Analysis of Heavy Metal Content in Soil and Plants in the Dumping Ground of Magnesite Mining Factory Jelšava-Lubeník (Slovakia)
Abstract
1. Introduction
2. Materials and Methods
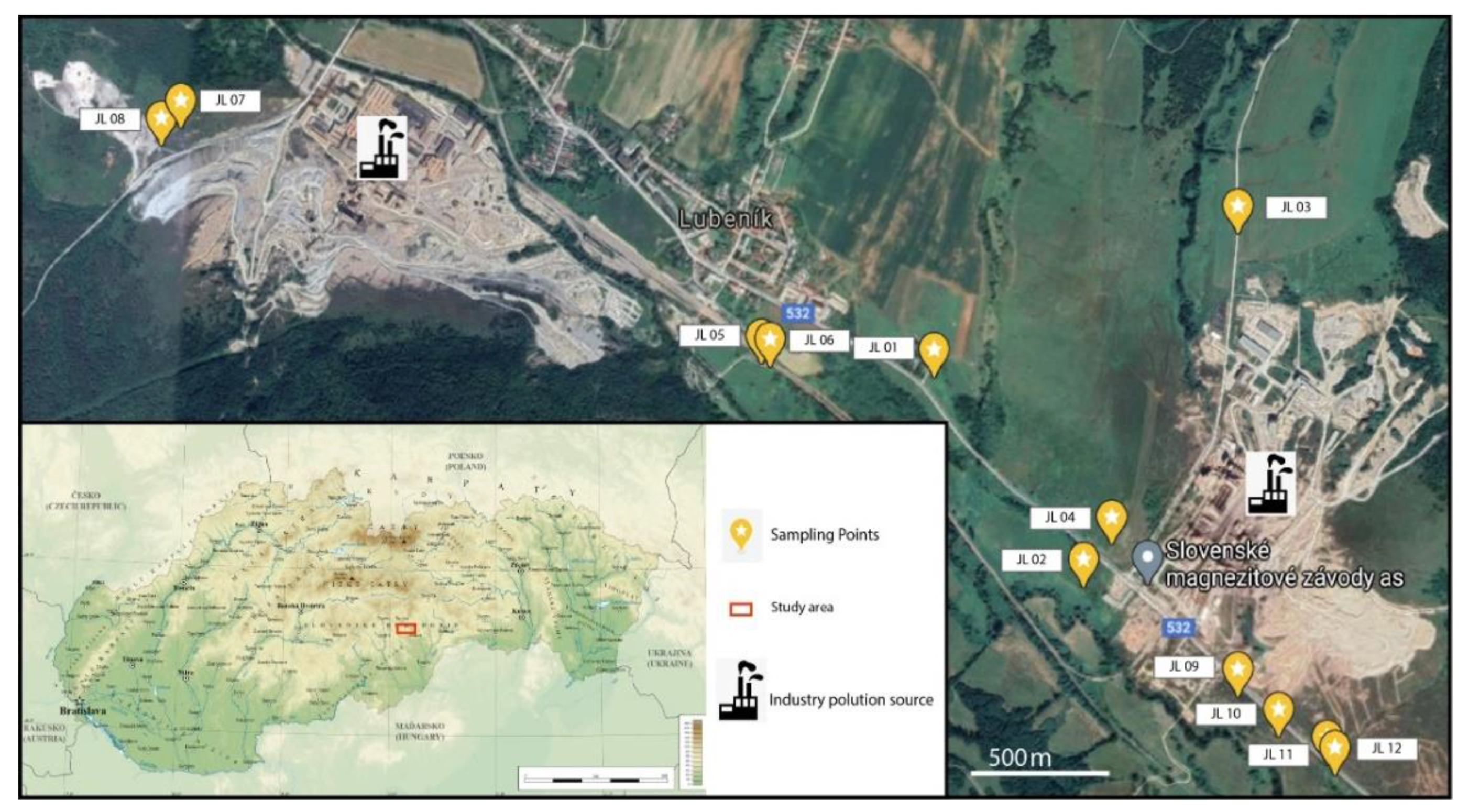
2.1. Study Area
2.2. Sampling Procedure
2.3. Analytical Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, C.; Hsu, P.C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; Available online: https://books.google.sk/books?id=bS-9x8TdXB8C&hl=sk&lr= (accessed on 5 January 2021).
- Błońska, E.; Lasota, J.; Gruba, P. Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecol. Res. 2016, 31, 655–664. [Google Scholar] [CrossRef]
- Gąsiorek, M.; Kowalska, J.; Mazurek, R.; Pająk, M. Comprehensive assessment of heavy metal pollution in topsoil of historical urban park on an example of the Planty Park in Krakow (Poland). Chemosphere 2017, 179, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Dradrach, A.; Karczewska, A.; Kocowicz, A. The distribution of sequentially extracted Cu, Pb, and Zn fractions in Podzol profiles under dwarf pine of different stages of degradation in subalpine zone of Karkonosze Mts (central Europe). J. Soils Sediments 2018, 18, 2387–2398. [Google Scholar] [CrossRef]
- Roba, C.; Roşu, C.; Piştea, I.; Ozunu, A.; Baciu, C. Heavy metal content in vegetables and fruits cultivated in Baia Mare mining area (Romania) and health risk assessment. Environ. Sci. Pollut. Res. 2016, 23, 6062–6073. [Google Scholar] [CrossRef]
- Nawab, J.; Li, G.; Khan, S.; Sher, H.; Aamir, M.; Shamshad, I.; Khan, A.; Khan, M.A. Health risk assessment from contaminated foodstuffs: A field study in chromite mining-affected areas northern Pakistan. Environ. Sci. Pollut. Res. 2016, 23, 12227–12236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Fan, L.; Chen, T.; Bai, Y.; Yu, Q.; Liu, Y. Assessment of multiple exposure to chemical elements and health risks among residents near Huodehong lead-zinc mining area in Yunnan, Southwest China. Chemosphere 2017, 174, 613–627. [Google Scholar] [CrossRef]
- Nuapia, Y.; Chimuka, L.; Cukrowska, E. Assessment of heavy metals in raw food samples from open markets in two African cities. Chemosphere 2018, 196, 339–346. [Google Scholar] [CrossRef]
- Wahsha, M.; Nadimi-Goki, M.; Fornasier, F.; Al-Jawasehr, R.; Hussein, E.I.; Bini, C. Microbial enzymes as an early warning management tool for monitoring mining site soils. Catena 2017, 148, 40–45. [Google Scholar] [CrossRef]
- Wu, B.; Hou, S.; Peng, D.; Wang, Y.; Wang, C.; Xu, F.; Xu, H. Response of soil micro-ecology to different levels of cadmium in alkaline soil. Ecotoxicol. Environ. Saf. 2018, 166, 116–122. [Google Scholar] [CrossRef]
- Halecki, W.; Gąsiorek, M. Seasonal variability of microbial biomass phosphorus in urban soils. Sci. Total Environ. 2015, 502, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Solgi, E. Contamination of two heavy metals in topsoils of the Urban Parks Asadabad. Arch. Hyg. Sci. 2016, 5, 92–101. Available online: http://jhygiene.muq.ac.ir/article-1-90-en.html (accessed on 5 January 2021).
- Zhou, H.; Zhou, X.; Zeng, M.; Liao, B.H.; Liu, L.; Yang, W.T.; Wu, Y.M.; Qiu, Q.Y.; Wang, Y.J. Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotoxicol. Environ. Saf. 2014, 101, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P. Slovak magnesite (in Slovak). Enviromagazín 2008, 13, 9. Available online: https://www.enviromagazin.sk/enviro2008/enviro6/06_slovensky.pdf (accessed on 5 January 2021).
- Jesenák, K. Wanderings around Slovakia with Karol Jesenák. Magnesite mining (in Slovak). 2014. Available online: https://fns.uniba.sk/fileadmin/prif/chem/kag/Zam-Jesenak/DnesnaSkola/dnesna_skola_apr_2014_Potulky_po_Slovensku_s_Karolom_Jesenakom_O_tazbe_magnezitu.pdf (accessed on 6 January 2021).
- Raman, N. Mycorrhizal status of plant species colonizing a magnesite mine spoil in India. Biol. Fertil. Soils 1993, 16, 76–78. [Google Scholar] [CrossRef]
- Machin, J.; Navas, A. Soil pH changes induced by contamination by magnesium oxides dust. Land Degrad. Dev. 2000, 11, 37–50. [Google Scholar] [CrossRef]
- Kautz, G.; Zimmer, M.; Zach, P.; Kulfan, J.; Topp, W. Suppression of soil microorganisms by emissions of a magnesite plant in the Slovak Republic. Water Air Soil Pollut. 2001, 125, 121–132. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Q.; Zeng, D.; Hu, Y.; Yu, Z. Remediation of a magnesium contaminated soil by chemical amendments and leaching. Land Degrad. Dev. 2015, 26, 613–619. [Google Scholar] [CrossRef]
- Ďurža, O. Impact of magnesite exploitation and processing on environment in Slovakia. Environment 2008, 42, 48–52. Available online: http://publikacie.uke.sav.sk/sites/default/files/2008_1_048_052_durza_0.pdf (accessed on 8 January 2021).
- Fazekaš, J.; Fazekašová, D.; Hronec, O.; Benková, E.; Boltižiar, M. Contamination of soil and vegetation at a magnesite mining area in Jelšava-Lubeník (Slovakia). Ekológia 2018, 37, 101–111. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef]
- Babst-Kostecka, A.; Schat, H.; Saumitou-Laprade, P.; Grodzińska, K.; Bourceaux, A.; Pauwels, M.; Frérot, H. Evolutionary dynamics of quantitative variation in an adaptive trait at the regional scale: The case of zinc hyperaccumulation in Arabidopsis halleri. Mol. Ecol. 2018, 27, 3257–3273. [Google Scholar] [CrossRef] [PubMed]
- El Khalil, H.; El Hamiani, O.; Bitton, G.; Ouazzani, N.; Boularbah, A. Heavy metal contamination from mining sites in South Morocco: Monitoring metal content and toxicity of soil runoff and groundwater. Environ. Monit. Assess. 2008, 136, 147–160. [Google Scholar] [CrossRef]
- Moroń, D.; Grześ, I.M.; Skórka, P.; Szentgyörgyi, H.; Laskowski, R.; Potts, S.G.; Woyciechowski, M. Abundance and diversity of wild bees along gradients of heavy metal pollution. J. Appl. Ecol. 2012, 49, 118–125. [Google Scholar] [CrossRef]
- Azarbad, H.; Niklińska, M.; van Gestel, C.A.M.; van Straalen, N.M.; Röling, W.F.M.; Laskowski, R. Microbial community structure and functioning along metal pollution gradients. Environ. Toxicol. Chem. 2013, 32, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, J.; Li, M.; Qin, Y.; Bao, X.; Wang, C.; Cui, D.; Xiang, O.; Ma, L.Q. Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: Oxidative stress, cell cycle arrest and apoptosis. Sci. Total Environ. 2021, 756, 143951. [Google Scholar] [CrossRef] [PubMed]
- Divan, A.M., Jr.; de Oliveira, P.L.; Perry, C.T.; Atz, V.L.; Azzarini-Rostirola, L.N.; Raya-Rodriguez, M.T. Using wild plant species as indicators for the accumulation of emissions from a thermal power plant, Candiota, South Brazil. Ecol. Indic. 2009, 9, 1156–1162. [Google Scholar] [CrossRef]
- Siedlecka, A.; Tukendorf, A.; Skórzyńska-Polit, E.; Maksymiec, W.; Wójcik, M.; Baszyński, T.; Krupa, Z. Angiosperms (Asteraceae, Convolvulaceae, Fabaceae and Poaceae; other than Brassicaceae). In Metals in the Environment. Analysis by Biodiversity; Prasad, M.N.V., Ed.; Marcel Dekket, Inc.: New York, NY, USA, 2001; pp. 171–217. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.E. Chemical Analysis of Ecological Material, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1989; 368 p. [Google Scholar]
- Szabová, T.; Leščinská, M.; Gondová, A. Heavy metal cumulation in crops after the sewage sludge application. Acta Montan. Slovaca 1998, 3, 478–481. Available online: https://core.ac.uk/download/pdf/27184684.pdf (accessed on 10 January 2021).
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. Available online: https://pubmed.ncbi.nlm.nih.gov/11741035/ (accessed on 10 January 2021). [CrossRef] [PubMed]
- Reeves, R.D. Hyperaccumulation of trace elements by plants. In Phytoremediation of Metal-Contaminated Soils; Morel, J.L., Echevarria, G., Goncharova, N., Eds.; Springer: New York, NY, USA, 2006; pp. 1–25. [Google Scholar] [CrossRef]
- Harrier, L.A.; Watson, C.A. The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest. Manag. Sci. 2004, 60, 149–157. [Google Scholar] [CrossRef]
- Janoušková, M.; Pavlíková, D.; Vosátka, M. Potential contribution of arbuscular mycorrhiza to cadmium immobilisation in soil. Chemosphere 2006, 65, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kenelley, E.D. A fern that hyperaccumulates arsenic—a hardy, versatile, fast-growing plant helps to remove arsenic from contaminated soils. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U. Enhancing phytoextraction: The effect of chemical soil manipulation on mobility, plant accumulation and leaching of heavy metals. J. Environ. Qual. 2003, 32, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Scragg, A. Environmental Biotechnology; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Boguská, Z.; Fazekašová, D.; Angelovičová, L. Diversity of vegetation on contaminated substrates. In Proceedings of the 17th International Conference on Environment and Mineral Processing, VŠB TU, Ostrava, Czech Republic, 6–8 June 2013. [Google Scholar]
- Baldantoni, D.; Alfani, A.; Di Tommasi, P.; Bartoli, G.; Virzo de Santo, A. Assessment of macro and microelement accumulation capability of two aquatic plants. Environ. Pollut. 2004, 130, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.M.; Han, J.D.; Shen, A.L.; Ping, X.Y.; Qian, P.L.; Li, C.J.; Shi, L.Y.; Chen, Y.Q. Uptake and distribution of N, P and heavy metals in three dominant salt marsh macrophytes from Yangtze River estuary, China. Mar. Environ. Res. 2007, 64, 21–37. [Google Scholar] [CrossRef]
- Bonanno, G.; Lo Giudice, R. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Minkina, T.; Fedorenko, G.; Nevidomskaya, D.; Fedorenko, A.; Chaplygin, V.; Mandzhieva, S. Morphological and anatomical changes of Phragmites australis Cav. due to the uptake and accumulation of heavy metals from polluted soils. Sci. Total Environ. 2018, 636, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, M.A.; Pietrini, F.; Fiore, F.; Petrilli, L.; Massacci, A. Antioxidant response to cadmium in Phragmites australis plants. Plant. Physiol. Biochem. 2002, 40, 977–982. [Google Scholar] [CrossRef]
- Ederli, L.; Reale, L.; Ferranti, F.; Pasqualini, S. Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol. Plant. 2004, 121, 66–74. [Google Scholar] [CrossRef]
- Weis, J.S.; Glover, T.; Weis, P. Interactions of metals affect their distribution in tissues of Phragmites australis. Environ. Pollut. 2004, 131, 409–415. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal pollution in aquatic ecosystems and its phytoremediation using wetland plants: An ecosustainable approach. Int. J. Phytorem. 2008, 10, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.P.; Human, L.R.D.; Adams, J.B. Wetland plants as indicators of heavy metal contamination. Mar. Pollut. Bull. 2015, 92, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Cicero-Fernández, D.; Peña-Fernández, M.; Expósito-Camargo, J.A.; Antizar-Ladislao, B. Long-term (two annual cycles) phytoremediation of heavy metal-contaminated estuarine sediments by Phragmites australis. N. Biotechnol. 2017, 38, 56–64. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, F.; Yu, G.; Song, C.; Geng, Z.; Zhuang, P. Removal of Cu, Zn, Pb, and Cr from yangtze estuary using the Phragmites australis artificial floating wetlands. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Su, F.; Wang, T.; Zhang, H.; Song, Z.; Feng, X.; Zhang, K. The distribution and enrichment characteristics of copper in soil and Phragmites australis of Liao River estuary wetland. Environ. Monit. Assess. 2018, 190, 365. [Google Scholar] [CrossRef]
- Minkina, T.M.; Mandzhieva, S.S.; Chaplygin, V.A.; Bauer, T.V.; Burachevskaya, M.V.; Nevidomskaya, D.G.; Sushkova, S.N.; Sherstnev, A.K.; Zamulina, I.V. Content and distribution of heavy metals in herbaceous plants under the effect of industrial aerosol emissions. J. Geochem. Explor. 2017, 174, 113–120. [Google Scholar] [CrossRef]
- Minkina, T.M.; Mandzhieva, S.S.; Chaplygin, V.A.; Nazarenko, O.G.; Maksimov, A.Y.; Zamulina, I.V.; Bukachevskaya, M.V.; Sushkova, S.N. Accumulation of heavy metals by forb stepe vegetation according to long-term monitoring data. Arid Ecosyst. 2018, 8, 190–202. [Google Scholar] [CrossRef]
- Enya, O.; Lin, C.; Qin, J. Heavy metal contamination status in soil-plant system in the Upper Mersey Estuarine Floodplain, Northwest England. Mar. Pollut. Bul. 2019, 146, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Issaka, S.; Ashraf, M.A. Phytorestoration of mine spoiled: “Evaluation of natural phytoremediation process occurring at ex-tin mining catchment”. In Phytorestoration of Abandoned Mining and Oil Drilling Sites; Bauddh, K., Korstad, J., Sharma, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 219–248. [Google Scholar] [CrossRef]
- Cadiz, N.M.; Davies, M.S.; de Guzman, C.C. Root growth characteristics of Ocimum sanctum and Festuca rubra cv. Merlin in response to cadmium, lead and zinc. Philipp. Agric. Sci. 1995, 78, 331–342. Available online: https://agris.fao.org/agris-search/search.do?recordID=PH9710113 (accessed on 15 February 2021).
- Cadiz, N.M.; de Guzman, C.C.; Davies, M.S. Tolerance strategies of plants to heavy metals: Cellular changes, accumulation pattern and intracellular localization of Cd, Pb and Zn in Festuca rubra L. cv. Merlin (Red Fescue) and Ocimum sanctum (Holy Basil). Philipp. Agric. Sci. 1999, 82, 5–24. Available online: https://agris.fao.org/agris-search/search.do?recordID=PH9610541 (accessed on 15 February 2021).
- Patra, D.K.; Acharya, S.; Pradhan, C.; Patra, H.K. Poaceae plants as potential phytoremediators of heavy metals and eco-restoration in contaminated mining sites. Environ. Technol. Innov. 2021, 21. [Google Scholar] [CrossRef]
- Pérez-de-mora, A.; Burgos, P.; Madejón, E.; Cabrera, F.; Jaeckel, P.; Schloter, M. Microbial community structure and function in a soil contaminated by heavy metals: Effects of plant growth and different amendments. Soil Biol. Biochem. 2006, 38, 327–341. [Google Scholar] [CrossRef]
- Parraga-Aguado, I.; Gonzáles-Alcaraz, M.N.; Schulin, R.; Conesa, H.M. The potential use of Piptatherum miliaceum for the phytomanagement of mine tailings in semiarid areas: Role of soil fertility and plant competition. J. Environ. Manage. 2015, 158, 74–84. [Google Scholar] [CrossRef]
- Gajic, G.; Djurdjevic, L.; Kostic, O.; Jaric, S.; Mitrovic, M.; Stevanovic, B.; Pavlovic, P. Assessment of the phytoremediation potential and an adaptive response of Festuca rubra L. Sown on fly ash deposits: Native grass has a pivotal role in Eco restoration management. Ecol. Eng. 2016, 93, 250–261. [Google Scholar] [CrossRef]
- Gajic, G.; Djurdjevic, L.; Kostic, O.; Jaric, S.; Mitrovic, M.; Pavlovic, P. Ecological potential of plants for phytoremediation and Eco restoration of fly ash deposits and mine wastes. Front. Environ. Sci. 2018, 6, 124. [Google Scholar] [CrossRef]
- Gajic, G.; Mitrovic, M.; Pavlovic, P. Eco restoration of fly ash deposits by native plant species at thermal power stations in Serbia. In Phytomanagement of Polluted Sites, Market Opportunities in Sustainable Phytoremediation; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amstredam, The Netherlands, 2019; pp. 113–177. [Google Scholar] [CrossRef]
- Mazúr, E.; Lukniš, M. Regional Division SSR (in Slovak); Geografický Ústav SAV: Bratislava, Slovakia, 1980. [Google Scholar]
- Klinda, J.; Mičík, T.; Némethová, M.; Slámková, M. Environmental Regionalisation of the Slovak Republic 2016; Ministry of Environment of the Slovak Republic: Bratislava, Slovakia, 2016; Available online: https://www.minzp.sk/files/environmentalna-regionalizacia-sr.pdf (accessed on 12 January 2021).
- Lapin, M.; Faško, P.; Melo, M.; Šťastný, P.; Tomlain, J. Climate Areas. Atlas of the Slovak Republic; Ministry of Environment of the Slovak Republic: Bratislava, Slovakia, 2002. [Google Scholar]
- Slovak Technical Standard ISO 10381-6. Soil Quality—Sampling—Part 6: Guidance on the Collection, Handling and Storage of Soil Under Aerobic Conditions for the Assessment of Microbiological Processes, Biomass and Diversity in the Laboratory. Available online: https://www.iso.org/standard/43691.html (accessed on 15 November 2020).
- Ministry of Agriculture of the Slovak Republic. Ministry of Agriculture Decree No. 338/2005; Ministry of Agriculture of the Slovak Republic: Bratislava, Slovakia, 2006. [Google Scholar]
- Act of the National Council of the Slovak Republic No. 220/2004 Coll. Available online: https://www.mpsr.sk/zakon-c-220-2004-z-z/27-23-27-8366/ (accessed on 20 November 2020).
- Kobza, J.; Barančíková, G.; Čumová, L.; Dodok, R.; Hrivňaková, K.; Makovníková, J. Methods of Determining Indicators of Agrochemical Soil Properties; SSCRI: Bratislava, Slovakia, 2011. [Google Scholar]
- Martin, M.; Bonifacio, E.; Hossain, K.M.J.; Huq, S.M.I.; Barberis, E. Arsenic fixation and mobilization in the soils of the Ganges and Meghna floodplains. Impact of pedoenvironmental properties. Geoderma 2014, 228–229, 132–141. [Google Scholar] [CrossRef]
- Yang, D.; Zeng, D.H.; Zhang, J.; Li, L.J.; Mao, R. Chemical and microbial properties in contaminated soils around a magnesite mine in northeast China. Land Degrad. Dev. 2012, 23, 256–262. [Google Scholar] [CrossRef]
- Hronec, O.; Adamišin, P. Management of Areas Revitalization with Innovative Processes. In Proceedings of the International Scientific Conference Sustainability-Environment-Safety 2014, Bratislava, Slovakia, 2014. Available online: http://www.sszp.eu/wp-content/uploads/2014_konf_SES__p-115__Adamisin-Hronec_.pdf (accessed on 13 February 2021).
- Tian, K.; Huang, B.; Xing, Z.; Hu, W. Geochemical baseline establishment and ecological risk evaluation of heavy metals in greenhouse soils from Dongtai. China. Ecol. Indic. 2017, 72, 510–520. [Google Scholar] [CrossRef]
- Šefčík, P.; Pramuka, S.; Gluch, A. Assessment of soil contamination in Slovakia according index of geoaccumulation. Agriculture 2008, 54, 119–130. Available online: https://agris.fao.org/agris-search/search.do?recordID=SK2008000265 (accessed on 13 February 2021).
- Xu, Z.Q.; Ni, S.; Tuo, X.G.; Zhang, C.J. Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ. Sci. Technol. 2008, 31, 112–115. [Google Scholar]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400. [Google Scholar] [CrossRef]
- Kobza, J. Current state and development of micronutrients in agricultural soils of Slovakia. Agrochémia 2018, 2, 15–21. Available online: http://agrochemia.uniag.sk/pdf/agrochemia_2_2018_kobza_3.pdf (accessed on 13 February 2021).
- Bobro, M.; Hančuľák, J. Mineralogical properties of imission sedimens in the areas of magnesite industry. Acta Montan. Slovaca 1997, 2, 240–243. Available online: https://core.ac.uk/download/pdf/26668833.pdf (accessed on 14 February 2021).
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of Soil Heavy Metal Pollution on Food Safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar] [CrossRef] [PubMed]
- Čurlík, J.; Šefčík, P. Geochemický atlas Slovenskej republiky/Geochemical atlas of the Slovak Republic; SSCRI: Bratislava, Slovakia, 1999. [Google Scholar]
- Ma, Y.; Egodawatta, P.; McGree, J.; Liu, A.; Goonetilleke, A. Human health risk assessment of heavy metals in urban stormwater. Sci. Total Environ. 2016, 557, 764–772. [Google Scholar] [CrossRef]
- Doležalová Weissmannová, H.; Mihočová, S.; Chovanec, P.; Pavlovský, J. Potential Ecological Risk and Human Health Risk Assessment of Heavy Metal Pollution in Industrial Affected Soils by Coal Mining and Metallurgy in Ostrava, Czech Republic. Int. J. Environ. Res. Public Health. 2019, 16, 4495. [Google Scholar] [CrossRef]
- Dercová, K.; Makovníková, J.; Barančíková, G.; Žuffa, J. Bioremediation of Soil and Wastewater Contaminated with Toxic Metals. Chem. Listy 2005, 99, 682–693. Available online: http://www.chemicke-listy.cz/ojs3/index.php/chemicke-listy/article/view/1984/1984 (accessed on 15 February 2021).
- Sun, Z.; Xie, X.; Wang, P.; Cheng, H. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total. Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef]
- Baker, A.; McGrath, S.; Reeves, R.; Smith, J. Metal hyper-accumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In Phytoremediation of Contaminated Soil and Water; Terry, N., Banuelos, G., Eds.; Lewis Publisher: London, UK, 2000; pp. 85–107. [Google Scholar]
- Lesage, E.; Rousseau, D.P.L.; Meers, E.; Tack, F.M.G.; de Pauw, N. Accumulation of metals in a horizontal subsurface flow constructed wetland treating domestic wastewater in Flanders, Belgium. Sci. Total Environ. 2007, 380, 102–115. [Google Scholar] [CrossRef]
- Vymazal, J.; Švehla, J.; Kröpfelová, L.; Chrastný, V. Trace metals in Phragmites australis and Phalaris arundinacea growing in constructed and natural wetlands. Sci. Total Environ. 2007, 380, 154–162. [Google Scholar] [CrossRef]
- Chaplygin, V.; Minkina, T.; Mandzhieva, S.; Burachevskaya, M.; Sushkova, S.; Poluektov, E.; Antonenko, E.; Kumacheva, V. The effect of technogenic emissions on the heavy metals accumulation by herbaceous plants. Environ. Monit. Assess. 2018, 190. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements in Soils and Plants; Springer: Berlin, Germany, 2007. [Google Scholar]
- Venkatesan, S.; Jayaganesh, S. Characterisation of Magnesium Toxicity, its Influence on Amino Acid Synthesis Pathway and Biochemical Parameters of Tea. Res. J. Phytochem. 2010, 4, 67–77. [Google Scholar] [CrossRef]
- Zeleňáková, E.; Hajdúk, J.; Holub, Z. Obsah horčíka a iných prvkov v rastlinách (Agropyrum repens P. Beauv., Secale cereale L., Triticum aestivum L.) ovplyvnených magnezitovými exhalačnými splodinami/Content of magnesium and other elements in plants (Agropyrum repens P. Beauv., Secale cereale L., Triticum aestivum L.) affected by magnesite exhalation fumes. Biológia 1966, 21, 365–375. [Google Scholar] [PubMed]
- Hronec, O.; Hajdúk, J. Remarkable resistance of Phragmites australis (Cav.) Trin. growing on soils intoxicated by magnesium immissions. Ekologia 1998, 17, 117–124. [Google Scholar]
- Blanár, D.; Petrasova, A. Desmatodon cernuus (Huebener) Bruch & Schimp—A new species in the bryoflora of Slovakia. Reussia 2007, 4, 79–106. Available online: https://www.researchgate.net/publication/296695052 (accessed on 14 February 2021).
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]



| Year | Parameter | Mean | Median | Minimum | Maximum | Standard Deviation | Limit Value * |
|---|---|---|---|---|---|---|---|
| 2019 | pH/KCl | 8.05 | 7.63 | 6.58 | 9.27 | 0.97 | - |
| Cr | 168.58 | 104.50 | 85.00 | 793.00 | 197.86 | 70.00 | |
| As | 25.83 | 21.00 | 13.00 | 63.00 | 14.31 | 25.00 | |
| Mn | 1341.67 | 1350.00 | 400.00 | 2200.00 | 505.35 | - | |
| Mg | 31,500.00 | 18,350.00 | 9800.00 | 83,100.00 | 25,749.07 | - | |
| Hg | 0.07 | 0.07 | 0.01 | 0.14 | 0.03 | 0.50 | |
| Cd | 0.52 | 0.40 | 0.40 | 1.00 | 0.19 | 0.70 | |
| Pb | 29.92 | 30.50 | 22.00 | 45.00 | 6.86 | 70.00 | |
| Zn | 84.17 | 77.50 | 61.00 | 113.00 | 16.02 | 150.00 | |
| Cu | 27.58 | 28.00 | 16.00 | 45.00 | 9.28 | 60.00 | |
| Ni | 32.58 | 30.50 | 21.00 | 54.00 | 10.07 | 50.00 | |
| 2020 | pH/KCl | 8.14 | 7.90 | 6.73 | 9.32 | 0.95 | - |
| Cr | 152.83 | 102.50 | 78.00 | 377.00 | 108.53 | 70.00 | |
| As | 27.92 | 21.50 | 11.00 | 84.00 | 20.45 | 25.00 | |
| Mn | 1266.67 | 1300.00 | 600.00 | 1900.00 | 396.19 | - | |
| Mg | 24,891.67 | 19,200.00 | 8400.00 | 57,200.00 | 17,033.73 | - | |
| Hg | 0.07 | 0.06 | 0.03 | 0.10 | 0.03 | 0.50 | |
| Cd | 0.40 | 0.40 | 0.40 | 0.40 | 0.00 | 0.70 | |
| Pb | 28.92 | 29.50 | 16.00 | 39.00 | 7.61 | 70.00 | |
| Zn | 81.83 | 82.00 | 52.00 | 108.00 | 13.59 | 150.00 | |
| Cu | 27.17 | 27.00 | 13.00 | 49.00 | 12.04 | 60.00 | |
| Ni | 31.92 | 28.50 | 19.00 | 47.00 | 9.77 | 50.00 |
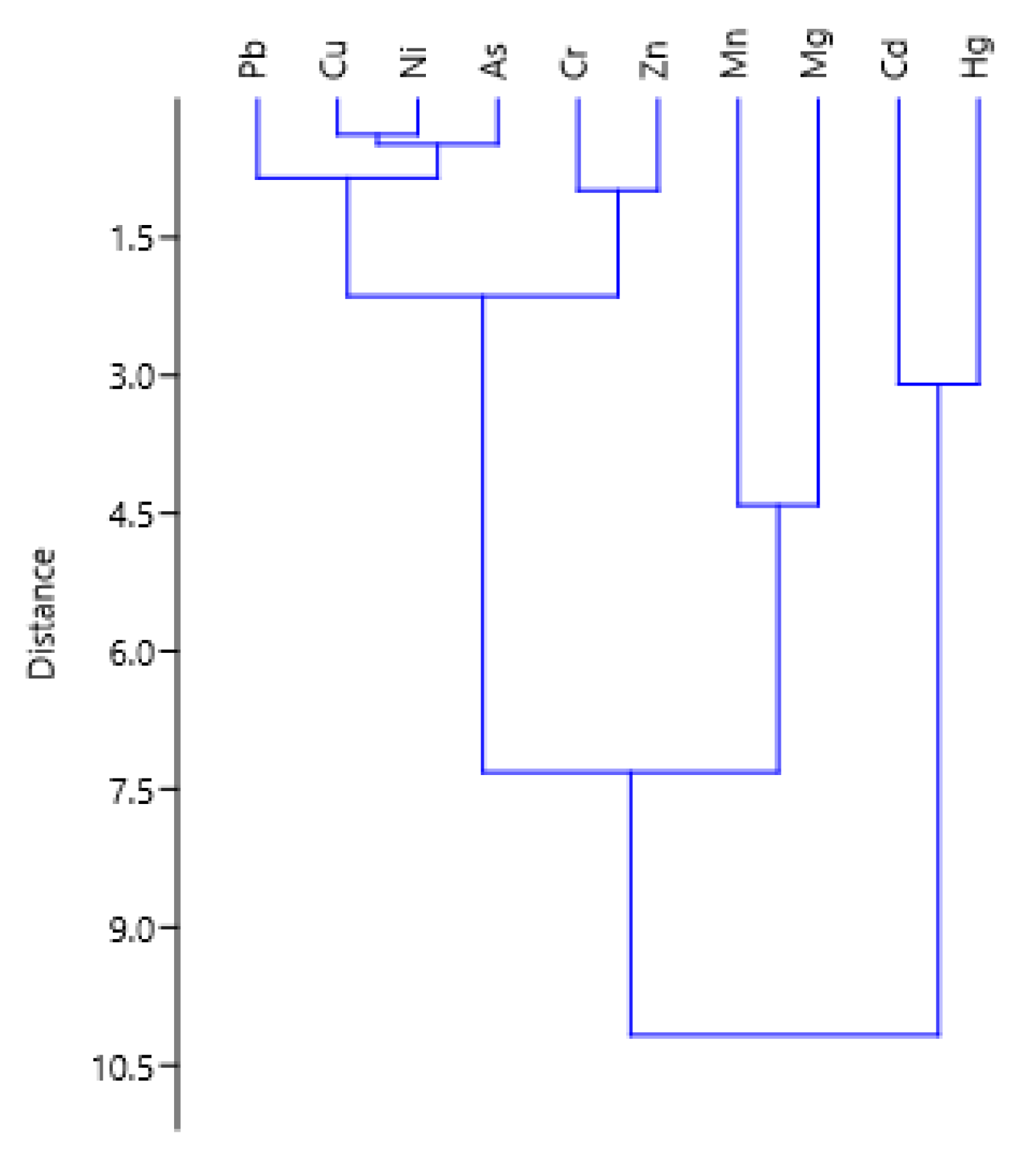
| Parameter | Cd | Pb | Cr | Zn | Cu | As | Ni | Mn | Mg | pH/KCl |
|---|---|---|---|---|---|---|---|---|---|---|
| Hg | 0.044 | 0.559 | −0.252 | −0.355 | −0.315 | −0.282 | −0.354 | 0.633 * | −0.090 | 0.115 |
| Cd | 0.004 | 0.143 | 0.344 | 0.345 | 0.485 | 0.376 | 0.196 | 0.152 | 0.231 | |
| Pb | −0.343 | −0.364 | −0.656 * | −0.551 | −0.501 | 0.139 | −0.466 | −0.228 | ||
| Cr | 0.582 * | 0.042 | 0.111 | 0.056 | −0.474 | −0.168 | −0.509 | |||
| Zn | 0.316 | 0.420 | 0.284 | −0.186 | 0.245 | −0.329 | ||||
| Cu | 0.924 ** | 0.924 ** | 0.259 | 0.723 ** | 0.586 * | |||||
| As | 0.935 ** | 0.234 | 0.581 * | 0.420 | ||||||
| Ni | 0.155 | 0.470 | 0.393 | |||||||
| Mn | 0.531 | 0.594 * | ||||||||
| Mg | 0.762 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štofejová, L.; Fazekaš, J.; Fazekašová, D. Analysis of Heavy Metal Content in Soil and Plants in the Dumping Ground of Magnesite Mining Factory Jelšava-Lubeník (Slovakia). Sustainability 2021, 13, 4508. https://doi.org/10.3390/su13084508
Štofejová L, Fazekaš J, Fazekašová D. Analysis of Heavy Metal Content in Soil and Plants in the Dumping Ground of Magnesite Mining Factory Jelšava-Lubeník (Slovakia). Sustainability. 2021; 13(8):4508. https://doi.org/10.3390/su13084508
Chicago/Turabian StyleŠtofejová, Lenka, Juraj Fazekaš, and Danica Fazekašová. 2021. "Analysis of Heavy Metal Content in Soil and Plants in the Dumping Ground of Magnesite Mining Factory Jelšava-Lubeník (Slovakia)" Sustainability 13, no. 8: 4508. https://doi.org/10.3390/su13084508
APA StyleŠtofejová, L., Fazekaš, J., & Fazekašová, D. (2021). Analysis of Heavy Metal Content in Soil and Plants in the Dumping Ground of Magnesite Mining Factory Jelšava-Lubeník (Slovakia). Sustainability, 13(8), 4508. https://doi.org/10.3390/su13084508






