Ecological Vulnerability of Adult Female Marine Turtles as Indicators of Opportunities for Regional Socioecosystem Management in the Southern Gulf of Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Satellite Tracking Data
2.3. Ecological Vulnerability Assessment
2.4. Spatial Configuration in Terms of Territorial Administration
3. Results
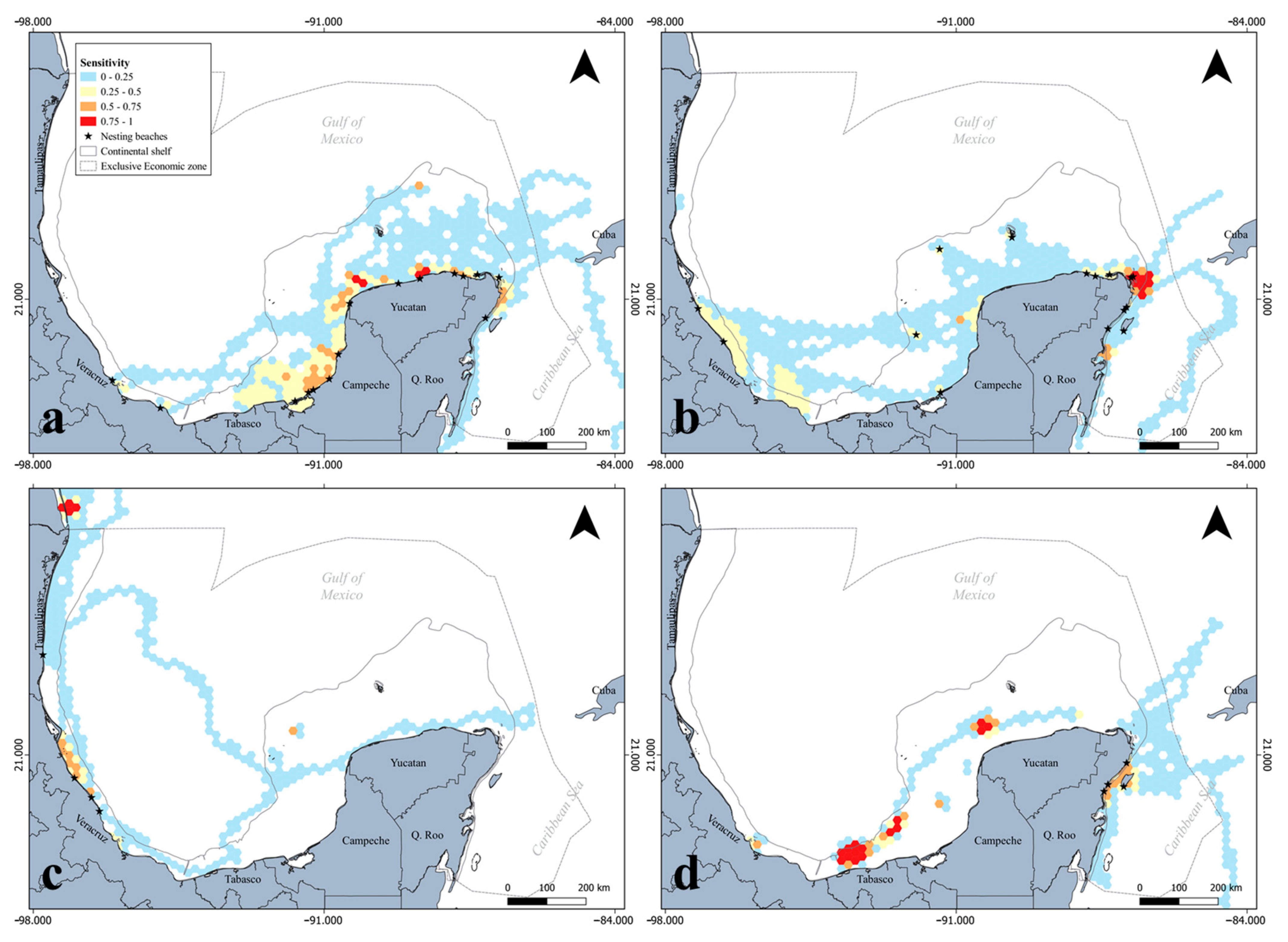
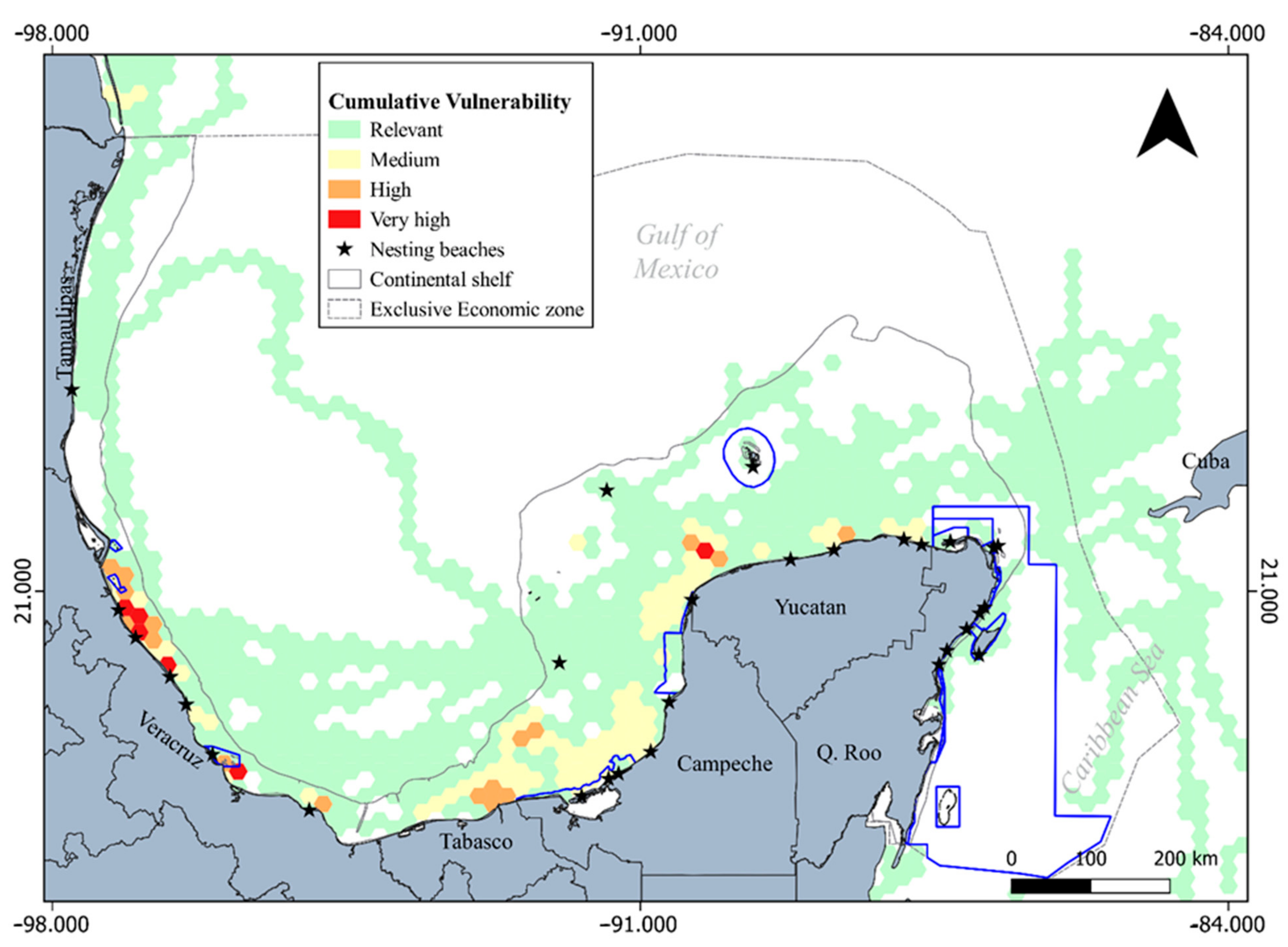
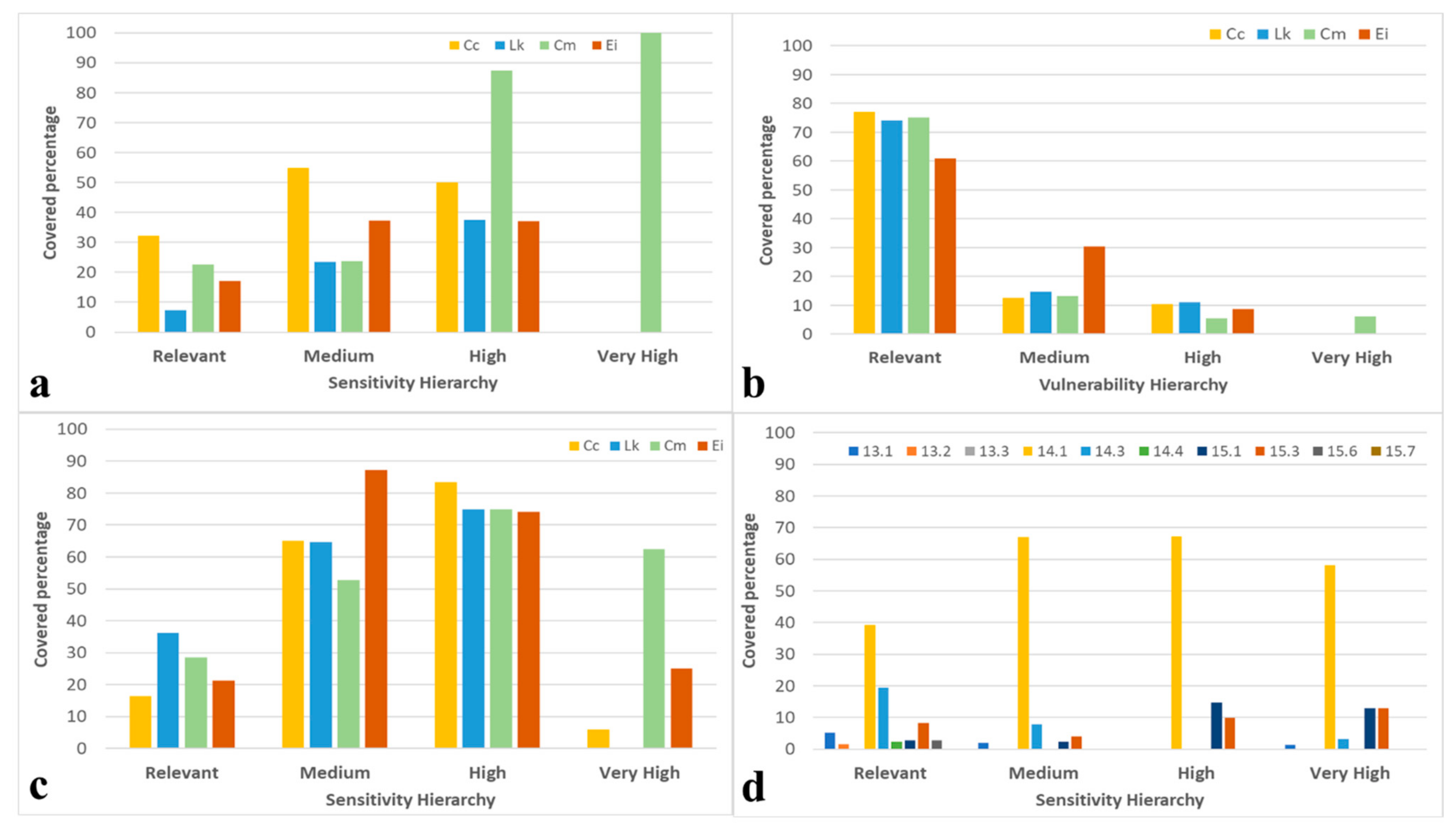
3.1. Ecological Vulnerability Assessment
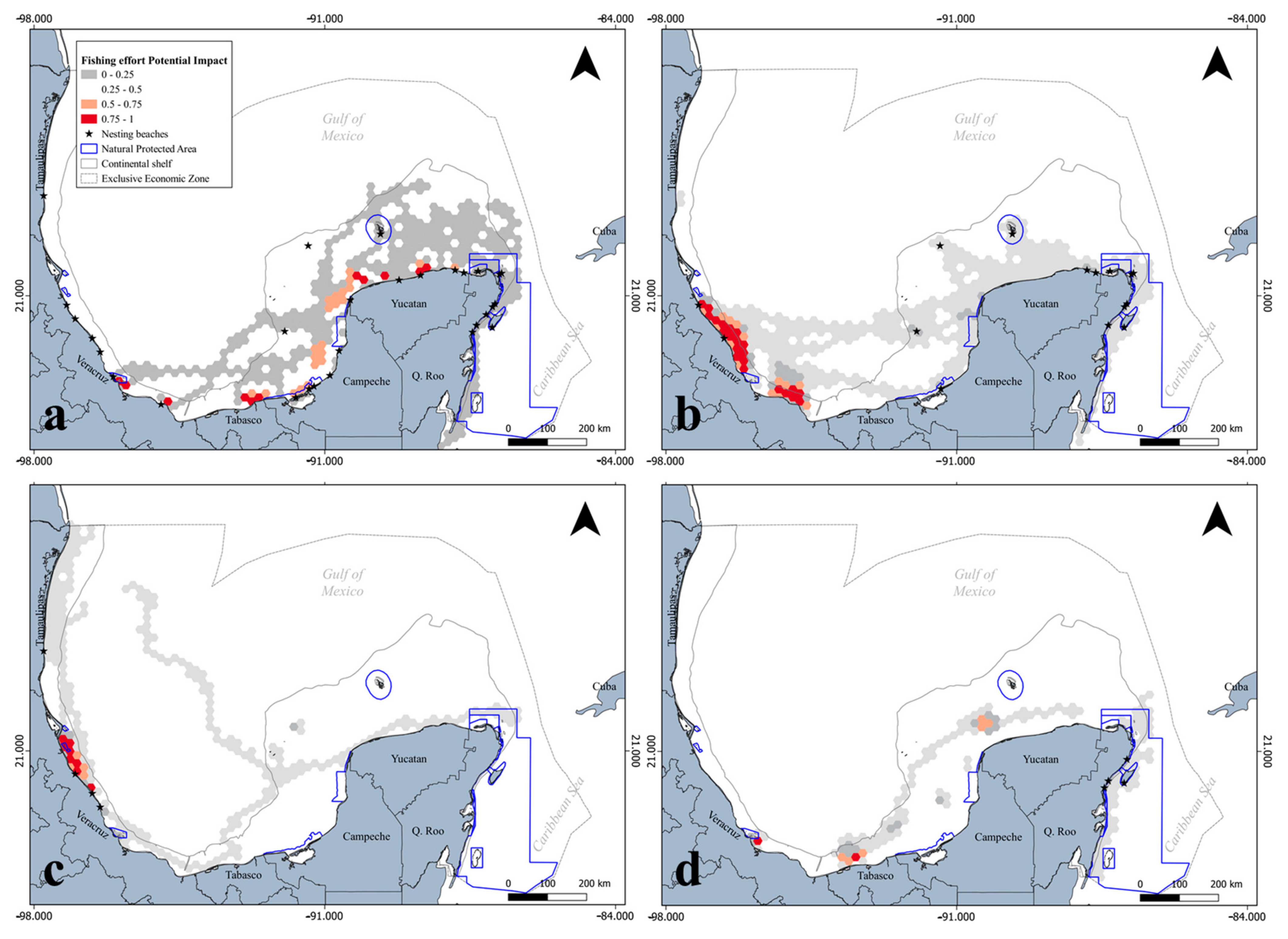
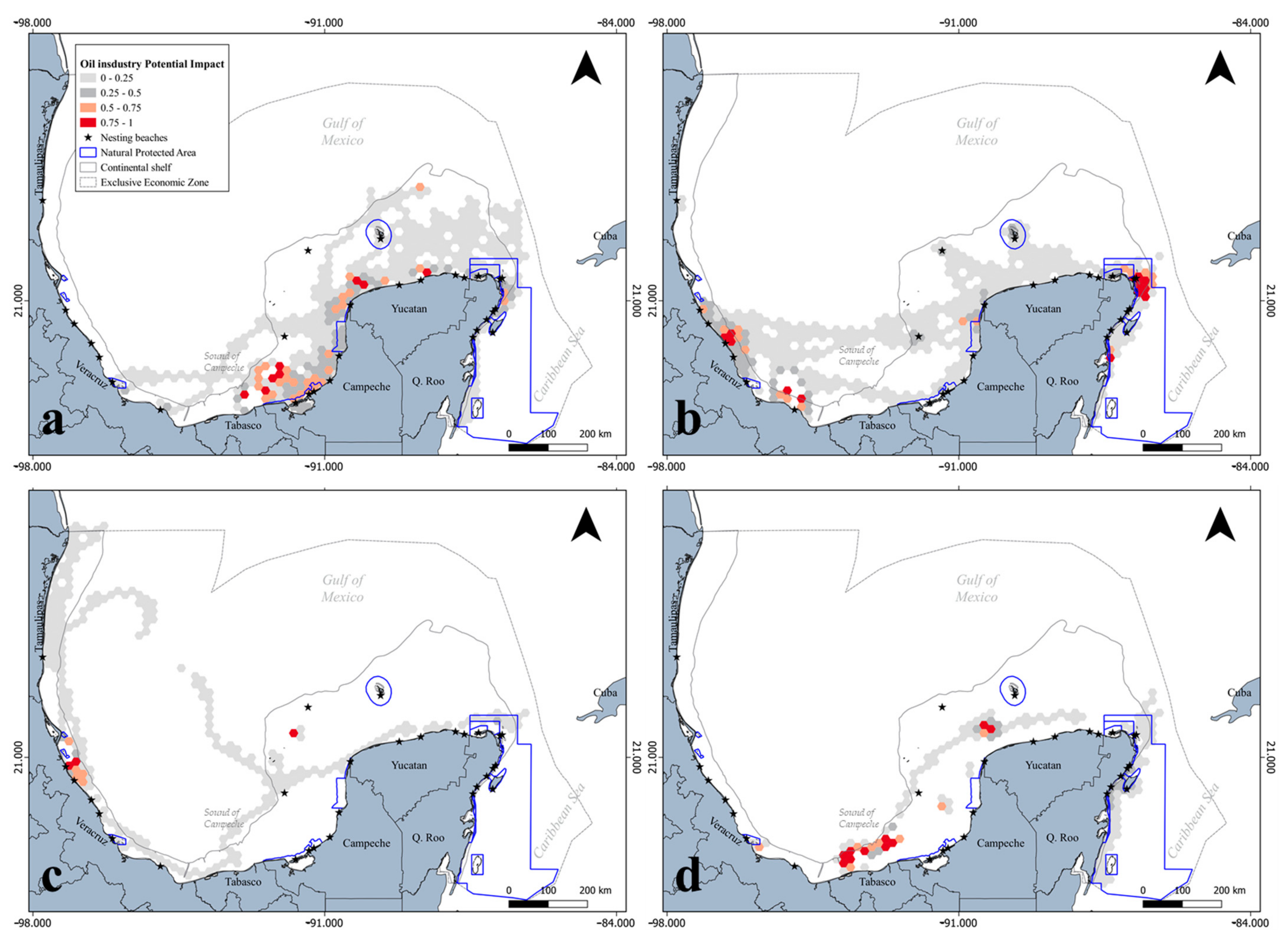
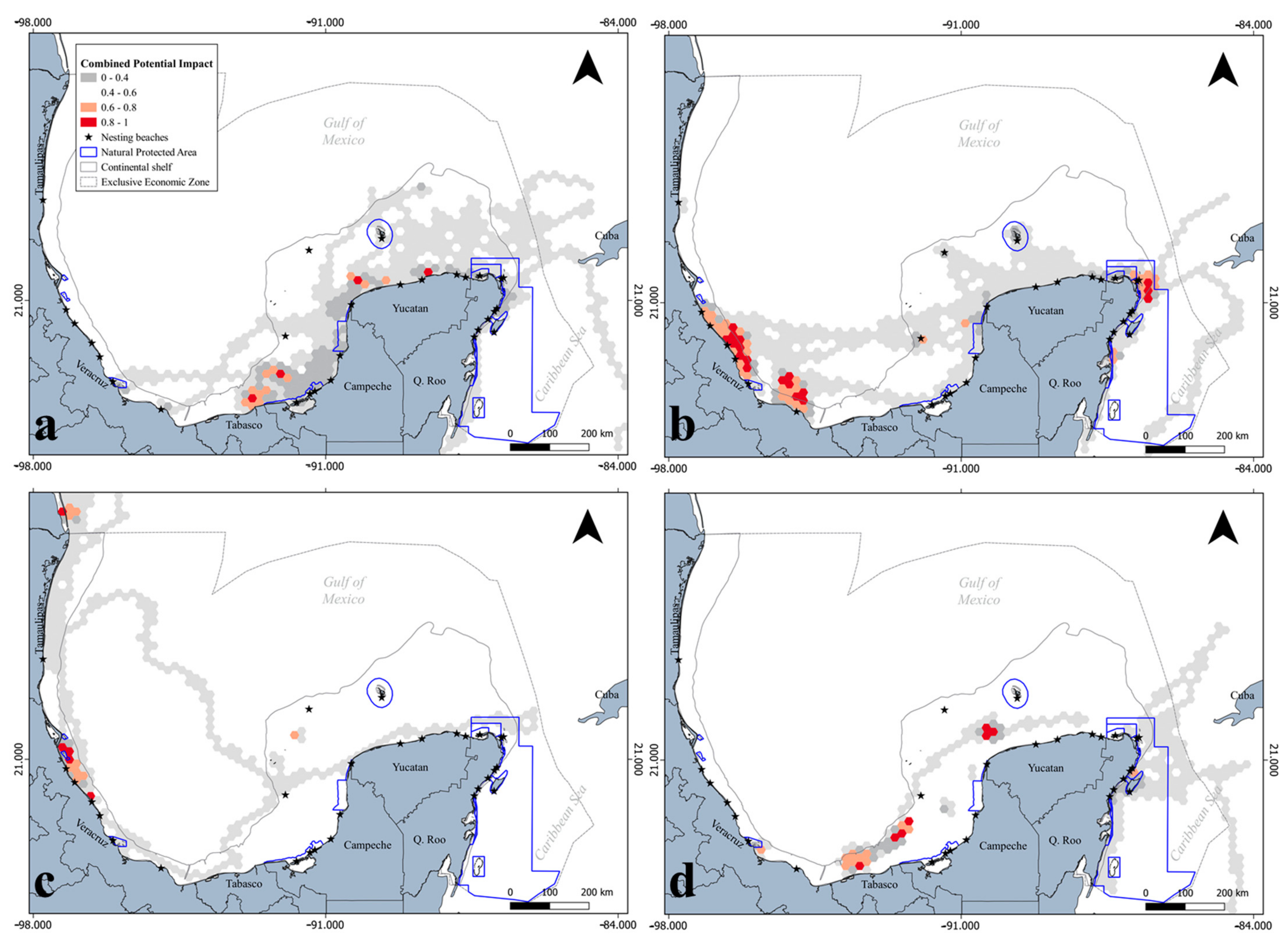
3.2. Spatial Configuration in Terms of Territorial Administration
4. Discussion
4.1. Marine Turtle Ecological Vulnerability
4.2. Territorial Administration Implications for Marine Biota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Moberg, F.; Rönnbäck, P. Ecosystem Services of the Tropical Seascape: Interactions, Substitutions and Restoration. Ocean Coast. Manag. 2003, 46, 27–46. [Google Scholar] [CrossRef]
- Guannel, G.; Arkema, K.; Ruggiero, P.; Verutes, G. The Power of Three: Coral Reefs, Seagrasses and Mangroves Protect Coastal Regions and Increase Their Resilience. PLoS ONE 2016, 11, e0158094. [Google Scholar] [CrossRef]
- Challenger, A.; Cordova, A.; Lazos Chavero, E.; Equihua, M.; Maass, M. Opportunities and Obstacles to Socioecosystem-Based Environmental Policy in Mexico: Expert Opinion at the Science-Policy Interface. Ecol. Soc. 2018, 23, art31. [Google Scholar] [CrossRef]
- García-Jácome, L.G.; García-Frapolli, E.; Bonilla-Moheno, M.; Rangel-Rivera, C.E.; Benítez, M.; Ramos-Fernández, G. Multiple Resource Use Strategies and Resilience of a Socio-Ecosystem in a Natural Protected Area in the Yucatan Peninsula, Mexico. Front. Sustain. Food Syst. 2020, 4, 522657. [Google Scholar] [CrossRef]
- Timpane-Padgham, B.L.; Beechie, T.; Klinger, T. A Systematic Review of Ecological Attributes that Confer Resilience to Climate Change in Environmental Restoration. PLoS ONE 2017, 12, e0173812. [Google Scholar] [CrossRef]
- Bouchard, S.S.; Bjorndal, K.A. Sea Turtles as Biological Transporters of Nutrients and Energy from Marine to Terrestrial Ecosystems. Ecology 2000, 81, 2305–2313. [Google Scholar] [CrossRef]
- Bjorndal, K.A.; Jackson, J.B.C. Roles of sea turtles in marine ecosystems: Reconstructing the past. In The Biology of Sea Turtles; CRC Press: Boca Raton, FL, USA, 2003; Volume II. [Google Scholar]
- Friedman, W.R.; Halpern, B.S.; McLeod, E.; Beck, M.W.; Duarte, C.M.; Kappel, C.V.; Levine, A.; Sluka, R.D.; Adler, S.; O’Hara, C.C.; et al. Research Priorities for Achieving Healthy Marine Ecosystems and Human Communities in a Changing Climate. Front. Mar. Sci. 2020, 7, 5. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Schmitz, O.J.; Flecker, A.S.; Lafferty, K.D.; Sih, A.; Atwood, T.B.; Gallagher, A.J.; Irschick, D.J.; Skubel, R.; Cooke, S.J. Ecosystem Function and Services of Aquatic Predators in the Anthropocene. Trends Ecol. Evol. 2019, 34, 369–383. [Google Scholar] [CrossRef] [PubMed]
- De Lange, H.J.; Sala, S.; Vighi, M.; Faber, J.H. Ecological Vulnerability in Risk Assessment—A Review and Perspectives. Sci. Total Environ. 2010, 408, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine Top Predators as Climate and Ecosystem Sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef]
- Caro, T.M.; O’Doherty, G. On the Use of Surrogate Species in Conservation Biology. Conserv. Biol. 1999, 13, 805–814. [Google Scholar] [CrossRef]
- Hunter, M.; Westgate, M.; Barton, P.; Calhoun, A.; Pierson, J.; Tulloch, A.; Beger, M.; Branquinho, C.; Caro, T.; Gross, J.; et al. Two Roles for Ecological Surrogacy: Indicator Surrogates and Management Surrogates. Ecol. Indic. 2016, 63, 121–125. [Google Scholar] [CrossRef]
- Wabnitz, C.; Balazs, G.; Beavers, S.; Bjorndal, K.; Bolten, A.; Christensen, V.; Hargrove, S.; Pauly, D. Ecosystem Structure and Processes at Kaloko Honokohau, Focusing on the Role of Herbivores, Including the Green Sea Turtle Chelonia mydas, in Reef Resilience. Mar. Ecol. Prog. Ser. 2010, 420, 27–44. [Google Scholar] [CrossRef]
- Hays, G.C.; Alcoverro, T.; Christianen, M.J.A.; Duarte, C.M.; Hamann, M.; Macreadie, P.I.; Marsh, H.D.; Rasheed, M.A.; Thums, M.; Unsworth, R.K.F.; et al. New Tools to Identify the Location of Seagrass Meadows: Marine Grazers as Habitat Indicators. Front. Mar. Sci. 2018, 5, 9. [Google Scholar] [CrossRef]
- Gradzens, C.; Marsh, H.; Fuentes, M.M.P.B.; Limpus, C.J.; Shimada, T.; Hamann, M. Satellite tracking of sympatric marine megafauna can inform the biological basis for species co-management. PLoS ONE 2014, e98944. [Google Scholar] [CrossRef]
- Zacharias, M.A.; Roff, J.C. A Hierarchical Ecological Approach to Conserving Marine Biodiversity. Conserv. Biol. 2000, 14, 1327–1334. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Maxwell, S.M.; Hazen, E.L.; Bograd, S.J.; Halpern, B.S.; Breed, G.A.; Nickel, B.; Teutschel, N.M.; Crowder, L.B.; Benson, S.; Dutton, P.H.; et al. Cumulative Human Impacts on Marine Predators. Nat. Commun. 2013, 4, 2688. [Google Scholar] [CrossRef]
- Cuevas, E.; Liceaga-Correa, M.; Uribe-Martínez, A. Ecological Vulnerability of Two Sea Turtle Species in the Gulf of Mexico: An Integrated Spatial Approach. Endanger. Species Res. 2019, 40, 337–356. [Google Scholar] [CrossRef]
- Ramos, R.; González-Solís, J. Trace Me If You Can: The Use of Intrinsic Biogeochemical Markers in Marine Top Predators. Front. Ecol. Environ. 2012, 10, 258–266. [Google Scholar] [CrossRef]
- Cuevas, E. Dimensiones espacial y temporal de los procesos de selección de hábitats críticos por las tortugas marinas. Rev. Biol. Mar. Oceanogr. 2017, 52, 187–199. [Google Scholar] [CrossRef]
- Frazier, J. Marine Turtles: The Role of Flagship Species in Interactions between People and the Sea. Mast 2005, 3, 5–38. [Google Scholar]
- Kalinkat, G.; Cabral, J.S.; Darwall, W.; Ficetola, G.F.; Fisher, J.L.; Giling, D.P.; Gosselin, M.-P.; Grossart, H.-P.; Jähnig, S.C.; Jeschke, J.M.; et al. Flagship Umbrella Species Needed for the Conservation of Overlooked Aquatic Biodiversity: Freshwater Flagship Umbrella Species. Conserv. Biol. 2017, 31, 481–485. [Google Scholar] [CrossRef]
- Wallace, B.; Stacy, B.; Cuevas, E.; Holyoake, C.; Lara, P.; Marcondes, A.; Miller, J.; Nijkamp, H.; Pilcher, N.; Robinson, I.; et al. Oil Spills and Sea Turtles: Documented Effects and Considerations for Response and Assessment Efforts. Endanger. Species Res. 2020, 41, 17–37. [Google Scholar] [CrossRef]
- Valverde, R.A.; Holzwart, K.R. Sea turtles of the Gulf of Mexico. In Habitats and Biota of the Gulf of Mexico: Before the Deepwater Horizon Oil Spill; Springer: New York, NY, USA, 2017; pp. 1189–1351. [Google Scholar]
- McConkey, K.R.; O’Farrill, G. Cryptic Function Loss in Animal Populations. Trends Ecol. Evol. 2015, 30, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Bolten, A.B.; Crowder, L.B.; Dodd, M.G.; MacPherson, S.L.; Musick, J.A.; Schroeder, B.A.; Witherington, B.E.; Long, K.J.; Snover, M.L. Quantifying Multiple Threats to Endangered Species: An Example from Loggerhead Sea Turtles. Front. Ecol. Environ. 2011, 9, 295–301. [Google Scholar] [CrossRef]
- Lovich, J.E.; Ennen, J.R.; Agha, M.; Gibbons, J.W. Where Have All the Turtles Gone, and Why Does It Matter? BioScience 2018, 68, 771–781. [Google Scholar] [CrossRef]
- Tisdell, C.; Wilson, C. Ecotourism for the survival of sea turtles and other wildlife. Biodivers. Conserv. 2002, 11, 1521–1538. [Google Scholar] [CrossRef]
- Richardson, K.L.; Gold-Bouchot, G.; Schlenk, D. The Characterization of Cytosolic Glutathione Transferase from Four Species of Sea Turtles: Loggerhead (Caretta Caretta), Green (Chelonia Mydas), Olive Ridley (Lepidochelys Olivacea), and Hawksbill (Eretmochelys imbricata). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 279–284. [Google Scholar] [CrossRef]
- Jackson, J.B.C. Reefs since Columbus. Coral Reefs 1997, 16, S23–S32. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Hoey, A.S.; Bellwood, D.R. The Role of Turtles as Coral Reef Macroherbivores. PLoS ONE 2012, 7, e39979. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.N. Predation on pelagic coelenterates: A review. J. Mar. Biol. Assoc. UK 2005, 85, 523–536. [Google Scholar] [CrossRef]
- Johnson, R.A.; Gulick, A.G.; Bolten, A.B.; Bjorndal, K.A. Blue Carbon Stores in Tropical Seagrass Meadows Maintained under Green Turtle Grazing. Sci. Rep. 2017, 7, 13545. [Google Scholar] [CrossRef]
- Johnson, R.A.; Gulick, A.G.; Constant, N.; Bolten, A.B.; Smulders, F.O.H.; Christianen, M.J.A.; Nava, M.I.; Kolasa, K.; Bjorndal, K.A. Seagrass Ecosystem Metabolic Carbon Capture in Response to Green Turtle Grazing across Caribbean Meadows. J. Ecol. 2020, 108, 1101–1114. [Google Scholar] [CrossRef]
- Hannan, L.B.; Roth, J.D.; Ehrhart, L.M.; Weishampel, J.F. Dune Vegetation Fertilization by Nesting Sea Turtles. Ecology 2007, 88, 1053–1058. [Google Scholar] [CrossRef]
- Madden, D.; Ballestero, J.; Calvo, C.; Carlson, R.; Christians, E.; Madden, E. Sea Turtle Nesting as a Process Influencing a Sandy Beach Ecosystem. Biotropica 2008, 40, 758–765. [Google Scholar] [CrossRef]
- Vander Zanden, H.B.; Bjorndal, K.A.; Inglett, P.W.; Bolten, A.B. Marine-Derived Nutrients from Green Turtle Nests Subsidize Terrestrial Beach Ecosystems. Biotropica 2012, 44, 294–301. [Google Scholar] [CrossRef]
- Hilborn, R.; Sinclair, A.R.E. Biodiversity Protection in the 21st Century Needs Intact Habitat and Protection from Overexploitation Whether inside or Outside Parks. Conserv. Lett. 2021, 14, e12830. [Google Scholar] [CrossRef]
- Ware, M.; Fuentes, M. Leave No Trace Ordinances for Coastal Species Management: Influences on Sea Turtle Nesting Success. Endanger. Species Res. 2020, 41, 197–207. [Google Scholar] [CrossRef]
- Liceaga-Correa, M.A.; Uribe-Martínez, A.; Cuevas, E. Vulnerabilidad ecológica de tortugas marinas ante múltiples amenazas y derrames de petróleo de gran escala en el golfo de México. In Vulnerabilidad Ecológica del Golfo de México Ante Derrames a Gran Escala; CICESE, CINVESTAV, UNAM: Ensenada, Mexico, 2020; Volume II, pp. 17–41. [Google Scholar]
- Uribe-Martínez, A.; Liceaga-Correa, M.; de los, A.; Cuevas, E. Critical In-Water Habitats for Post-Nesting Sea Turtles from the Southern Gulf of Mexico. J. Mar. Sci. Eng. 2021, 9, 793. [Google Scholar] [CrossRef]
- Dow Piniak, W.; Eckert, K. Sea Turtle Nesting Habitat in the Wider Caribbean Region. Endanger. Species Res. 2011, 15, 129–141. [Google Scholar] [CrossRef][Green Version]
- Cuevas, E.; Abreu-Grobois, F.; Guzmán-Hernández, V.; Liceaga-Correa, M.; van Dam, R. Post-Nesting Migratory Movements of Hawksbill Turtles Eretmochelys imbricata in Waters Adjacent to the Yucatan Peninsula, Mexico. Endanger. Species Res. 2008, 10, 123–133. [Google Scholar] [CrossRef]
- Méndez, D.; Cuevas, E.; Navarro, J.; González-Garza, B.I.; Guzmán-Hernández, V. Rastreo satelital de las hembras de tortuga blanca Chelonia mydas y evaluación de sus ámbitos hogareños en la costa norte de la península de Yucatán, México. Rev. Biol. Mar. Oceanogr. 2013, 48, 497–509. [Google Scholar] [CrossRef]
- Vázquez, M.; Cuevas, E. Hábitats críticos de tortuga blanca (Chelonia mydas) y carey (Eretmochelys imbricata) en la península de Yucatán y su coincidencia espacial con zonas de pesca artesanal. In El Uso del Conocimiento de las Tortugas Marinas Como Herramienta Para la Restauración de Sus Poblaciones y Hábitats Asociados en México; Universidad Autónoma del Carmen: Ciudad del Carmen, Mexico, 2019; pp. 57–62. [Google Scholar]
- Gallegos-Fernández, S.A.; Cuevas, E.; de los Ángeles Liceaga-Correa, M. Procesos metodológicos para la colocación de transmisores satelitales en tortugas marinas de caparazón duro en playas de anidación. Rev. Biol. Mar. Oceanogr. 2018, 53, 147. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lopez, R.; Malardé, J.P.R.; Gaspar, P. Improving Argos doppler location using multiple-model Kalman filtering. IEEE Trans. Geosci. Remote Sens. 2013, 52, 4744–4755. [Google Scholar] [CrossRef]
- Boyd, J.D.; Brightsmith, D.J. Error Properties of Argos Satellite Telemetry Locations Using Least Squares and Kalman Filtering. PLoS ONE 2013, 8, e63051. [Google Scholar] [CrossRef]
- Shaver, D.J.; Hart, K.M.; Fujisaki, I.; Rubio, C.; Sartain, A.R.; Peña, J.; Burchfield, P.M.; Gamez, D.G.; Ortiz, J. Foraging Area Fidelity for Kemp’s Ridleys in the Gulf of Mexico. Ecol. Evol. 2013, 3, 2002–2012. [Google Scholar] [CrossRef]
- Shaver, D.J.; Hart, K.M.; Fujisaki, I.; Rubio, C.; Sartain-Iverson, A.R.; Peña, J.; Gamez, D.G.; de Jesus Gonzales Diaz Miron, R.; Burchfield, P.M.; Martinez, H.J.; et al. Migratory Corridors of Adult Female Kemp’s Ridley Turtles in the Gulf of Mexico. Biol. Conserv. 2016, 194, 158–167. [Google Scholar] [CrossRef]
- Iverson, A.R.; Benscoter, A.M.; Fujisaki, I.; Lamont, M.M.; Hart, K.M. Migration Corridors and Threats in the Gulf of Mexico and Florida Straits for Loggerhead Sea Turtles. Front. Mar. Sci. 2020, 7, 208. [Google Scholar] [CrossRef]
- Freitas, C.; Lydersen, C.; Fedak, M.A.; Kovacs, K.M. A Simple New Algorithm to Filter Marine Mammal Argos Locations. Mar. Mammal Sci. 2008, 24, 315–325. [Google Scholar] [CrossRef]
- Hart, K.M.; Lamont, M.M.; Iverson, A.R.; Smith, B.J. The Importance of the Northeastern Gulf of Mexico to Foraging Loggerhead Sea Turtles. Front. Mar. Sci. 2020, 7, 330. [Google Scholar] [CrossRef]
- Granizo, T.; Molina, M.E.; Secaira, E.; Herrera, B. Manual de Planificación Para la Conservación de Áreas, PCA, 1st ed.; The Nature Conservancy/USAID: Quito, Ecuador, 2006. [Google Scholar]
- Zacharias, M.A.; Gregr, E.J. Sensitivity and Vulnerability in Marine Environments: An Approach to Identifying Vulnerable Marine Areas. Conserv. Biol. 2005, 19, 86–97. [Google Scholar] [CrossRef]
- Wilkinson, T.; Wiken, E.; Bezaury-Creel, J.; Hourigan, T.; Agardy, T.; Herrmann, H.; Janishevski, L.; Madden, C.; Morgan, L.; Padilla, M. Marine ecoregions of North America. In Geospatial_Data_Presentation_Form: Vector Digital Data; Commission for Environmental Cooperation: Montreal, QC, Canada, 2009. [Google Scholar]
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad; Comisión Nacional de Áreas Naturales Protegidas. The nature conservancy—Programa México; Pronatura. In Sitios Prioritarios Marinos para la Conservación de la Biodiversidad; CONABIO: Ciudad de México, Mexico, 2007. [Google Scholar]
- Diario Oficial de la Federación. Acuerdo por el Que Se Expide la Parte Marina del Programa de Ordenamiento Ecológico Marino y Regional del Golfo de México y Mar Caribe y Se da a Conocer la Parte Regional del Propio Programa (Continúa en la Segunda Sección). Available online: http://dof.gob.mx/nota_detalle.php?codigo=5279084&fecha=24/11/2012 (accessed on 10 October 2021).
- Hart, K.M.; Lamont, M.M.; Fujisaki, I.; Tucker, A.D.; Carthy, R.R. Common Coastal Foraging Areas for Loggerheads in the Gulf of Mexico: Opportunities for Marine Conservation. Biol. Conserv. 2012, 145, 185–194. [Google Scholar] [CrossRef]
- Hays, G.C.; Bailey, H.; Bograd, S.J.; Bowen, W.D.; Campagna, C.; Carmichael, R.H.; Casale, P.; Chiaradia, A.; Costa, D.P.; Cuevas, E.; et al. Translating Marine Animal Tracking Data into Conservation Policy and Management. Trends Ecol. Evol. 2019, 34, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Edgar, G.J. Does the Global Network of Marine Protected Areas Provide an Adequate Safety Net for Marine Biodiversity?: EDITORIAL. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 313–316. [Google Scholar] [CrossRef]
- Love, M.; Robbins, C.; Baldera, A.; Eastman, S.; Bolten, A.; Hardy, R.; Herren, R.; Metz, T.; Zanden, H.B.V.; Wallace, B.; et al. Restoration without Borders: An Assessment of Cumulative Stressors to Guide Large-Scale, Integrated Restoration of Sea Turtles in the Gulf of Mexico. Ocean. Conserv. Rep. 2017. [Google Scholar] [CrossRef]
- Hart, K.M.; Iverson, A.R.; Fujisaki, I.; Lamont, M.M.; Bucklin, D.; Shaver, D.J. Marine Threats Overlap Key Foraging Habitat for Two Imperiled Sea Turtle Species in the Gulf of Mexico. Front. Mar. Sci. 2018, 5, 336. [Google Scholar] [CrossRef]
- Cuevas, E.; Guzmán-Hernández, V.; Uribe-Martínez, A.; Raymundo-Sánchez, A.; Herrera-Pavon, R. Identification of Potential Sea Turtle Bycatch Hotspots Using a Spatially Explicit Approach in the Yucatan Peninsula, Mexico. Chelonian Conserv. Biol. 2018, 17, 78–93. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación. PROYECTO de Modificación a La Norma Oficial Mexicana NOM-029-PESC-2006, Pesca Responsable de Tiburones y Rayas. Especificaciones Para Su Aprovechamiento; 2015. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5381585&fecha=11/02/2015 (accessed on 10 October 2021).
- Finkbeiner, E.M.; Wallace, B.P.; Moore, J.E.; Lewison, R.L.; Crowder, L.B.; Read, A.J. Cumulative Estimates of Sea Turtle Bycatch and Mortality in USA Fisheries between 1990 and 2007. Biol. Conserv. 2011, 144, 2719–2727. [Google Scholar] [CrossRef]
- Putman, N.F.; Hawkins, J.; Gallaway, B.J. Managing Fisheries in a World with More Sea Turtles. Proc. R. Soc. B 2020, 287, 20200220. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación. NORMA Oficial Mexicana NOM-002-SAG/PESC-2013, Para Ordenar el Aprovechamiento de las Especies de Camarón en Aguas Dejurisdicción Federal de los Estados Unidos Mexicanos. 2013. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5306294&fecha=11/07/2013 (accessed on 10 October 2021).
- Diario Oficial de la Federación. NORMA Oficial Mexicana NOM-061-SAG-PESC/SEMARNAT-2016, Especificaciones Técnicas de los Excluidores de Tortugas Marinasutilizados por la Flota de Arrastre Camaronera en Aguas de Jurisdicción Federal de los Estados Unidos Mexicanos; FAO: Rome, Italy, 2016. [Google Scholar]
- Raborn, S.W.; Gallaway, B.J.; Cole, J.G.; Gazey, W.J.; Andrews, K.I. Effects of Turtle Excluder Devices (TEDs) on the Bycatch of Three Small Coastal Sharks in the Gulf of Mexico Penaeid Shrimp Fishery. N. Am. J. Fish. Manag. 2012, 32, 333–345. [Google Scholar] [CrossRef]
- Willems, T.; Depestele, J.; De Backer, A.; Hostens, K. Ray Bycatch in a Tropical Shrimp Fishery: Do Bycatch Reduction Devices and Turtle Excluder Devices Effectively Exclude Rays? Fish. Res. 2016, 175, 35–42. [Google Scholar] [CrossRef]
- Bojórquez-Tapia, L.A.; Ponce-Díaz, G.; Pedroza-Páez, D.; Díaz-de-León, A.J.; Arreguín-Sánchez, F. Application of Exploratory Modeling in Support of Transdisciplinary Inquiry: Regulation of Fishing Bycatch of Loggerhead Sea Turtles in Gulf of Ulloa, Mexico. Front. Mar. Sci. 2021, 8, 643347. [Google Scholar] [CrossRef]
- Secretaría de Agricultura y Desarrollo Rural; Comisión Nacional de Acuacultura y Pesca. Plan de Acciones Emergentes Implementadas por el Gobierno Mexicano Para la Conservación de Tortugas Marinas, 1st ed.; SADER: Ciudad de Mexico, Mexico, 2021. [Google Scholar]
- Yaghmour, F. Are oil spills a key mortality factor for marine turtles from the eastern coast of the United Arab Emirates? Mar. Pollut. Bull. 2019, 149, 110624. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación. ACUERDO Secretarial Número 249, por el Cual Se Expide la Versión Abreviada del Plan Nacional de Contingencia para Derrames de Hidrocarburos y Sustancias Nocivas Potencialmente Peligrosas en las Zonas Marinas Mexicanas. 2016. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5458067&fecha=24/10/2016 (accessed on 10 October 2021).
- Frazão Santos, C.; Michel, J.; Neves, M.; Janeiro, J.; Andrade, F.; Orbach, M. Marine Spatial Planning and Oil Spill Risk Analysis: Finding Common Grounds. Mar. Pollut. Bull. 2013, 74, 73–81. [Google Scholar] [CrossRef]
- Fetissov, M.; Aps, R.; Goerlandt, F.; Jänes, H.; Kotta, J.; Kujala, P.; Szava-Kovats, R. Next-Generation Smart Response Web (NG-SRW): An Operational Spatial Decision Support System for Maritime Oil Spill Emergency Response in the Gulf of Finland (Baltic Sea). Sustainability 2021, 13, 6585. [Google Scholar] [CrossRef]
- García-Aguilar, M.C.; Romo-Curiel, A.E.; Ramírez-León, M.R.; Ramírez-Mendoza, Z.; Fajardo-Yamamoto, A.; Sosa-Nishizaki, O. Modelación espacial de la coocurrencia entre los cetáceos y derrames de petróleo en el Golfo de México. In Vulnerabilidad Ecológica del Golfo de México Ante Derrames a Gran Escala; CICESE, CINVESTAV, UNAM: Ensenada, Mexico, 2020; Volume II, pp. 75–118. [Google Scholar]
- Sosa-Nishizaki, O.; Romo-Curiel, A.E.; Ramírez-Mendoza, Z.; Fajardo-Yamamoto, A.; García-Aguilar, M.C.; Ramírez-León, M.R. Evaluación de la vulnerabilidad de los peces pelágicos ante escenarios de derrame de petróleo profundos en el Golfo de México. In Vulnerabilidad Ecológica del Golfo de México Ante Derrames a Gran Escala; CICESE, CINVESTAV, UNAM: Ensenada, Mexico, 2020; Volume II, pp. 119–152. [Google Scholar]
- McDonald, T.; Schroeder, B.; Stacy, B.; Wallace, B.; Starcevich, L.; Gorham, J.; Tumlin, M.; Cacela, D.; Rissing, M.; McLamb, D.; et al. Density and Exposure of Surface-Pelagic Juvenile Sea Turtles to Deepwater Horizon Oil. Endanger. Species Res. 2017, 33, 69–82. [Google Scholar] [CrossRef]
- Takeshita, R.; Sullivan, L.; Smith, C.; Collier, T.; Hall, A.; Brosnan, T.; Rowles, T.; Schwacke, L. The Deepwater Horizon Oil Spill Marine Mammal Injury Assessment. Endanger. Species Res. 2017, 33, 95–106. [Google Scholar] [CrossRef]
- Wallace, B.; Stacy, B.; Rissing, M.; Cacela, D.; Garrison, L.; Graettinger, G.; Holmes, J.; McDonald, T.; McLamb, D.; Schroeder, B. Estimating Sea Turtle Exposures to Deepwater Horizon Oil. Endanger. Species Res. 2017, 33, 51–67. [Google Scholar] [CrossRef]
- Scholz, A.; Bonzon, K.; Fujita, R.; Benjamin, N.; Woodling, N.; Black, P.; Steinback, C. Participatory Socioeconomic Analysis: Drawing on Fishermen’s Knowledge for Marine Protected Area Planning in California. Mar. Policy 2004, 28, 335–349. [Google Scholar] [CrossRef]
- Lopes, R.; Videira, N. Valuing Marine and Coastal Ecosystem Services: An Integrated Participatory Framework. Ocean Coast. Manag. 2013, 84, 153–162. [Google Scholar] [CrossRef]
- Lauerburg, R.A.M.; Diekmann, R.; Blanz, B.; Gee, K.; Held, H.; Kannen, A.; Möllmann, C.; Probst, W.N.; Rambo, H.; Cormier, R.; et al. Socio-Ecological Vulnerability to Tipping Points: A Review of Empirical Approaches and Their Use for Marine Management. Sci. Total Environ. 2020, 705, 135838. [Google Scholar] [CrossRef] [PubMed]
- Planque, B.; Mullon, C.; Arneberg, P.; Eide, A.; Fromentin, J.; Heymans, J.J.; Hoel, A.H.; Niiranen, S.; Ottersen, G.; Sandø, A.B.; et al. A Participatory Scenario Method to Explore the Future of Marine Social-ecological Systems. Fish Fish. 2019, 20, 434–451. [Google Scholar] [CrossRef]
- Sowman, M.; Raemaekers, S. Socio-Ecological Vulnerability Assessment in Coastal Communities in the BCLME Region. J. Mar. Syst. 2018, 188, 160–171. [Google Scholar] [CrossRef]
- Campbell, L.M.; Gray, N.J. Area Expansion versus Effective and Equitable Management in International Marine Protected Areas Goals and Targets. Mar. Policy 2019, 100, 192–199. [Google Scholar] [CrossRef]
- Revuelta, O.; Hawkes, L.; León, Y.; Godley, B.; Raga, J.; Tomás, J. Evaluating the Importance of Marine Protected Areas for the Conservation of Hawksbill Turtles Eretmochelys imbricata Nesting in the Dominican Republic. Endanger. Species Res. 2015, 27, 169–180. [Google Scholar] [CrossRef]
- Dawson, T.M.; Formia, A.; Agamboué, P.D.; Asseko, G.M.; Boussamba, F.; Cardiec, F.; Chartrain, E.; Doherty, P.D.; Fay, J.M.; Godley, B.J.; et al. Informing Marine Protected Area Designation and Management for Nesting Olive Ridley Sea Turtles Using Satellite Tracking. Front. Mar. Sci. 2017, 4, 312. [Google Scholar] [CrossRef]
- Fuentes, M.M.P.B.; Gillis, A.J.; Ceriani, S.A.; Guttridge, T.L.; Van Zinnicq Bergmann, M.P.M.; Smukall, M.; Gruber, S.H.; Wildermann, N. Informing Marine Protected Areas in Bimini, Bahamas by Considering Hotspots for Green Turtles (Chelonia mydas). Biodivers. Conserv. 2019, 28, 197–211. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Thums, M.; Fossette, S.; Wilson, P.; Shimada, T.; Tucker, A.D.; Pendoley, K.; Waayers, D.; Guinea, M.L.; Loewenthal, G.; et al. Multiple Satellite Tracking Datasets Inform Green Turtle Conservation at a Regional Scale. Divers. Distrib. 2021, 27, 249–266. [Google Scholar] [CrossRef]
- Roberts, K.E.; Smith, B.J.; Burkholder, D.; Hart, K.M. Evaluating the Use of Marine Protected Areas by Endangered Species: A Habitat Selection Approach. Ecol. Solut. Evid. 2021, 2, e12035. [Google Scholar] [CrossRef]
- Shaver, D.J.; Frandsen, H.R.; George, J.A.; Gredzens, C. Green Turtle (Chelonia mydas) Nesting Underscores the Importance of Protected Areas in the Northwestern Gulf of Mexico. Front. Mar. Sci. 2020, 7, 673. [Google Scholar] [CrossRef]
- Santos, A.J.B.; Bellini, C.; Santos, E.A.P.; Sales, G.; Ramos, R.; Vieira, D.H.G.; Marcovaldi, M.A.; Gillis, A.; Wildermann, N.; Mills, M.; et al. Effectiveness and Design of Marine Protected Areas for Migratory Species of Conservation Concern: A Case Study of Post-Nesting Hawksbill Turtles in Brazil. Biol. Conserv. 2021, 261, 109229. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación. Ley General del Equilibrio Ecológico y la Protección al Ambiente. 2021. Available online: http://www.diputados.gob.mx/LeyesBiblio/pdf/LGEEPA.pdf (accessed on 10 October 2021).
- Polanco Trujillo, L.D.A.; Gutiérrez Aguirre, M.A. Evaluación de enfoques metodológicos que analizan la efectividad de las áreas naturales protegidas de Quintana Roo, México. Teoría Prax. 2013, 9, 59–82. [Google Scholar] [CrossRef]
- Cuevas, E.; Liceaga-Correa, M.A. Santuarios para tortugas marinas: Propuestas. In La Costa del Estado de Yucatán: Un Espacio de Reflexión Sobre la Relación Sociedad-Naturaleza, en el Contexto de Su Ordenamiento Ecológico Territorial; Plaza y Valdes: Ciudad de México, Mexico, 2014; Volume 1, pp. 303–310. [Google Scholar]
- Bolio Ortiz, H.J.; Bolio Ortiz, J.P.; Lara Farfan, F.D.L.L.; Hernandez Rugerio, A.D. Economía y sustentabilidad. Propuesta de evaluación del Programa de Ordenamiento Ecológico Costero de Yucatán. Rev. Logos Cienc. Tecnol. 2016, 8, 136–147. [Google Scholar] [CrossRef]
- Fernández, R.J.L.; Gil, A.M.; Franco, J.F.S. La racionalidad económica en los nuevos criterios de regulación ecológica del Programa de Ordenamiento Ecológico del Territorio Costero del Estado de Yucatán. Paradig. Económico 2017, 9, 79–102. [Google Scholar]
- Fish, M.R.; Côté, I.M.; Horrocks, J.A.; Mulligan, B.; Watkinson, A.R.; Jones, A.P. Construction Setback Regulations and Sea-Level Rise: Mitigating Sea Turtle Nesting Beach Loss. Ocean Coast. Manag. 2008, 51, 330–341. [Google Scholar] [CrossRef]
- Crowder, L.; Norse, E. Essential Ecological Insights for Marine Ecosystem-Based Management and Marine Spatial Planning. Mar. Policy 2008, 32, 772–778. [Google Scholar] [CrossRef]
- Toonen, H.M.; van Tatenhove, J.P.M. Marine Scaping: The Structuring of Marine Practices. Ocean Coast. Manag. 2013, 75, 43–52. [Google Scholar] [CrossRef]
- Long, R.D.; Charles, A.; Stephenson, R.L. Key Principles of Marine Ecosystem-Based Management. Mar. Policy 2015, 57, 53–60. [Google Scholar] [CrossRef]
- Maass, M.; Balvanera, P.; Bourgeron, P.; Equihua, M.; Baudry, J.; Dick, J.; Forsius, M.; Halada, L.; Krauze, K.; Nakaoka, M.; et al. Changes in Biodiversity and Trade-Offs among Ecosystem Services, Stakeholders, and Components of Well-Being: The Contribution of the International Long-Term Ecological Research Network (ILTER) to Programme on Ecosystem Change and Society (PECS). Ecol. Soc. 2016, 21, art31. [Google Scholar] [CrossRef]
- Murphy, S.E.; Farmer, G.; Katz, L.; Troëng, S.; Henderson, S.; Erdmann, M.V.; Corrigan, C.; Gold, B.; Lavoie, C.; Quesada, M.; et al. Fifteen Years of Lessons from the Seascape Approach: A Framework for Improving Ocean Management at Scale. Conserv. Sci. Pract. 2021, 3, e423. [Google Scholar] [CrossRef]
- Queffelec, B.; Bonnin, M.; Ferreira, B.; Bertrand, S.; Teles Da Silva, S.; Diouf, F.; Trouillet, B.; Cudennec, A.; Brunel, A.; Billant, O.; et al. Marine Spatial Planning and the Risk of Ocean Grabbing in the Tropical Atlantic. ICES J. Mar. Sci. 2021, fsab006. [Google Scholar] [CrossRef]
- Esty, D.C. (Ed.) Global Environmental Governance: Options & Opportunities; Yale School of Forestry & Environmental Studies: New Haven, CT, USA, 2002; ISBN 978-0-9707882-2-1. [Google Scholar]
- Herrmann, H. El papel de las organizaciones de la sociedad civil en el manejo costero de México. In El Manejo Costero en México; Universidad Autónoma de Quintana Roo: Campeche, Mexico, 2004; pp. 115–132. [Google Scholar]
- Bultitude, K.; Rodari, P.; Weitkamp, E. Bridging the Gap between Science and Policy: The Importance of Mutual Respect, Trust and the Role of Mediators. Online J. Sci. Commun. 2012, 11, C01. [Google Scholar] [CrossRef]
- Rodríguez Cardozo, L. El Desarrollo de Las ONG de México y Su Coincidencia Con Los Objetivos Para El Desarrollo Sostenible de Naciones Unidas. Ciriec-España 2017, 91, 59–84. [Google Scholar] [CrossRef]
- Parviainen, T.; Lehikoinen, A.; Kuikka, S.; Haapasaari, P. How Can Stakeholders Promote Environmental and Social Responsibility in the Shipping Industry? WMU J. Marit. Aff. 2018, 17, 49–70. [Google Scholar] [CrossRef]
- Rose, N.A.; Parsons, E.C.M. “Back off, Man, I’m a Scientist!” When Marine Conservation Science Meets Policy. Ocean Coast. Manag. 2015, 115, 71–76. [Google Scholar] [CrossRef]
- Holmes, J.; Clark, R. Enhancing the Use of Science in Environmental Policy-Making and Regulation. Environ. Sci. Policy 2008, 11, 702–711. [Google Scholar] [CrossRef]
- Fuentes, M.M.P.B.; Limpus, C.J.; Hamann, M. Vulnerability of Sea Turtle Nesting Grounds to Climate Change: VULNERABILITY ASSESSMENT FRAMEWORK. Glob. Chang. Biol. 2011, 17, 140–153. [Google Scholar] [CrossRef]
- North, M.A. A method for implementing a statistically significant number of data classes in the jenks algorithm. In Proceedings of the 2009 Sixth International Conference on Fuzzy Systems and Knowledge Discovery, Tianjin, China, 14–16 August 2009; pp. 35–38. [Google Scholar]
- Worton, B.J. Kernel Methods for Estimating the Utilization Distribution in Home-Range Studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Schofield, G.; Dimadi, A.; Fossette, S.; Katselidis, K.A.; Koutsoubas, D.; Lilley, M.K.S.; Luckman, A.; Pantis, J.D.; Karagouni, A.D.; Hays, G.C. Satellite Tracking Large Numbers of Individuals to Infer Population Level Dispersal and Core Areas for the Protection of an Endangered Species. Divers. Distrib. 2013, 19, 834–844. [Google Scholar] [CrossRef]
- Calenge, C. The Package “Adehabitat” for the R Software: A Tool for the Analysis of Space and Habitat Use by Animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Comisión Nacional de Áreas Naturales Protegidas. Programa de Acción para la Conservación de la Especie Eretmochelys imbricata. 2009. Available online: https://www.gob.mx/cms/uploads/attachment/file/350214/PACE_Tortuga_Carey.pdf (accessed on 10 October 2021).
- Comisión Nacional de Áreas Naturales Protegidas. Programa de Acción para la Conservación de la Especie Chelonia mydas. 2011. Available online: https://www.gob.mx/conanp/documentos/programa-de-accion-para-la-conservacion-de-la-especie-tortuga-verde-negra-chelonia-mydas (accessed on 10 October 2021).
- Saaty, T.L. Decision Making with the Analytic Hierarchy Process. Int. J. Serv. Sci. 2008, 1, 83–98. [Google Scholar] [CrossRef]
- Goepel, K.D. Implementing the analytic hierarchy process as a standard method for multi-criteria decision making in corporate enterprises—A new AHP excel template with multiple inputs. In Proceedings of the International Symposium on the Analytic Hierarchy Process; Creative Decisions Foundation Kuala Lumpur: Kuala Lumpur, Malaysia, 2013. [Google Scholar]
- Diario Oficial de la Federación. Acuerdo por el Que Se da a Conocer la Actualización de la Carta Nacional Pesquera. 2012. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5265388&fecha=24/08/2012 (accessed on 10 October 2021).
- Noguez-Fuentes, J.F.; Dreyfus-León, M.J. Análisis de la pesca de atún con palangre en el Golfo de México durante las fases de luna nueva y llena Analysis of the long-line tuna fishery in the Gulf of Mexico during the new and full moon phases. Hidrobiológica 2007, 17, 91–99. [Google Scholar]
- Comision Nacional de Acuacultura y Pesca. Anuario Estadístico de Acuacultura y Pesca. 2014. Available online: https://www.gob.mx/conapesca/documentos/anuario-estadistico-de-acuacultura-y-pesca (accessed on 10 October 2021).
- Hawkes, L.; Broderick, A.; Godfrey, M.; Godley, B. Climate Change and Marine Turtles. Endanger. Species Res. 2009, 7, 137–154. [Google Scholar] [CrossRef]
- Patrício, A.R.; Varela, M.R.; Barbosa, C.; Broderick, A.C.; Catry, P.; Hawkes, L.A.; Regalla, A.; Godley, B.J. Climate Change Resilience of a Globally Important Sea Turtle Nesting Population. Glob. Chang. Biol. 2019, 25, 522–535. [Google Scholar] [CrossRef]
- Ocean Biology Processing Group. MODIS Aqua Level 3 SST MID-IR Monthly 4 Km Nighttime; Version 2014.0; PO.DAAC: Pasadena, CA, USA, 2016. [Google Scholar]
- Fuentes, M.; Abbs, D. Effects of Projected Changes in Tropical Cyclone Frequency on Sea Turtles. Mar. Ecol. Prog. Ser. 2010, 412, 283–292. [Google Scholar] [CrossRef]
- Dewald, J.R.; Pike, D.A. Geographical Variation in Hurricane Impacts among Sea Turtle Populations. J. Biogeogr. 2014, 41, 307–316. [Google Scholar] [CrossRef]
- NOAA Hurricane Tracks: Cumulative—1950–2020. 2021. Available online: https://sos.noaa.gov/catalog/datasets/hurricane-tracks-cumulative/ (accessed on 10 October 2021).
- Servicio Meteorologico Nacional. Etapas de Evolución-Ciclones Tropicales. Available online: https://smn.conagua.gob.mx/es/ciclones-tropicales/etapas-de-evolucion (accessed on 10 October 2021).
- Department of Commerce through NOAA’s Office for Coastal Management. Gulf of Mexico Vessel Density. 2013. Available online: https://www.fisheries.noaa.gov/inport/item/48932 (accessed on 10 October 2021).
- Comision Nacional de Hidrocarburos. Pozos y Licitaciones del Portal de Información Técnica v2.1.4 2018; Nacional de Hidrocarburos: Ciudad de México, Mexico, 2018. [Google Scholar]
- Comisión Nacional de Hidrocarburos. Áreas Contractuales a Través de las Rondas Sujetas a Concurso por Licitación 2018; Comisión Nacional de Hidrocarburos: Ciudad de México, Mexico, 2018. [Google Scholar]
- Nelms, S.E.; Piniak, W.E.D.; Weir, C.R.; Godley, B.J. Seismic Surveys and Marine Turtles: An Underestimated Global Threat? Biol. Conserv. 2016, 193, 49–65. [Google Scholar] [CrossRef]
- Comision Nacional de Hidrocarburos. Zonas Sísmicas 2D y 3D 2018; Nacional de Hidrocarburos: Ciudad de México, Mexico, 2018. [Google Scholar]
- Diario Oficial de la Federación Quinta Sección, Poder Ejecutivo, Secretaría de Energía. Decreto por el Que Se Establece la Zona de Salvaguarda Denominada Arrecifes de Coral del Golfo de México y Caribe Mexicano. 2016. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5464472&fecha=07/12/2016 (accessed on 10 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liceaga-Correa, M.d.l.A.; Uribe-Martínez, A.; Cuevas, E. Ecological Vulnerability of Adult Female Marine Turtles as Indicators of Opportunities for Regional Socioecosystem Management in the Southern Gulf of Mexico. Sustainability 2022, 14, 184. https://doi.org/10.3390/su14010184
Liceaga-Correa MdlA, Uribe-Martínez A, Cuevas E. Ecological Vulnerability of Adult Female Marine Turtles as Indicators of Opportunities for Regional Socioecosystem Management in the Southern Gulf of Mexico. Sustainability. 2022; 14(1):184. https://doi.org/10.3390/su14010184
Chicago/Turabian StyleLiceaga-Correa, María de los Angeles, Abigail Uribe-Martínez, and Eduardo Cuevas. 2022. "Ecological Vulnerability of Adult Female Marine Turtles as Indicators of Opportunities for Regional Socioecosystem Management in the Southern Gulf of Mexico" Sustainability 14, no. 1: 184. https://doi.org/10.3390/su14010184
APA StyleLiceaga-Correa, M. d. l. A., Uribe-Martínez, A., & Cuevas, E. (2022). Ecological Vulnerability of Adult Female Marine Turtles as Indicators of Opportunities for Regional Socioecosystem Management in the Southern Gulf of Mexico. Sustainability, 14(1), 184. https://doi.org/10.3390/su14010184




