Conceptual Model of Ecosystem Service Flows from Carbon Dioxide to Blue Carbon in Coastal Wetlands: An Empirical Study Based on Yancheng, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Process
2.2. Analysis Methods
3. Results
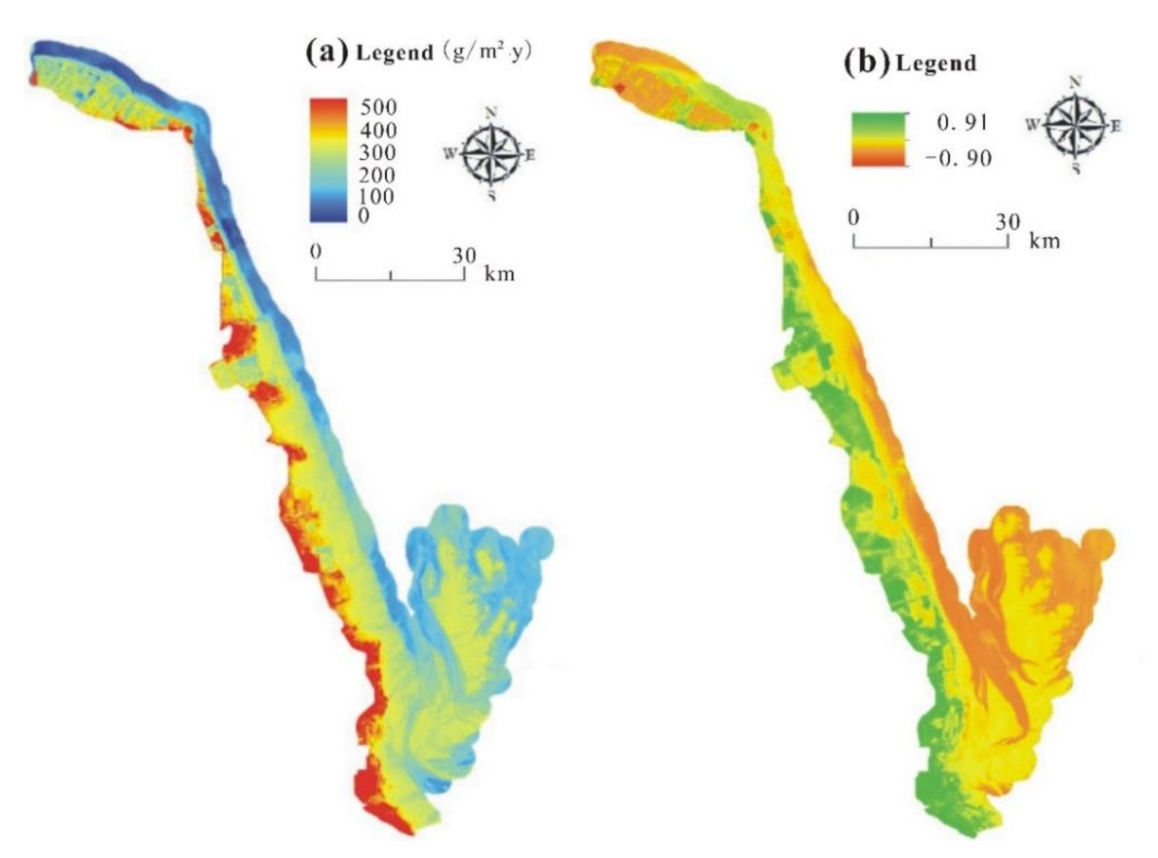
3.1. The Horizontal Distribution Pattern of Aboveground Vegetation NPP in the Study Area
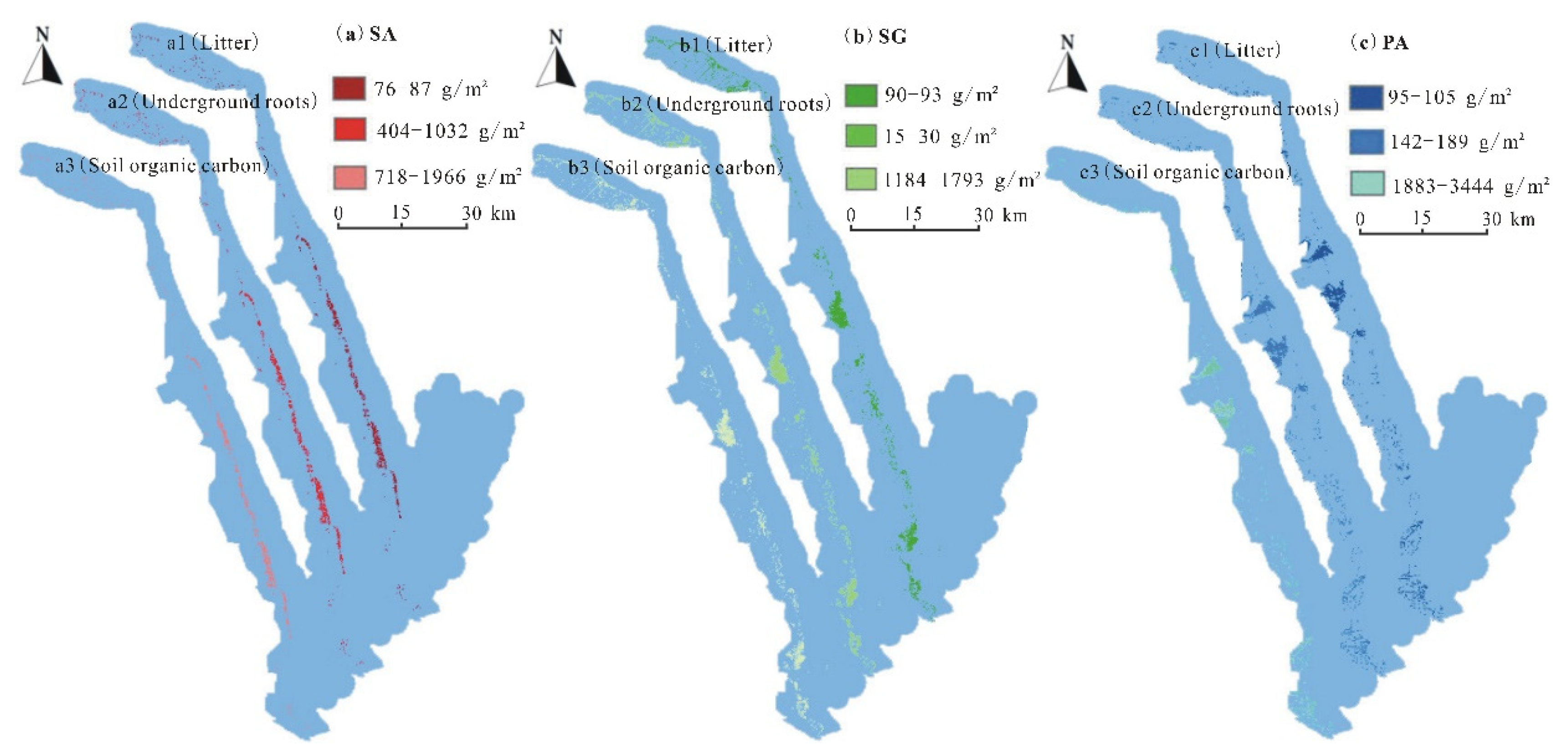
3.2. The Perpendicular Distribution Pattern of Blue Carbon in the Study Area
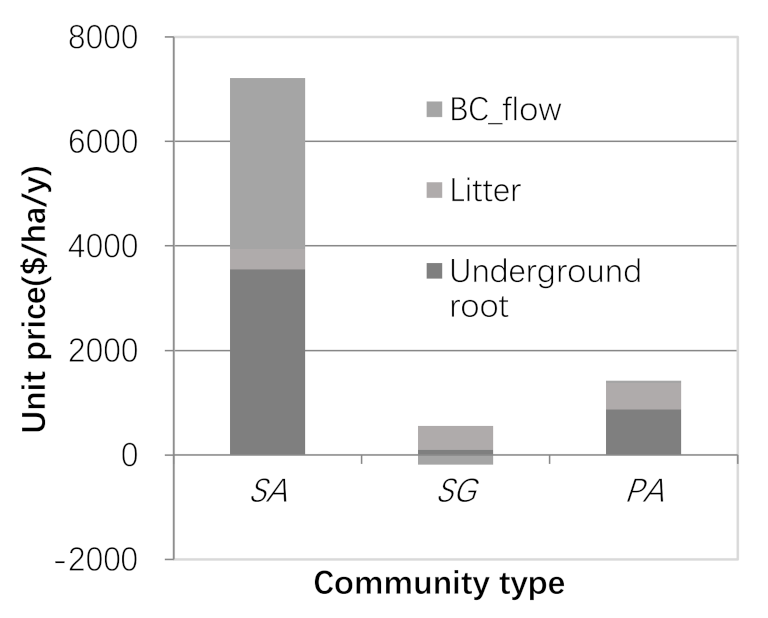
3.3. Carbon Sequestration Capacity and Carbon Sequestration Value
4. Discussion
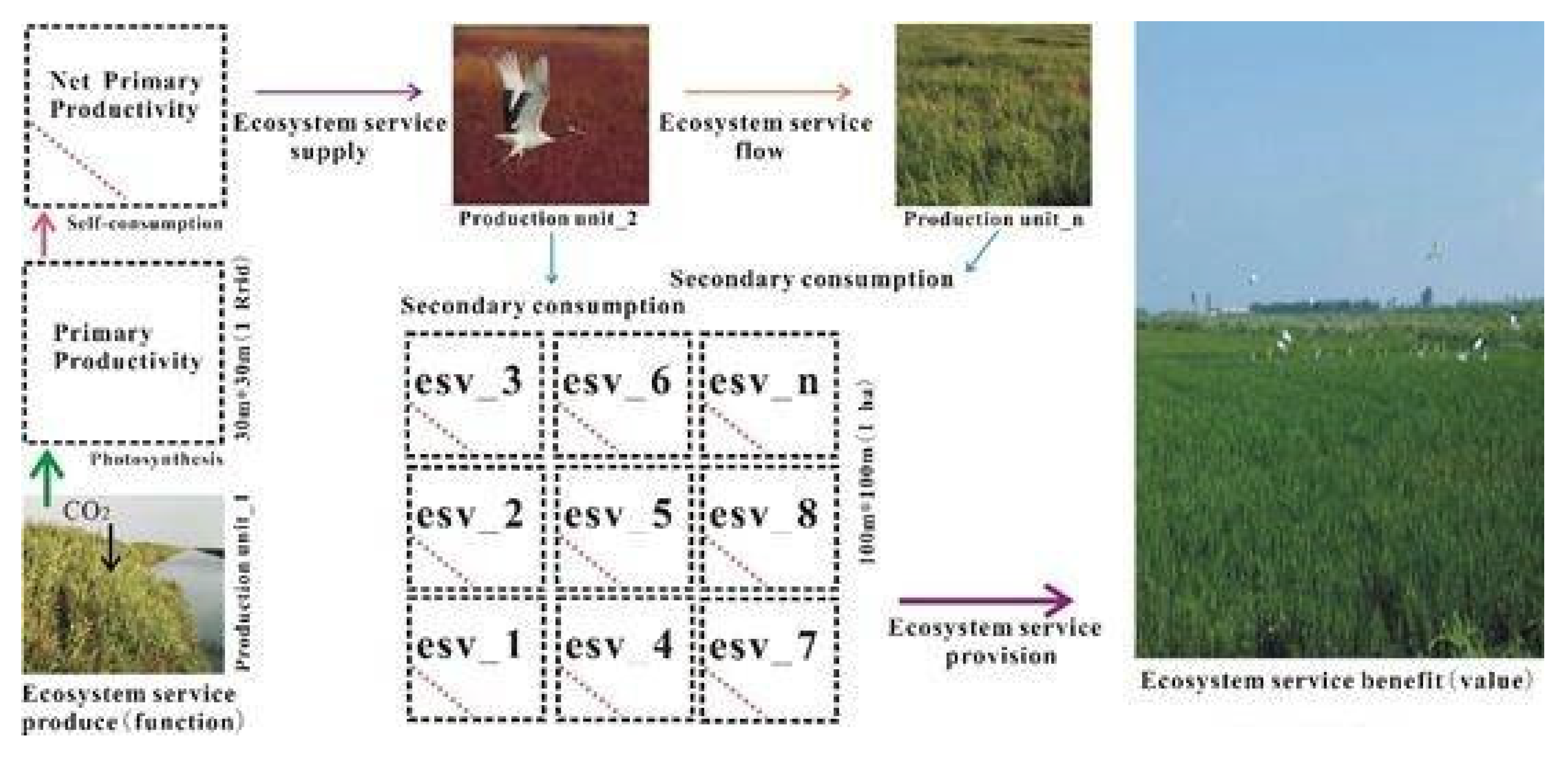
4.1. The Conversion Process of the “Ecosystem Services Function-Ecosystem Services Value”
4.2. Uncertainty Analysis of Carbon Cycle in Coastal Wetland
5. Conclusions
- The aboveground NPP of coastal wetland vegetation exhibited gradient changes from low to high or from high to low in a direction perpendicular to the coastline. Using the light use efficiency model, we obtained an aboveground NPP of coastal wetland vegetation that ranged between 200 and 500 g/m2∙y in Yancheng, China.
- The “blue carbon” in coastal wetland vegetation showed a three-dimensional distribution pattern. The “blue carbon” content of P. australis and S. alterniflora communities gradually increased from the ground surface to the underground area (litter < underground roots < soil) in Yancheng, China. The surface soil of each plant community contained a higher content of “blue carbon” compared with the aboveground shoots, litter, and underground roots.
- Coastal wetlands absorb carbon dioxide and convert black carbon to blue carbon, which is of great significance to the regulation of regional microclimates. This process reflects the conceptual essence of ecosystem service flow from ecosystem service function to ecosystem service value. In Yancheng, China, Phragmites australis and Spartina alterniflora belong to the blue carbon supply type coastal wetland, with an average annual outflow carbon sequestration value of 40.9 $∙ha−2 and 3272.3 $∙ha−2, while Suaeda salsa (8024 $∙ha−2∙y−1) belongs to the blue carbon benefit coastal wetland, and the annual inflow carbon sequestration value is 190.7 $∙ha−2.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.Z.; Wang, S.; Zou, W. Chinese marine ecosystem services value: Regional and structural equilibrium analysis. Ocean Coast. Manag. 2016, 125, 70–83. [Google Scholar] [CrossRef]
- Zang, Z.; Zou, X.Q.; Zuo, P.; Song, Q.C.; Wang, C.L.; Wang, J.J. Impact of landscape patterns on ecological vulnerability and ecosystem service values: An empirical analysis of Yancheng Nature Reserve in China. Ecol. Indic. 2017, 72, 142–152. [Google Scholar] [CrossRef]
- Hillebrand, H.; Matthiessen, B. Biodiversity in a complex world: Consolidation and progress in functional biodiversity research. Ecol. Lett. 2009, 12, 1405–1419. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Bagstad, K.J.; Johnson, G.W.; Voigt, B.; Villa, F. Spatial dynamics of ecosystem service flows: A comprehensive approach to quantifying actual services. Ecosyst. Serv. 2013, 4, 117–125. [Google Scholar] [CrossRef]
- Serna-Chavez, H.M.; Schulp, C.J.; Bodegom, P.M.; Bouten, W.; Verburg, P.H.; Davidson, M.D. A quantitative framework for assessing spatial flows of ecosystem services. Ecol. Indic. 2014, 39, 24–33. [Google Scholar] [CrossRef]
- Jones, L.; Norton, L.; Austin, Z.; Browne, A.L.; Donovan, D.; Emmett, B.A. Stocks and flows of natural and human-derived capital in ecosystem services. Land. Use Policy 2016, 52, 151–162. [Google Scholar] [CrossRef]
- Mitchell, M.G.; Bennett, E.M.; Gonzalez, A. Linking landscape connectivity and ecosystem service provision: Current knowledge and research gaps. Ecosystems 2013, 16, 894–908. [Google Scholar] [CrossRef]
- Luck, G.W.; Harrington, R.; Harrison, P.A. Quantifying the contribution of organisms to the provision of ecosystem services. BioScience 2016, 59, 223–235. [Google Scholar] [CrossRef]
- van der Meulen, E.S.; Braat, L.C.; Brils, J.M. Abiotic flows should be inherent part of ecosystem services classification. Ecosyst. Serv. 2016, 19, 1–5. [Google Scholar] [CrossRef]
- Schirpke, U.; Scolozzi, R.; De Marco, C.; Tappeiner, U. Mapping beneficiaries of ecosystem services flows from Natura 2000 sites. Ecosyst. Serv. 2014, 9, 170–179. [Google Scholar] [CrossRef]
- Gopal, B. A conceptual framework for environmental flows assessment based on ecosystem services and their economic valuation. Ecosyst. Serv. 2016, 21, 53–58. [Google Scholar] [CrossRef]
- Villamagna, A.M.; Angermeier, P.L.; Bennett, E.M. Capacity, pressure, demand, and flow: A conceptual framework for analyzing ecosystem service provision and delivery. Ecol. Complex. 2013, 15, 114–121. [Google Scholar] [CrossRef]
- Baró, F.; Palomo, I.; Zulian, G. Mapping ecosystem service capacity, flow and demand for landscape and urban planning: A case study in the Barcelona metropolitan region. Land. Use Policy 2016, 57, 405–417. [Google Scholar] [CrossRef]
- Lukas, E.V.; Daniel, D.; Paulo, P. Mapping the ecosystem service delivery chain: Capacity, flow, and demand pertaining to aesthetic experiences in mountain landscapes. Sci. Total Environ. 2017, 574, 422–436. [Google Scholar]
- Ullman, R.; Bilbao-Bastida, V.; Grimsditch, G. Including Blue Carbon in climate market mechanisms. Ocean Coast. Manag. 2013, 83, 15–18. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Hughes, A.R.; Kimbro, D.L. Loss of ‘blue carbon’ from coastal salt marshes following habitat disturbance. PLoS ONE 2013, 8, e69244. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.R.; Yang, T.T.; Jia, Y.L.; He, N.P.; Zhuang, J. New insight into global blue carbon estimation under human activity in land-sea interaction area: A case study of China. Earth Sci. Rev. 2016, 159, 36–46. [Google Scholar] [CrossRef]
- Fisher, B.; Turner, R.K.; Morling, P. Defining and classifying ecosystem systems service for decision making. Ecol. Econ. 2009, 68, 643–653. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering co2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Grimsditch, G.; Alder, J.; Nakamura, T.; Kenchington, R.; Tamelander, J. The blue carbon special edition—Introduction and overview. Ocean Coast. Manag. 2013, 83, 1–4. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marba, N.; et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef] [PubMed]
- Sifleet, S.; Pendleton, L.; Murray, B.C. State of the Science on Coastal Blue Carbon: A Summary for Policy Makers. Nicholas Institute for Environmental Policy Solutions. Ph.D. Thesis, Duke University, Durham, NC, USA, 2011. [Google Scholar]
- Bian, J.H.; Li, A.N.; Deng, W. Estimation and analysis of net primary productivity of Ruoergai wetland in China for the recent 10 years based on remote sensing. Procedia Environ. Sci. 2010, 2, 288–301. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Pan, Z.Y.; He, H.; Yu, D.Y.; Hu, H.B. Simulation of maximum light use efficiency for some typical vegetation types in China. Chin. Sci. Bull. 2006, 51, 457–463. [Google Scholar] [CrossRef]
- Howard, J.; Hoyt, S.; Isensee, K.; Pidgeon, E.; Telszewski, M.E. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows; Conservation Int.; Intergovernmental Oceanographic Commission of UNESCO; Int. Union for Conservation of Nature: Rome, Italy, 2014; p. 181. [Google Scholar]
- Zang, Z. Analysis of intrinsic value and estimating losses of “blue carbon” in coastal wetlands: A case study of Yancheng, China. Ecosyst. Health Sustain. 2019, 5, 216–225. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Baker, E. Geographical Analysis of Lung Cancer Mortality Rate and PM2.5 Using Global Annual Average PM2.5 Grids from MODIS and MISR Aerosol Optical Depth. J. Geosci. Environ. Prot. 2017, 5, 183–197. [Google Scholar] [CrossRef][Green Version]
- McHale, M.R.; Beck, S.M.; Pickett, S.T.A.; Childers, D.L.; Cadenasso, M.L.; Rivers, L., III; Swemmer, L.; Ebersohn, L.; Wayne, T.; Bunn, D.N. Democratization of ecosystem services—A radical approach for assessing nature’s benefits in the face of urbanization. Ecosys. Health Sustain. 2018, 4, 115–131. [Google Scholar] [CrossRef]
- Dang, H.Y.; Jiao, N. Perspectives of the microbial carbon pump with special references to microbial respiration and ecological efficiency. Biogeosciences 2014, 11, 3887–3898. [Google Scholar] [CrossRef]
- Jiao, N.; Robinson, C.; Azam, F.; Thomas, H.; Baltar, F.; Dang, H.; Hardman-Mountford, N.J.; Johnson, M.; Kirchman, D.L.; Koch, B.P.; et al. Mechanisms of microbial carbon sequestration in the ocean-future research directions. Biogeosciences 2014, 11, 7931–7990. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Allison, M.A.; Zhao, J.; Li, X.; Comeaux, R.S.; Feagin, R.A. Historical reconstruction of mangrove expansion in the Gulf of Mexico: Linking climate change with carbon sequestration in coastal wetlands. Estuar. Coast. Shelf Sci. 2013, 119, 7–16. [Google Scholar] [CrossRef]
- Li, C.; Wang, R.H.; Tan, M.L. Spatio-Temporal Changes in Vegetation Net Primary Productivity and Its Responses to Climatic Factors in Jiangsu Province, Eastern China. Sains Malays. 2019, 48, 2317–2324. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, C.H.; Pei, T.; Xie, Y.C. A comparative analysis of changes in the phasing of temperature and satellite-derived greenness at northern latitudes. J. Geogr. Sci. 2013, 23, 57–66. [Google Scholar] [CrossRef]
- Mu, S.J.; Yang, H.F.; Li, J.L.; Chen, Y.Z.; Gang, C.C.; Zhou, W.; Ju, W.M. Spatio-temporal dynamics of vegetation coverage and its relationship with climate factors in Inner Mongolia, China. J. Geogr. Sci. 2013, 23, 231–246. [Google Scholar] [CrossRef]
- Rezaei, S.A.; Gilkes, R.J. The effects of landscape attributes and plant community on soil chemical properties in rangelands. Geoderma 2005, 125, 167–176. [Google Scholar] [CrossRef]

| Quadrat | Plant Height (cm) | Plant Weight (g) | Litter Proportion | Crown-Root Proportion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | SG | PA | SA | SG | PA | SA | SG | PA | SA | SG | PA | |
| NEZ | 123.21 | 42.02 | 172.55 | 35.32 | 3.22 | 52.36 | 0.469 | 0.726 | 0.674 | 0.181 | 6.812 | 0.830 |
| NBZ | 128.13 | 45.13 | 156.23 | 38.53 | 3.35 | 49.65 | 0.453 | 0.708 | 0.621 | 0.185 | 6.803 | 0.838 |
| CZ | 135.00 | 53.25 | 155.40 | 39.12 | 3.18 | 45.66 | 0.425 | 0.721 | 0.607 | 0.463 | 8.372 | 0.907 |
| SBZ | 127.92 | 42.61 | 162.08 | 40.15 | 3.23 | 50.02 | 0.439 | 0.735 | 0.672 | 0.185 | 4.310 | 0.838 |
| SEZ | 125.65 | 44.45 | 172.86 | 36.62 | 3.41 | 51.27 | 0.488 | 0.719 | 0.657 | 0.300 | 5.900 | 1.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, Z. Conceptual Model of Ecosystem Service Flows from Carbon Dioxide to Blue Carbon in Coastal Wetlands: An Empirical Study Based on Yancheng, China. Sustainability 2021, 13, 4630. https://doi.org/10.3390/su13094630
Zang Z. Conceptual Model of Ecosystem Service Flows from Carbon Dioxide to Blue Carbon in Coastal Wetlands: An Empirical Study Based on Yancheng, China. Sustainability. 2021; 13(9):4630. https://doi.org/10.3390/su13094630
Chicago/Turabian StyleZang, Zheng. 2021. "Conceptual Model of Ecosystem Service Flows from Carbon Dioxide to Blue Carbon in Coastal Wetlands: An Empirical Study Based on Yancheng, China" Sustainability 13, no. 9: 4630. https://doi.org/10.3390/su13094630
APA StyleZang, Z. (2021). Conceptual Model of Ecosystem Service Flows from Carbon Dioxide to Blue Carbon in Coastal Wetlands: An Empirical Study Based on Yancheng, China. Sustainability, 13(9), 4630. https://doi.org/10.3390/su13094630





