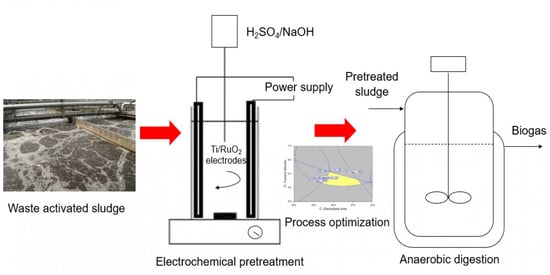
Process Optimization of Waste Activated Sludge in Anaerobic Digestion and Biogas Production by Electrochemical Pre-Treatment Using Ruthenium Oxide Coated Titanium Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Electrode Coating
2.2. Experimental Procedures and Statistical Analysis
2.3. Analytical Methodology
3. Results and Discussion
3.1. Disintegration and Dewaterability of WAS by Electrochemical Pre-Treatment
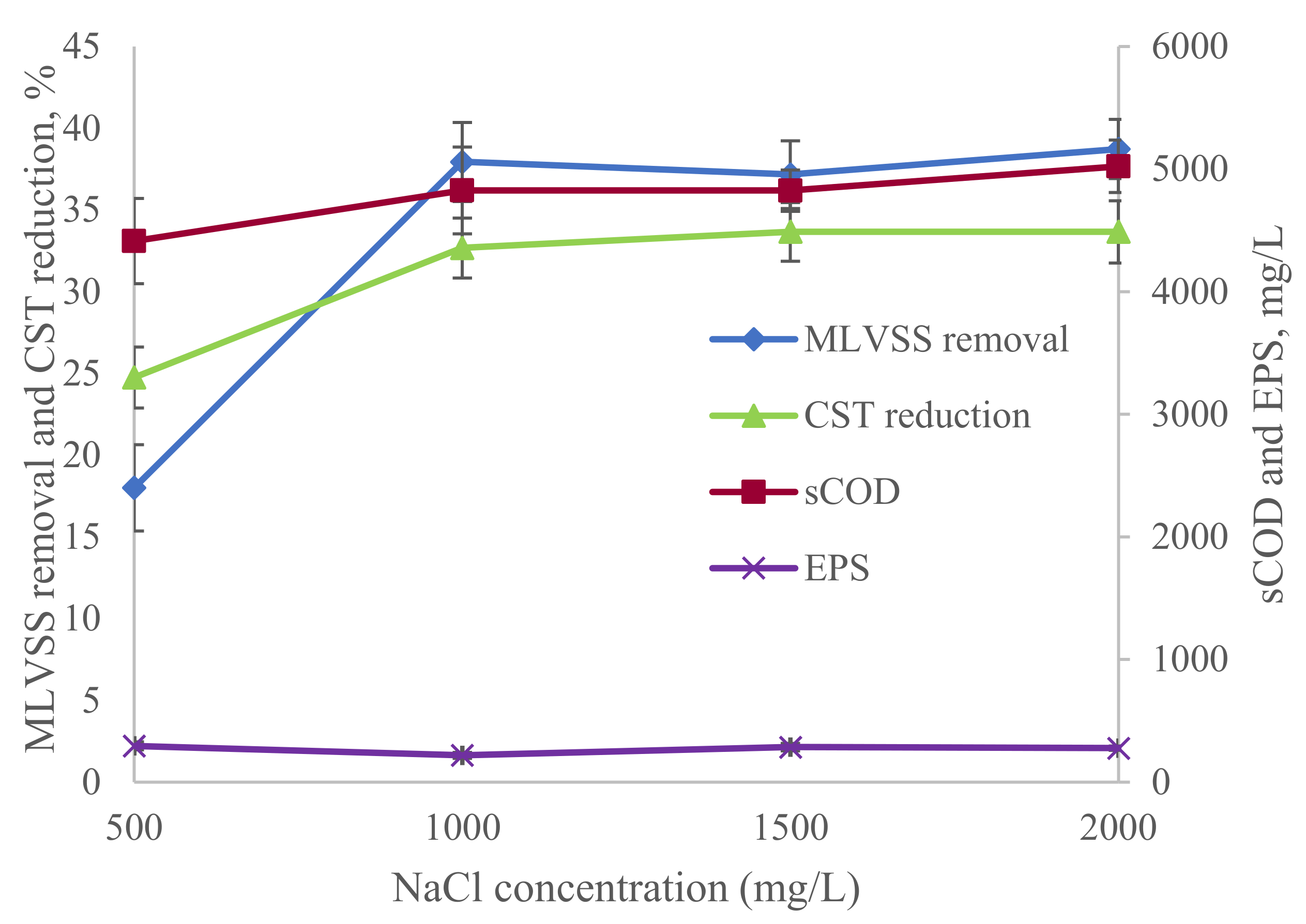
3.1.1. Process Analysis
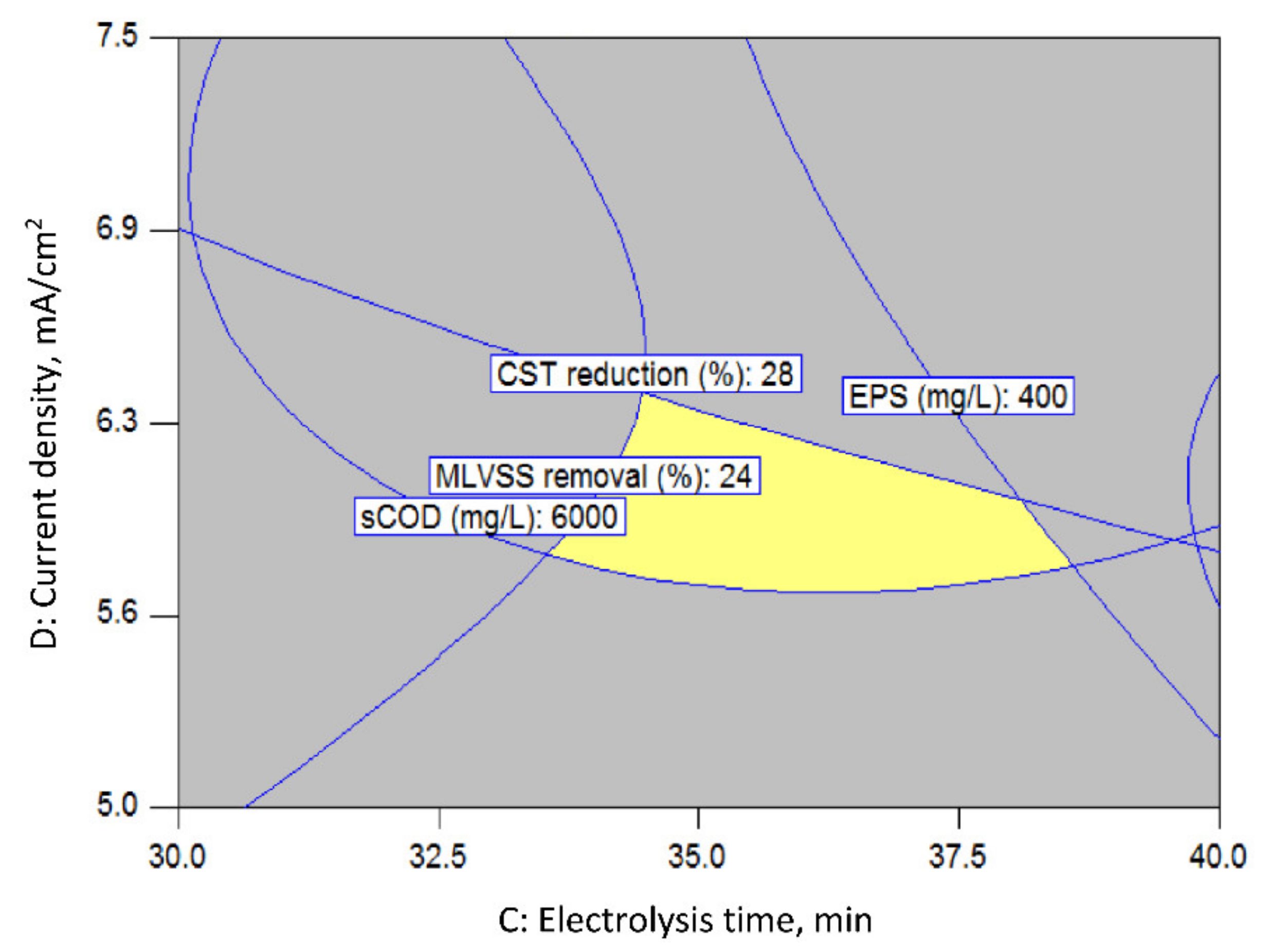
3.1.2. Process Optimization by Response Surface Methodology (RSM)
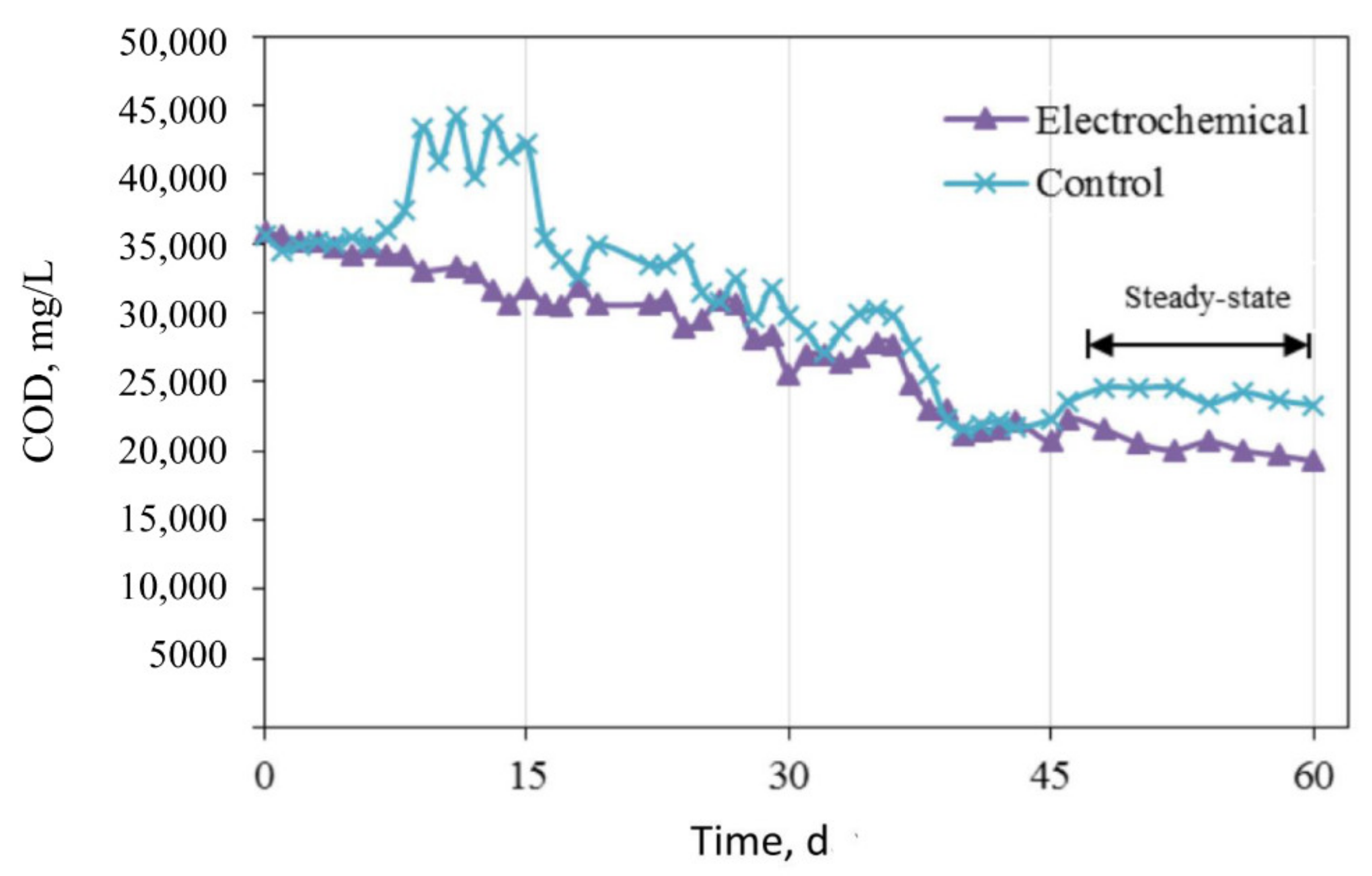
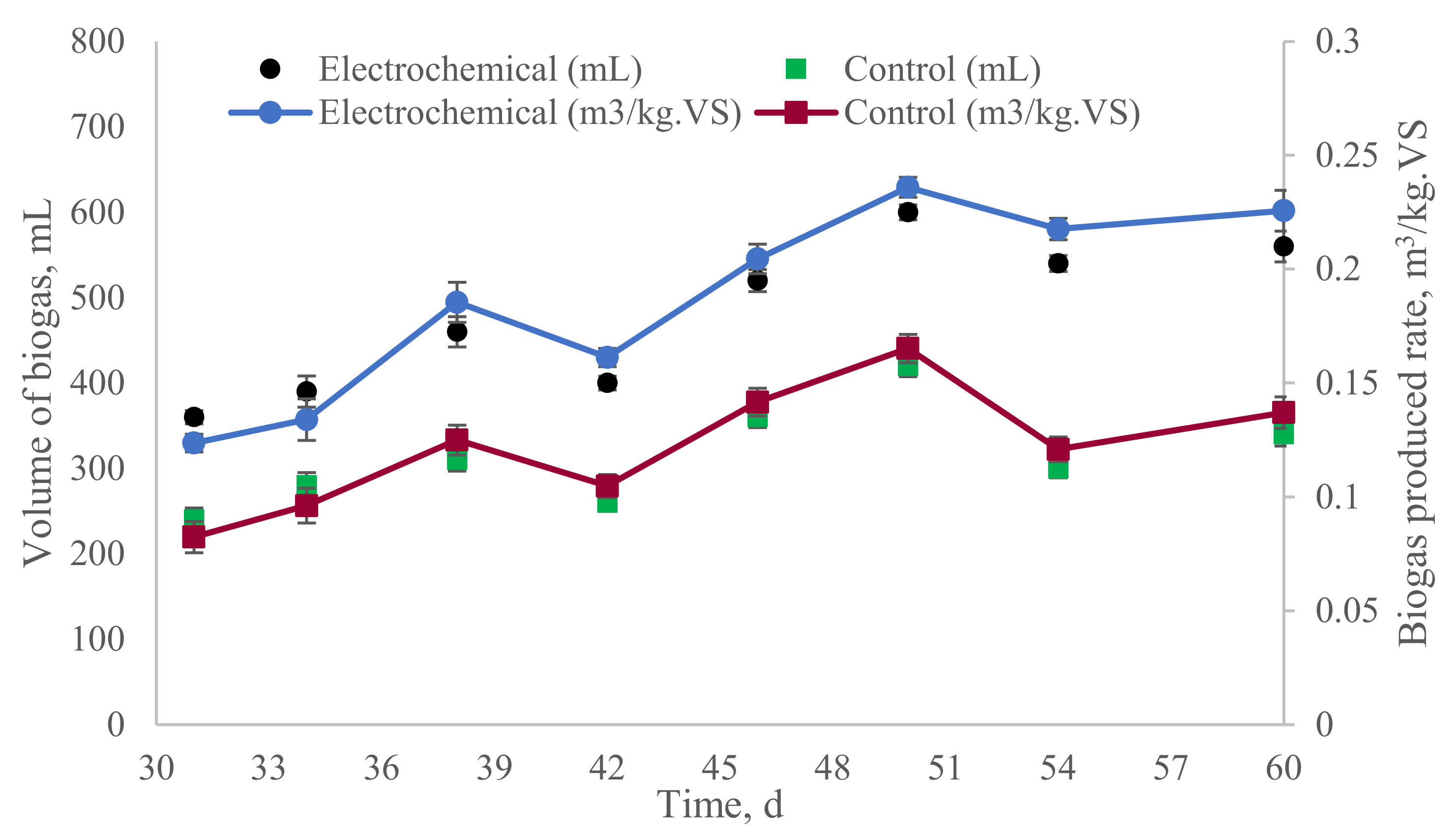
3.2. Anaerobic Digester Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tezel, U.; Tandukar, M.; Pavlostathis, S.G. Anaerobic Biotreatment of Municipal Sewage Sludge. In Comprehensive Biotechnology, 2nd ed.; Agathos, S., Ed.; Environmental Biotechnology and Safety; Elsevier: Amsterdam, The Netherlands, 2011; Volume 6. [Google Scholar]
- Mininni, G.; Laera, G.; Bertanza, G.; Canato, M.; Sbrilli, A. Mass and energy balances of sludge processing in reference and upgraded wastewater treatment plants. Environ. Sci. Pollut. Res. 2015, 22, 7203–7215. [Google Scholar] [CrossRef]
- Tomei, M.C.; Bertanza, G.; Canato, M.; Heimersson, S.; Laera, G.; Svanström, M. Techno-economic and environmental assessment of upgrading alternatives for sludge stabilization in municipal wastewater treatment plants. J. Clean Prod. 2016, 112, 3106–3115. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Wang, X.; Wu, J.; Li, F. Energy Self-sufficient Wastewater Treatment Plants: Feasibilities and Challenges. Energy Procedia 2017, 105, 3741–3751. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.; Delgenès, J.; Steyer, J.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Jäger, K.E.; Flemming, H.-C. Interaction between extracellular polysaccharides and enzymes. In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar]
- Forster, C.F. Bound water in sewage sludges and its relationship to sludge surfaces and sludge viscosities. J. Chem. Technol. Biotechnol. 2008, 33, 76–84. [Google Scholar] [CrossRef]
- Roberson, E.B.; Firestone, M.K. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wang, D.; Ma, T.; Bai, R. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. 2015, 83, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Heng, G.C.; Isa, M.H.; Lim, J.-W.; Ho, Y.-C.; Zinatizadeh, A.A.L. Enhancement of anaerobic digestibility of waste activated sludge using photo-Fenton pretreatment. Environ. Sci. Pollut. Res. 2017, 24, 27113–27124. [Google Scholar] [CrossRef]
- Kavitha, S.; Saranya, T.; Kaliappan, S.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. Accelerating the sludge disintegration potential of a novel bacterial strain Planococcus jake 01 by CaCl2 induced deflocculation. Bioresour. Technol. 2015, 175, 396–405. [Google Scholar] [CrossRef]
- Song, L.-J.; Zhu, N.-W.; Yuan, H.-P.; Hong, Y.; Ding, J. Enhancement of waste activated sludge aerobic digestion by electrochemical pre-treatment. Water Res. 2010, 44, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Sanin, F.D.; Vesilind, P.A. Effect of centrifugation on the removal of extracellular polymers and physical properties of activated sludge. Water Sci. Technol. 1994, 30, 117–127. [Google Scholar] [CrossRef]
- Karr, P.R.; Keinath, T.M. Influence of particle size on sludge dewatering. J. Water Pollut. Control. Fed. 1978, 50, 1911–1930. [Google Scholar]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA®. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Malpass, G.; Miwa, D.; Mortari, D.; Machado, S.; Motheo, A. Decolorisation of real textile waste using electrochemical techniques: Effect of the chloride concentration. Water Res. 2007, 41, 2969–2977. [Google Scholar] [CrossRef]
- Yaqub, A.; Isa, M.H.; Ajab, H. Electrochemical Degradation of Polycyclic Aromatic Hydrocarbons in Synthetic Solution and Produced Water Using a Ti/SnO2-Sb2O5-RuO2 Anode. J. Environ. Eng. 2015, 141, 04014074. [Google Scholar] [CrossRef]
- Santos, I.D.; Dezotti, M.; Dutra, A.J. Electrochemical treatment of effluents from petroleum industry using a Ti/RuO2 anode. Chem. Eng. J. 2013, 226, 293–299. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.-H.; Park, C.; Shin, E.-B. Electrochemical oxidation of polyvinyl alcohol using a RuO2/Ti anode. Desalination 2003, 155, 49–57. [Google Scholar] [CrossRef]
- Torresa, R.A.; Sarria, V.; Torres, W.; Peringera, P.; Pulgarina, C. Electrochemical treatment of industrial wastewater containing 5-amino-6-methyl-2-benzimidazolone: Toward an electrochemical-biological coupling. Water Res. 2003, 37, 3118–3124. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Costa, C.R.; Botta, C.M.; Espíndola, E.L.; Olivi, P. Electrochemical treatment of tannery wastewater using DSA® electrodes. J. Hazard. Mater. 2008, 153, 616–627. [Google Scholar] [CrossRef]
- Simond, O.; Schaller, V.; Comninellis, C. Theoretical model for the anodic oxidation of organics on metal oxide electrodes. Electrochim. Acta 1997, 42, 2009–2012. [Google Scholar] [CrossRef]
- Heng, G.C.; Isa, M.H. Electrochemical Disintegration of Activated Sludge Using Ti/RuO2 Anode. Appl. Mech. Mater. 2014, 567, 44–49. [Google Scholar] [CrossRef]
- Yu, B.; Xu, J.; Yuan, H.; Lou, Z.; Lin, J.; Zhu, N. Enhancement of anaerobic digestion of waste activated sludge by electrochemical pretreatment. Fuel 2014, 130, 279–285. [Google Scholar] [CrossRef]
- Ye, C.; Yuan, H.; Lou, Z.; Zhu, N. Combined Electrochemical and Hypochlorite Pretreatment for Improving Solubilization and Anaerobic Digestion of Waste-Activated Sludge: Effect of Hypochlorite Dosage. Energy Fuels 2016, 30, 2990–2996. [Google Scholar] [CrossRef]
- Erden, G. Disintegration of Biological Sludge by Electro-oxidation Process with Different Electrode Couples. Waste Biomass Valorization 2019, 11, 2701–2707. [Google Scholar] [CrossRef]
- Ajab, H.; Isa, M.H.; Yaqub, A. Electrochemical oxidation using ti/RuO2 anode for COD and PAHs removal from aqueous solution. Sustain. Mater. Technol. 2020, 26, e00225. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, B.; Cheng, P.; Zhu, N.; Yin, C.; Ying, L. Pilot-scale study of enhanced anaerobic digestion of waste activated sludge by electrochemical and sodium hypochlorite combination pretreatment. Int. Biodeterior. Biodegrad. 2016, 110, 227–234. [Google Scholar] [CrossRef]
- Ding, W.; Jin, W.; Zhou, X.; Yang, Q.; Chen, C.; Wang, Q. Role of extracellular polymeric substances in anaerobic granular sludge: Assessing dewaterability during Fe(II)-peroxydisulfate conditioning and granulation processes. J. Clean. Prod. 2020, 286, 124968. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Preece, D.A.; Montgomery, D.C. Design and Analysis of Experiments. Int. Stat. Rev. 1978, 46, 120. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, E.; Olivi, P. Preparation and characterization of Sb-doped SnO2 films with controlled stoichiometry from polymeric precursors. J. Phys. Chem. Solids 2003, 64, 1105–1112. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N.; Song, F. Dewaterability characteristics of sludge conditioned with surfactants pretreatment by electrolysis. Bioresour. Technol. 2011, 102, 2308–2315. [Google Scholar] [CrossRef]
- USEPA. Standards for the Use or Disposal of Sewage Sludge. In 40CFR PART 503; United States Environmental Protection Agency: Washington, DC, USA, 1993. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- HACH. Water Analysis Handbook, 4th ed.; Hach Company: Loveland, CO, USA, 2003. [Google Scholar]
- Ras, M.; Girbal-Neuhauser, E.; Paul, E.; Spérandio, M.; Lefebvre, D. Protein extraction from activated sludge: An analytical approach. Water Res. 2008, 42, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Zuriaga-Agustí, E.; Bes-Piá, A.; Mendoza-Roca, J.; Alonso-Molina, J. Influence of extraction methods on proteins and carbohydrates analysis from MBR activated sludge flocs in view of improving EPS determination. Sep. Purif. Technol. 2013, 112, 1–10. [Google Scholar] [CrossRef]
- Ammar, A.A.; Asmeret, A.B.; Teamrat, A.G. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar]
- SHARI. FAQ—Interpreting Lack of Fit; Stat-Teaser, News from Stat-Ease, Inc.: Minneapolis, MN, USA, 2004. [Google Scholar]
- Mohajeri, S.; Aziz, H.A.; Isa, M.H.; Zahed, M.A.; Bashir, M.J.K.; Adlan, M.N. Application of the central composite design for condition optimization for semi-aerobic landfill leachate treatment using electrochemical oxidation. Water Sci. Technol. 2010, 61, 1257–1266. [Google Scholar] [CrossRef]
- Ölmez, T. The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. J. Hazard. Mater. 2009, 162, 1371–1378. [Google Scholar] [CrossRef]
- Katsiris, N.; Kouzeli-Katsiri, A. Bound water content of biological sludges in relation to filtration and dewatering. Water Res. 1987, 21, 1319–1327. [Google Scholar] [CrossRef]
- Apul, O.G.; Atalar, I.; Zorba, G.T.; Sanin, F.D. The Dewaterability of Disintegrated Sludge Samples before and after Anaerobic Digestion. Dry. Technol. 2010, 28, 901–909. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Yuan, H.; Zhou, Q.; Gu, G. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res. 2007, 41, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.R.; Olivi, P. Effect of chloride concentration on the electrochemical treatment of a synthetic tannery wastewater. Electrochim. Acta 2009, 54, 2046–2052. [Google Scholar] [CrossRef]
- Scialdone, O.; Randazzo, S.; Galia, A.; Silvestri, G. Electrochemical oxidation of organics in water: Role of operative parameters in the absence and in the presence of NaCl. Water Res. 2009, 43, 2260–2272. [Google Scholar] [CrossRef]
- Feng, Y.; Smith, D.W.; Bolton, J.R. Photolysis of Aqueous Free Chlorine Species (HOCl and OCl¯) with 254 nm Ultraviolet Light. J. Environ. Eng. Sci. 2007, 6, 277–284. [Google Scholar] [CrossRef]
- Bougrier, C.; Albasi, C.; Delgenès, J.; Carrère, H. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. 2006, 45, 711–718. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, H.; Lin, J.; Yuan, W. Evaluation of thermal, thermal-alkaline, alkaline and electrochemical pretreatments on sludge to enhance anaerobic biogas production. J. Taiwan Inst. Chem. Eng. 2014, 45, 2531–2536. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gianico, A.; Gallipoli, A.; Mininni, G. The impact of sludge pre-treatments on mesophilic and thermophilic anaerobic digestion efficiency: Role of the organic load. Chem. Eng. J. 2015, 270, 362–371. [Google Scholar] [CrossRef]

| Parameter | Mean Value (Standard Deviation) |
|---|---|
| pH | 7.02 ( |
| Total solids (mg/L) | 41,500 (1301) |
| Volatile solids (mg/L) | 26,020 (572) |
| Mixed liquor suspended solids (mg/L) | 38,057 (1052) |
| Mixed liquor volatile suspended solids (mg/L) | 25,371(387) |
| Total chemical oxygen demand (mg/L) | 35,850 (271) |
| Soluble chemical oxygen demand (mg/L) | 935 (21) |
| Capillary suction time (s) | 114 (9) |
| Extracellular polymeric substances (EPS) (mg/L) 1 | 370 (12) |
| Independent Variable | Code | Levels and Ranges (Coded) | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| pH | A | 9 | 10 | 11 | 12 | 13 |
| Sludge concentration (mg/L) | B | 10,000 | 15,000 | 20,000 | 25,000 | 30,000 |
| Electrolysis time (min) | C | 10 | 20 | 30 | 40 | 50 |
| Current density (mA/cm2) | D | 2.5 | 5 | 7.5 | 10 | 12.5 |
| Response | Model (F Value) | PLOF | AP | R2 |
|---|---|---|---|---|
| MLVSS removal (%) | 0.0002 | 0.0126 | 11.393 | 0.8759 |
| sCOD | <0.0001 | 0.0059 | 11.544 | 0.9016 |
| CST reduction (%) | <0.0001 | 0.0055 | 15.074 | 0.8867 |
| EPS | <0.0001 | 0.0944 | 14.096 | 0.9199 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, G.C.; Isa, M.H.; Lock, S.S.M.; Ng, C.A. Process Optimization of Waste Activated Sludge in Anaerobic Digestion and Biogas Production by Electrochemical Pre-Treatment Using Ruthenium Oxide Coated Titanium Electrodes. Sustainability 2021, 13, 4874. https://doi.org/10.3390/su13094874
Heng GC, Isa MH, Lock SSM, Ng CA. Process Optimization of Waste Activated Sludge in Anaerobic Digestion and Biogas Production by Electrochemical Pre-Treatment Using Ruthenium Oxide Coated Titanium Electrodes. Sustainability. 2021; 13(9):4874. https://doi.org/10.3390/su13094874
Chicago/Turabian StyleHeng, Gan Chin, Mohamed Hasnain Isa, Serene Sow Mun Lock, and Choon Aun Ng. 2021. "Process Optimization of Waste Activated Sludge in Anaerobic Digestion and Biogas Production by Electrochemical Pre-Treatment Using Ruthenium Oxide Coated Titanium Electrodes" Sustainability 13, no. 9: 4874. https://doi.org/10.3390/su13094874
APA StyleHeng, G. C., Isa, M. H., Lock, S. S. M., & Ng, C. A. (2021). Process Optimization of Waste Activated Sludge in Anaerobic Digestion and Biogas Production by Electrochemical Pre-Treatment Using Ruthenium Oxide Coated Titanium Electrodes. Sustainability, 13(9), 4874. https://doi.org/10.3390/su13094874