15.6 ± 0.2 | 14.8 ± 0.1 | 12.4 ± 0.3 | 29.8 ± 0.3 | 28.0 ± 0.2 | 24.6 ± 0.5 | 4.0 ± 0.3 | 4.5 ± 0.4 | 5.4 ± 0.3 | ||
| M10S1 | 19.8 ± 0.4 | 16.4 ± 0.4 | 15.1 ± 0.3 | 35.5 ± 0.5 | 30.3 ± 0.5 | 28.1 ± 0.5 | 1.5 ± 0.2 | 3.2 ± 0.3 | 3.6 ± 0.4 | ||
| M10S2 | 19.5 ± 0.3 | 17.3 ± 0.3 | 15.3 ± 0.4 | 34.4 ± 0.4 | 32.1 ± 0.4 | 28.4 ± 0.6 | 3.4 ± 0.2 | 2.0 ± 0.1 | 3.6 ± 0.2 | ||
| M10S3 | 19.0 ± 0.2 | 14.6 ± 0.3 | 14.8 ± 0.2 | 34.5 ± 0.2 | 28.0 ± 0.4 | 27.8 ± 0.3 | 3.8 ± 0.3 | 3.6 ± 0.4 | 4.7 ± 0.4 | ||
| 15 | MR | 17.5 ± 0.2 | 17.6 ± 0.1 | 13.2 ± 0.9 | 31.5 ± 0.3 | 31.9 ± 0.2 | 25.9 ± 1.3 | 1.0 ± 0.4 | 2.8 ± 0.2 | 6.7 ± 1.0 | |
| M5S1 | 19.8 ± 0.2 | 16.5 ± 0.3 | 13.3 ± 0.3 | 34.8 ± 0.3 | 31.0 ± 0.4 | 25.9 ± 0.5 | 1.6 ± 0.2 | 4.5 ± 0.2 | 5.6 ± 0.5 | ||
| M5S2 | 17.5 ± 0.4 | 18.4 ± 0.2 | 15.1 ± 0.3 | 32.3 ± 0.5 | 33.3 ± 0.5 | 28.4 ± 0.4 | 3.0 ± 1.2 | 2.0 ± 0.2 | 4.1 ± 0.5 | ||
| M5S3 | 15.8 ± 0.3 | 14.8 ± 0.2 | 13.1 ± 0.5 | 30.0 ± 0.4 | 28.0 ± 0.4 | 25.5 ± 0.7 | 3.7 ± 0.5 | 4.4 ± 0.3 | 4.7 ± 0.6 | ||
| M10S1 | 19.9 ± 0.2 | 17.3 ± 0.5 | 16.0 ± 0.4 | 35.5 ± 0.2 | 31.6 ± 0.7 | 29.2 ± 0.4 | 1.5 ± 0.3 | 2.9 ± 0.4 | 2.9 ± 0.2 | ||
| M10S2 | 19.5 ± 0.2 | 18.0 ± 0.3 | 15.7 ± 0.3 | 34.4 ± 0.3 | 32.9 ± 0.4 | 28.9 ± 0.4 | 2.9 ± 0.2 | 1.9 ± 0.2 | 3.6 ± 0.3 | ||
| M10S3 | 19.4 ± 0.5 | 15.1 ± 0.3 | 15.1 ± 0.4 | 35.0 ± 0.7 | 28.4 ± 0.5 | 28.2 ± 0.6 | 3.4 ± 0.2 | 3.1 ± 0.2 | 4.4 ± 0.2 | ||
| 20 | MR | 17.7 ± 0.2 | 17.6 ± 0.2 | 13.9 ± 0.2 | 31.7 ± 0.3 | 31.8 ± 0.3 | 26.8 ± 0.3 | 1.0 ± 0.1 | 2.6 ± 0.2 | 5.7 ± 0.4 | |
| M5S1 | 19.8 ± 0.3 | 16.6 ± 0.3 | 14.3 ± 0.5 | 34.9 ± 0.4 | 31.0 ± 0.5 | 27.2 ± 0.7 | 1.4 ± 0.3 | 4.3 ± 0.4 | 4.3 ± 0.8 | ||
| M5S2 | 18.2 ± 0.5 | 18.6 ± 0.2 | 15.8 ± 0.4 | 33.1 ± 0.6 | 33.5 ± 0.6 | 29.2 ± 0.5 | 2.3 ± 1.2 | 1.6 ± 0.2 | 3.4 ± 0.4 | ||
| M5S3 | 16.4 ± 0.3 | 15.0 ± 0.2 | 13.9 ± 0.7 | 30.9 ± 0.4 | 28.2 ± 0.2 | 26.7 ± 1.0 | 2.6 ± 0.3 | 3.8 ± 0.3 | 3.5 ± 0.6 | ||
| M10S1 | 20.0 ± 0.1 | 18.8 ± 0.5 | 16.6 ± 0.5 | 36.5 ± 0.3 | 33.4 ± 0.6 | 30.0 ± 0.7 | 1.3 ± 0.3 | 2.1 ± 0.1 | 2.5 ± 0.3 | ||
| M10S2 | 19.8 ± 0.6 | 19.0 ± 0.3 | 15.7 ± 0.2 | 35.1 ± 0.3 | 33.9 ± 0.4 | 29.0 ± 0.2 | 2.1 ± 0.1 | 1.4 ± 0.2 | 3.4 ± 0.2 | ||
| M10S3 | 20.6 ± 0.8 | 15.4 ± 0.2 | 15.0 ± 0.3 | 36.1 ± 0.9 | 29.0 ± 0.3 | 28.0 ± 0.5 | 2.3 ± 0.1 | 2.9 ± 0.1 | 4.6 ± 0.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreiro, M.E.A.; da Silva, V.J.; Rodrigues, A.M.; Barbosa, E.P.d.A.; da Costa, F.P.; Menezes, R.R.; Neves, G.A.; Santana, L.N.d.L. Firing Parameters Effect on the Physical and Mechanical Properties of Scheelite Tailings-Containing Ceramic Masses. Sustainability 2022, 14, 333. https://doi.org/10.3390/su14010333
Carreiro MEA, da Silva VJ, Rodrigues AM, Barbosa EPdA, da Costa FP, Menezes RR, Neves GA, Santana LNdL. Firing Parameters Effect on the Physical and Mechanical Properties of Scheelite Tailings-Containing Ceramic Masses. Sustainability. 2022; 14(1):333. https://doi.org/10.3390/su14010333
Chicago/Turabian StyleCarreiro, Marcos Emmanuel Araújo, Valmir José da Silva, Alisson Mendes Rodrigues, Ester Pires de Almeida Barbosa, Fabiana Pereira da Costa, Romualdo Rodrigues Menezes, Gelmires Araújo Neves, and Lisiane Navarro de Lima Santana. 2022. "Firing Parameters Effect on the Physical and Mechanical Properties of Scheelite Tailings-Containing Ceramic Masses" Sustainability 14, no. 1: 333. https://doi.org/10.3390/su14010333
APA StyleCarreiro, M. E. A., da Silva, V. J., Rodrigues, A. M., Barbosa, E. P. d. A., da Costa, F. P., Menezes, R. R., Neves, G. A., & Santana, L. N. d. L. (2022). Firing Parameters Effect on the Physical and Mechanical Properties of Scheelite Tailings-Containing Ceramic Masses. Sustainability, 14(1), 333. https://doi.org/10.3390/su14010333









