Chemical and Biological Characteristics of Organic Amendments Produced from Selected Agro-Wastes with Potential for Sustaining Soil Health: A Laboratory Assessment
Abstract
1. Introduction
2. Materials and Methods
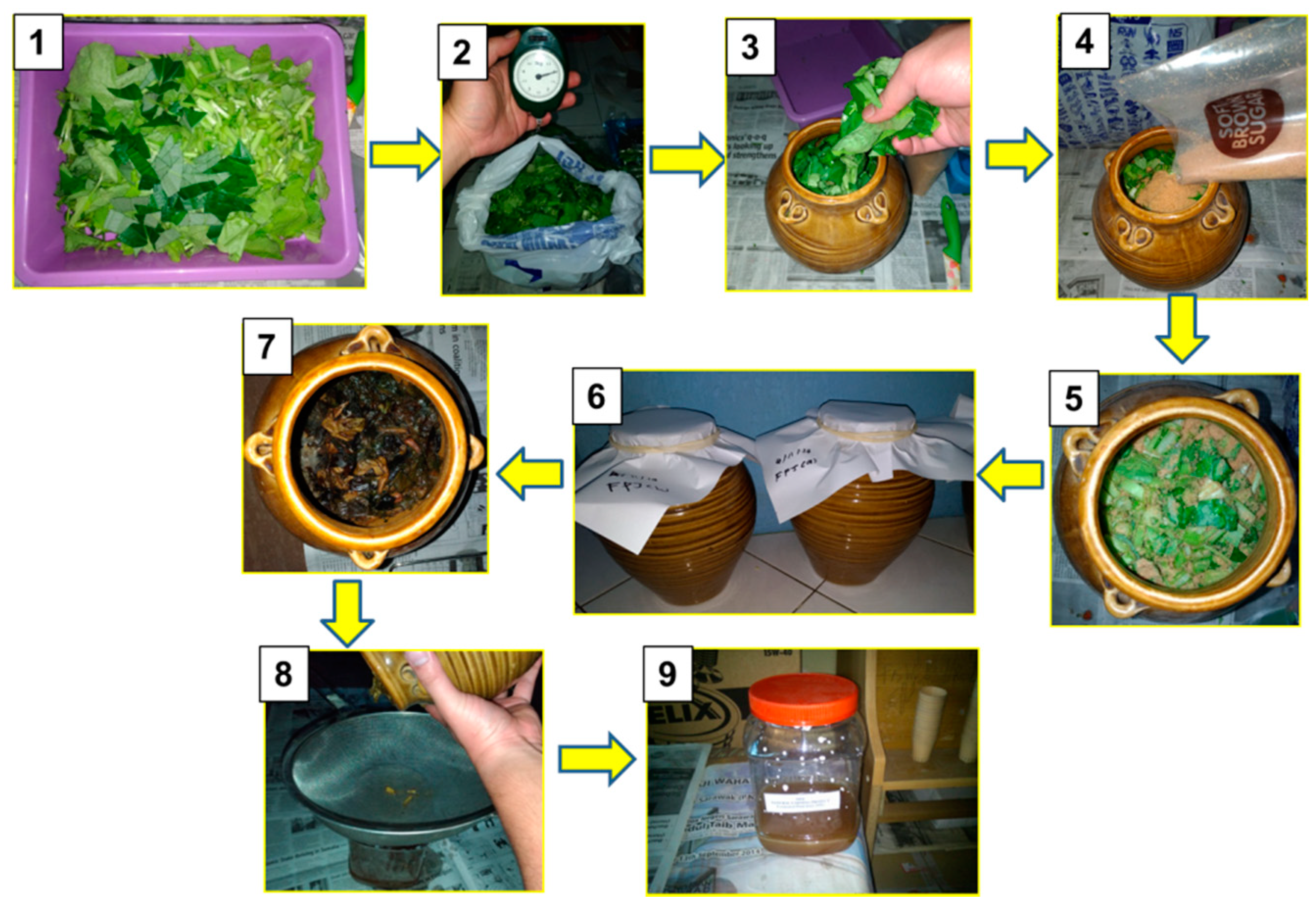
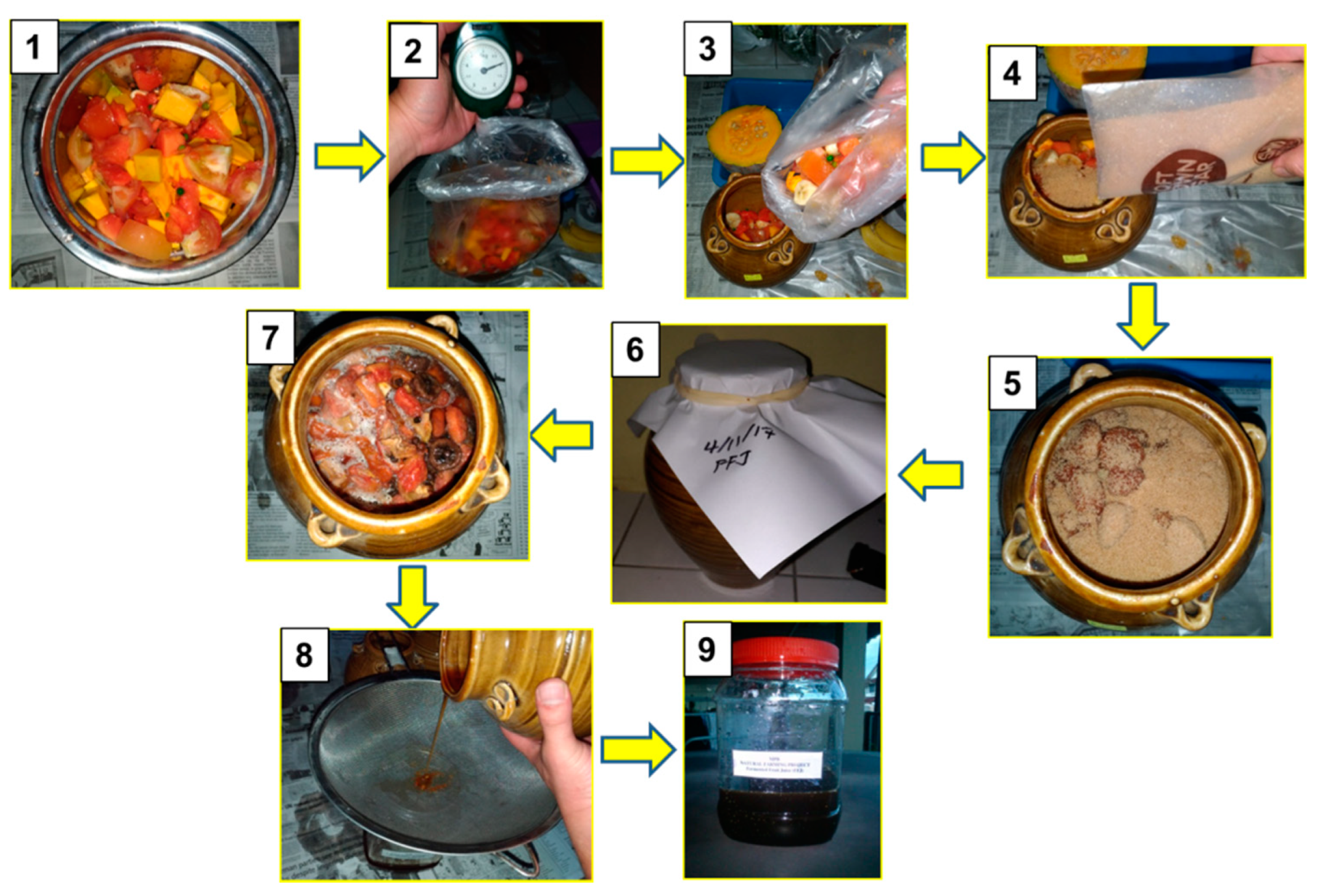
2.1. Production of the Fermented Juices
2.2. Chemical Analysis of the Fermented Juices
2.3. Identification of the Microorganisms and Screening for Phosphate and Potassium Solubilizers in the Fermented Juices
2.4. Biochar Production and Chemical Analysis
2.5. Compost Production and Chemical Analysis
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Chemical Characteristics of the Fermented Juices and Potential Benefits to Soil Health
3.2. Identification of Microorganisms in the Fermented Juices
3.3. Screening for Phosphate and Potassium Solubilizers in the Fermented Juices
3.4. Characteristics of the Palm Kernel Shell (PKS) Biochar and Potential Benefits to Soil Health
3.5. Characteristics of the Kitchen Waste Compost and Potential Benefits to Soil Health
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higa, T. Effective microorganisms and their role in Kyusei nature farming and sustainable agriculture. In Proceedings of the 2nd Conference on Effective Microorganisms, Kyusei Natural Farming Center, Saraburi, Thailand, 17–19 November 1993; pp. 1–6. [Google Scholar]
- Higa, T.; Wididana, G.N. Changes in the soil microflora induced by Effective Microorganisms. In Proceedings of the First International Conference on Kyusei Nature Farming; Parr, J.F., Hornick, S.B., Whitman, C.E., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 1991; pp. 153–162. [Google Scholar]
- Sharma, A.; Saha, N.T.; Arora, A.; Shah, R.; Nain, L. Efficient microorganism compost benefits plant growth and improves soil health in Calendula and Marigold. Hort. Plant J. 2017, 3, 67–72. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Urra Potential Benefits and Risks for Soil Health Derived from the Use of Organic Amendments in Agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Toscano, P.; Casacchia, T.; Diacono, M.; Montemurro, F. Composted Olive Mill By-Products: Compost Characterization and Application on Olive Orchards. J. Agric. Sci. Technol. 2013, 15, 627–638. [Google Scholar]
- Altieri, R.; Esposito, A. Evaluation of the fertilizing effect of olive mill waste compost in short-term crops. Int. Biodeterior. Biodegrad. 2010, 64, 124–128. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Paulus, A.D. Chapter 6: Planting and Maintenance. In Pepper Production Technology in Malaysia; Lai, K.F., Sim, S.L., Eds.; Malaysian Pepper Board: Kuching, Malaysia, 2011; pp. 106–109. [Google Scholar]
- Megir, G.; Paulus, A.D. Chapter 12: Organic Pepper Production. In Pepper Production Technology in Malaysia; Lai, K.F., Sim, S.L., Eds.; Malaysian Pepper Board: Kuching, Malaysia, 2011; pp. 229–233. [Google Scholar]
- Suhaimee, S.; Ibrahim, I.Z.; Abdul Wahab, M.A.M. Organic Agriculture in Malaysia; FFTC Agricultural Policy Articles; Production Policy: Taipei, Taiwan, 2016; Available online: https://ap.fftc.org.tw/article/1010 (accessed on 23 August 2020).
- Zamora, O.B.; Calub, B.M. Organic Agriculture Technologies and Systems Developed and Adapted by Farmers in the Phillippines; Department of Agriculture–Bureau of Agricultural Research and University of Philippines: Quezon City, Phillippines, 2016; p. 70. ISBN 978-971-0347-46-9.
- Aizawa, M.; Sekine, Y.; Shirai, Y. Determination of Total Nitrogen Content in Fertilizer by a Combustion Method: A Collaborative Study. Res. Rep. Fertil. 2010, 3, 11–18. [Google Scholar]
- Aoyama, K. Simultaneous Determination of Water-Soluble Principal Ingredients (W-P2O5, W-K2O, W-MgO, W-MnO and W-B2O3) in Liquid Fertilizer using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES). Res. Rep. Fertil. 2015, 8, 1–9. [Google Scholar]
- Kimura, Y.; Araya, H. Verification of Performance Characteristics of Testing Methods for Potassium Content in Fertilizer by Atomic Absorption Spectrometry. Res. Rep. Fertil. 2012, 5, 190–200. [Google Scholar]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Himedialabs.com. Pikovskayas Agar and Aleksandrow Agar HiMedia Laboratories Technical Data (M520). Revision 2. 2015. Available online: http://himedialabs.com/TD/M520.pdf (accessed on 22 April 2019).
- Wahi, R.; Sharifah Mona, A.A.; Sinin, H.; Zainab, N. Biochar production from agricultural wastes via low-temperature microwave carbonization. In Proceedings of the 2015 IEEE International RF and Microwave Conference (RFM 2015), Kuching, Sarawak, 14–16 December 2015. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar that is Used in Soil; IBI-STD-2.1; International Biochar Initiative: Canandaigua, NY, USA, 2015; p. 47. [Google Scholar]
- Cottenie, A. Soil testing and plant testing as a basis of fertilizer recommendation. FAO Soils Bull. 1980, 38, 70–73. [Google Scholar]
- Lawrinenko, M. Anion Exchange Capacity of Biochar. Master’s Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Tan, K.H. Soil Sampling, Preparation and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 672. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; Part 2; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.F., Dinauer, R.C., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Enders, A.; Lehnamm, J. Comparison of Wet-Digestion and Dry-Ashing Methods for Total Elemental Analysis of Biochar. Commun. Soil Sci. Plant Anal. 2012, 43, 1042–1052. [Google Scholar] [CrossRef]
- Kimpinski, J.; Gallant, C.E.; Henry, R.; MacLeod, J.A.; Sanderson, J.B.; Sturz, A.V. Effect of Compost and Manure Soil Amendments on Nematodes and on Yields of Potato and Barley: A 7-Year Study. J. Nematol. 2003, 35, 289–293. [Google Scholar] [PubMed]
- Zuraihah, I.I.; Aini, Z.; Faridah, M. Effects of IMO and EM application on soil nutrients, microbial population and crop yield. J. Trop. Agric. Food Sci. 2012, 40, 257–263. [Google Scholar]
- Yadav, A.N.; Kumar, R.; Kumar, S.; Kumar, V.; Sugitha, T.; Singh, B.; Chauahan, V.S.; Dhaliwal, H.S.; Saxena, A.K. Beneficial microbiomes: Biodiversity and potential biotechnological applications for sustainable agriculture and human health. J. Appl. Biol. Biotechnol. 2017, 5, 45–57. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Fassler, J.; Cooper, P. BLAST® Help Internet (Glossary). 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK62051/ (accessed on 26 April 2019).
- Fogg, C.N.; Kovats, D.E. 2014 ISCB Accomplishment by a Senior Scientist Award: Gene Myers. PLoS Comput. Biol. 2014, 10, e1003621. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B.; Jalloh, M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phosphate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B.; Jalloh, M.B. Effects of Selected Functional Bacteria on Maize Growth and Nutrient Use Efficiency. Microorganisms 2020, 8, 854. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus Solubilization by Bacillus Species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef]
- Talaat, N.B.; Ghoniem, A.E.; Abdelhamid, M.T.; Shawky, B.T. Effective microorganisms improve growth performance, alter nutrients acquisition and induce compatible solutes accumulation in common bean (Phaseolus vulgaris L.) plants subjected to salinity stress. Plant Growth Regul. 2015, 75, 281–295. [Google Scholar] [CrossRef]
- Lee, C.T.; Ismail, M.N.; Razali, F.; Muhamad, I.I.; Sarmidi, M.R.; Khamis, A.K. Application of effective microorganisms on soil and maize. J. Chem. Nat. Resour. Eng. 2008, 2, 1–13. [Google Scholar]
- Zimmermann, I. Trial with Charcoal as a Soil Amendment for Growing Lucerne; Unpublished Report; Polytechnic of Namibia: Windhoek, Namibia, 2008. [Google Scholar]
- Tedersoo, L.; Smith, M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef]
- Parr, J.F.; Hornick, S.B. Assessment of the Third International Conference on Kyusei Nature Farming: Round Table Discussion by USDA Scientists, 7 October 1993; Nature Farming Research and Development Foundation: Lompoc, CA, USA, 1994. [Google Scholar]
- Park, H.; Du Ponte, M.W. How to cultivate Indigenous Microorganisms. Biotechnology 2008, 9, 1–7. [Google Scholar]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschuk, M. Bacterial diversity at different stages of the com-posting process. BMC Microbiol. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Miller, S.A.; Ikeda, D.M.; Weinert, E., Jr.; Chang, K.; McGinn, J.M.; Keliihoomalu, C.; Duponte, M.W. Natural Farming: Fermented Plant Juice. Trop. Agric. Hum. Resour. 2013, 2, 1–7. [Google Scholar]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology, 1st ed.; Lehmann, J., Joseph, S., Eds.; Earthscan Publications Ltd.: London, UK, 2009; pp. 85–105. [Google Scholar]
- Abideen, Z.; Koyro, H.-W.; Huchzermeyer, B.; Gul, B.; Khan, M.A. Impact of a biochar or a biochar-compost mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere 2020, 30, 466–477. [Google Scholar] [CrossRef]
- Fischer, D.; Glaser, B. Synergisms between compost and biochar for sustainable soil amelioration, management of organic Waste. In Management-of-Organic-Waste/Synergism-between-Biochar-and-Compost-for-Sustainable-Soil-Amelioration; Kumar, S.E., Ed.; InTechOpen: Shanghai, China, 2012; pp. 167–198. Available online: https://www.intechopen.com/books/management-of-organic-waste/synergism-between-biochar-and-compost-for-sustainable-soil-amelioration (accessed on 12 June 2019).
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The ’Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Glaser, B. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 2012, 175, 410–422. [Google Scholar] [CrossRef]
- Hunt, J.; Du Ponte, M.; Sato, D.; Kawabata, A. The basics of Biochar: A Natural Soil Amendment. Soil Crop Manag. 2010, 30, 1–6. [Google Scholar]
- Malaysian Department of Environment. Contaminated Land Management and Control Guidelines No.1: Malaysian Recommended Site Screening Levels for Contaminated Land; Department of Environment Malaysia, Ministry of Natural Resources and Environment: Putrajaya, Malaysia, 2015; p. 67.
- Ross, S.M. Toxic Metals in Soil-Plant Systems, 1st ed.; Wiley: Chichester, UK, 1994; p. 484. [Google Scholar]
- Moreno-Jimenez, E.; Esteban, E.; Penalosa, J.M. The fate of Arsenic in the soil-plant system. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; Volume 215, pp. 1–37. [Google Scholar]
- Simarani, K.; Halmi, M.F.A.; Abdullah, R. Short-term effects of biochar amendment on soil microbial community in humid tropics. Arch. Agron. Soil Sci. 2018, 64, 1847–1860. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Amlinger, F.; Peyr, S.; Geszti, J.; Dreher, P.; Karlheinz, W.; Nortcliff, S. Beneficial Effects of Compost Application on Fertility and Productivity of Soils. Literature Study; Federal Ministry for Agriculture and Forestry, Environment and Water Management: Wien, Austria, 2007; p. 225. [Google Scholar]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Adugna, G. A review on impact of compost on soil properties, water use and crop productivity. Acad. Res. J. Agric. Sci. Res. 2016, 4, 93–104. [Google Scholar]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under or-ganic soil amendments and nitrogen fertilizations on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Subler, S.; Edwards, C.A.; Metzger, J. Growth of tomato plants in horticultural potting media amended with vermicomposts. Pedobiologia 2000, 43, 724–728. [Google Scholar]
- Huerta-Pujol, O.; Soliva, M.; Martinez-Farre, F.X.; Valero, J.; Lopez, M. Bulk density determination as a simple and complementary too in composting process control. Bioresour. Technol. 2010, 101, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Subler, S.; Edwards, C.A.; Metzger, J. Comparing vermicomposts and composts. Biocycle 1998, 39, 63–66. [Google Scholar]
- Khaliq, A.; Abbasi, M.K.; Hussain, T. Effects of integrated use of organic and inorganic nutrient sources with effective microorganisms (EM) on seed cotton yield in Pakistan. Bioresour. Technol. 2006, 97, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Marcote, I.; Hernández, T.; Garcı’a, C.; Polo, A. Influence of one or two successive annual applications of organic fertilizers on the enzyme activity of a soil under barley cultivation. Bioresour. Technol. 2001, 79, 147–154. [Google Scholar] [CrossRef]
- Azeem, M.; Hale, L.; Montgomery, J.; Crowley, D., Jr. Biochar and compost effects on soil microbial communities and nitrogen induced respiration in turfgrass soils. PLoS ONE 2020, 15, e0242209. [Google Scholar] [CrossRef] [PubMed]
| Fermented Juice | Total N (%) | Total P2O5 (%) | Total K2O (%) | Total MgO (%) | Total B2O3 (%) | Total Cu (%) | Total Zn (%) |
|---|---|---|---|---|---|---|---|
| FPJ | 0.29 ± 0.10 a | Trace | 0.28 ± 0.03 a | 0.20 ± 0.06 a | Trace | Trace | Trace |
| FFJ | 0.22 ± 0.07 a | Trace | 0.31 ± 0.08 a | 0.20 ± 0.04 a | Trace | Trace | Trace |
| Sample Name | Description | E-Value | Identity (%) |
|---|---|---|---|
| FPJ 1 | hypothetical protein HMPREF9209_0169 (Lactobacillus gasseri 224-1) | 2 × 10−50 | 96 |
| FPJ 2 | conserved hypothetical protein (Lactobacillus crispatus ST1) | 1 × 10−45 | 96 |
| FPJ 3 | hypothetical protein BTR22_20195 (Bacillus pseudofirmus) | 8 × 10−18 | 96 |
| FPJ 4 | hypothetical protein AF332_00860 (Sporosarcina globispora) | 8 × 10−18 | 96 |
| FPJ 5 | hypothetical protein BKP45_03105 (Anaerobacillus alkalidiazotrophicus) | 9 × 10−17 | 96 |
| FPJ 6 | hypothetical protein BN2127_JRS6_00905 (Bacillus subtilis) | 3 × 10−7 | 96 |
| FPJ 7 | hypothetical protein BN2127_JRS5_00115 (Bacillus amyloliquefaciens) | 2 × 10−33 | 96 |
| FPJ 8 | putative oRF16-lacZ fusion protein (Bacillus licheniformis) | 2 × 10−33 | 96 |
| FPJ 9 | Aspergillus niger strain BAB99 large subunit ribosomal RNA gene, partial sequence | 4 × 10−149 | 99 |
| FFJ 1 | Bacillus sp. PK-9 16S ribosomal RNA gene, partial sequence | 2 × 10−2 | 100 |
| FFJ 2 | Aspergillus tamarii isolate DTO 065-A4 internal transcribed spacer 1, partial sequence; 5.8S ribosomal RNA gene and internal transcribed spacer 2, complete sequence; and large subunit ribosomal RNA gene, partial sequence | 7 × 10−67 | 97 |
| FFJ 3 | Aspergillus oryzae DNA, verB gene transcriptional regulatory element binding region, strain: RIB 67 | 3 × 10−5 | 96 |
| FFJ 4 | hypothetical protein Tpal_2817, partial (Trichococcus palustris) | 3 × 10−54 | 97 |
| FFJ 5 | Talaromyces funiculosus strain SUF74 large subunit ribosomal RNA gene, partial sequence | 4 × 10−9 | 98 |
| FFJ 6 | Penicillium citrinum isolate 7H52B beta-tubulin (tub) gene, partial cds | 3 × 10−6 | 96 |
| FFJ 7 | Aspergillus niger BAB-1552 28S ribosomal RNA gene, partial sequence | 3 × 10−131 | 99 |
| FFJ 8 | Aspergillus terreus ATCC 1012 28S rRNA gene, partial sequence; from TYPE material | 8 × 10−14 | 97 |
| Fermented Juice | Species | P Solubilization | K Solubilization |
|---|---|---|---|
| FPJ | Lactobacillus gasseri | +++ | --- |
| Lactobacillus crispatus | +++ | --- | |
| Bacillus pseudofirmus | +++ | +++ | |
| Sporosarcina globispora | +++ | +++ | |
| Anaerobacillus alkalidiazotrophicus | +++ | --- | |
| Bacillus subtilis | +++ | +++ | |
| Bacillus amyloliquefaciens | +++ | +++ | |
| Bacillus licheniformis | +++ | +++ | |
| Aspergillus niger | +++ | +++ | |
| FFJ | Bacillus sp. PK-9 | +++ | +++ |
| Aspergillus tamarii | +++ | --- | |
| Aspergillus oryzae | +++ | --- | |
| Trichococcus palustris | +++ | --- | |
| Talaromyces funiculosus | +++ | --- | |
| Penicillium citrinum | +++ | --- | |
| Aspergillus niger | +++ | +++ | |
| Aspergillus terreus | +++ | +++ |
| Property | Value Obtained |
|---|---|
| pH | 10.1 |
| CEC (cmolc kg−1) | 12.10 |
| AEC (cmolc kg−1) | 7.22 |
| ASH (%) | 5.22 |
| C (%) | 84.03 |
| N (%) | 0.21 |
| P (%) | ND (<0.001) |
| K (%) | 0.52 |
| Ca (mg kg−1) | 6977 |
| S (mg kg−1) | ND (<0.1) |
| Al (mg kg−1) | 1.50 |
| B (mg kg−1) | 3.50 |
| As (mg kg−1) | ND (<0.1) |
| Cd (mg kg−1) | ND (<0.1) |
| Cu (mg kg−1) | ND (<0.1) |
| Pb (mg kg−1) | ND (<0.1) |
| Hg (mg kg−1) | ND (<0.1) |
| Ni (mg kg−1) | ND (<0.1) |
| Zn (mg kg−1) | ND (<0.1) |
| Co (mg kg−1) | ND (<0.1) |
| Mn (mg kg−1) | 2.35 |
| Cr (mg kg−1) | ND (<0.1) |
| Fe (mg kg−1) | ND (<0.1) |
| Property | Value Obtained |
|---|---|
| pH (water) | 7.82 |
| EC (dS m−1) | 3.60 |
| Moisture (%) | 42.10 |
| C (%) | 27.89 |
| N (%) | 1.12 |
| C/N Ratio | 24.90 |
| P (%) | 10.23 |
| K (%) | 7.32 |
| Ca (%) | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulok, K.M.T.; Ahmed, O.H.; Khew, C.Y.; Zehnder, J.A.M.; Jalloh, M.B.; Musah, A.A.; Abdu, A. Chemical and Biological Characteristics of Organic Amendments Produced from Selected Agro-Wastes with Potential for Sustaining Soil Health: A Laboratory Assessment. Sustainability 2021, 13, 4919. https://doi.org/10.3390/su13094919
Sulok KMT, Ahmed OH, Khew CY, Zehnder JAM, Jalloh MB, Musah AA, Abdu A. Chemical and Biological Characteristics of Organic Amendments Produced from Selected Agro-Wastes with Potential for Sustaining Soil Health: A Laboratory Assessment. Sustainability. 2021; 13(9):4919. https://doi.org/10.3390/su13094919
Chicago/Turabian StyleSulok, Kevin Muyang Tawie, Osumanu Haruna Ahmed, Choy Yuen Khew, Jarroop Augustine Mercer Zehnder, Mohamadu Boyie Jalloh, Adiza Alhassan Musah, and Arifin Abdu. 2021. "Chemical and Biological Characteristics of Organic Amendments Produced from Selected Agro-Wastes with Potential for Sustaining Soil Health: A Laboratory Assessment" Sustainability 13, no. 9: 4919. https://doi.org/10.3390/su13094919
APA StyleSulok, K. M. T., Ahmed, O. H., Khew, C. Y., Zehnder, J. A. M., Jalloh, M. B., Musah, A. A., & Abdu, A. (2021). Chemical and Biological Characteristics of Organic Amendments Produced from Selected Agro-Wastes with Potential for Sustaining Soil Health: A Laboratory Assessment. Sustainability, 13(9), 4919. https://doi.org/10.3390/su13094919








