Assessing Yield Response and Relationship of Soil Boron Fractions with Its Accumulation in Sorghum and Cowpea under Boron Fertilization in Different Soil Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling, Processing, and Characterization
2.2. Greenhouse Study and Growth Conditions
2.3. Harvesting and Biomass Measurement
2.4. Boron Sequential Extraction
2.5. Sample Processing for Total B Concentration in Plant and Soil
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Soil
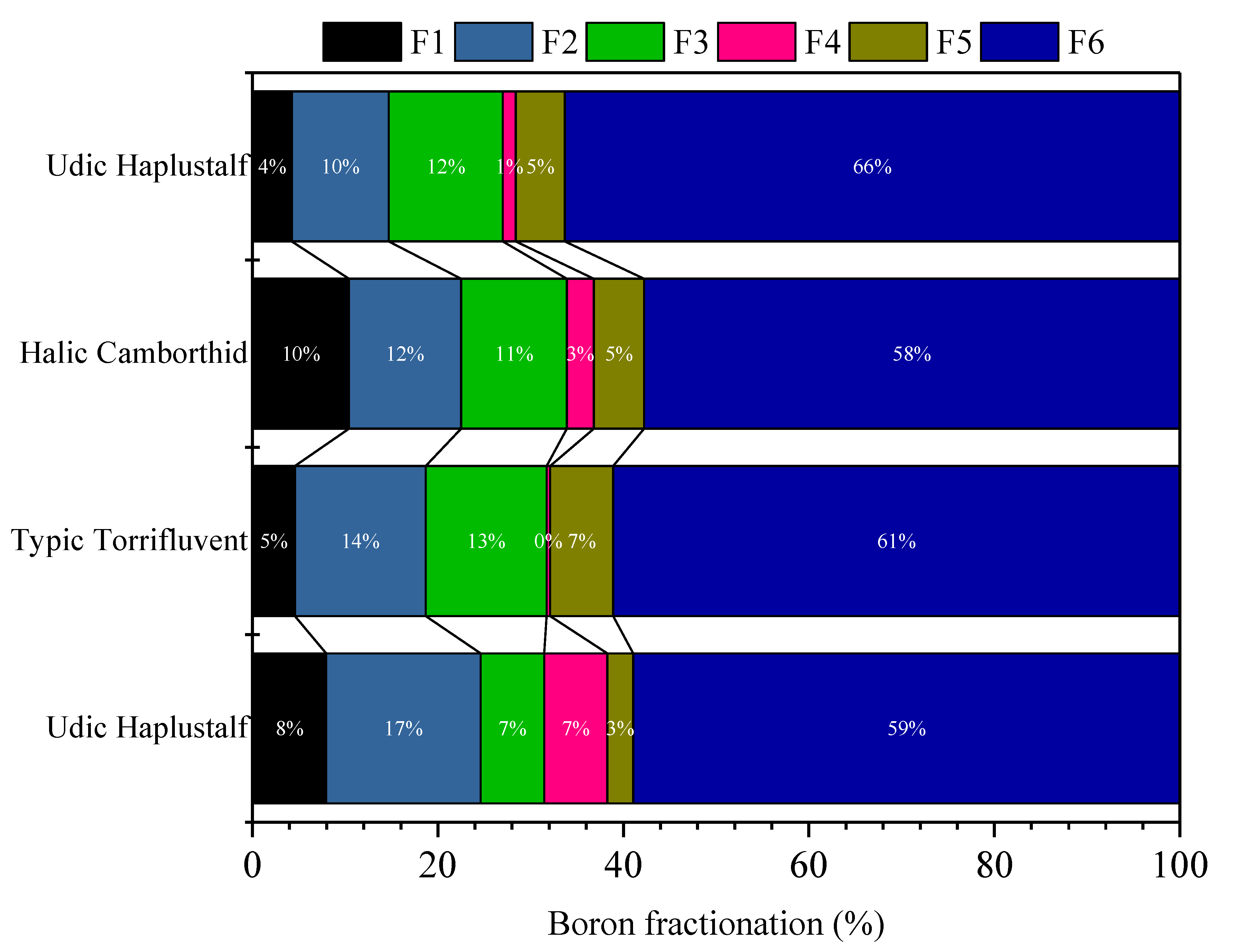
3.2. Boron (B) Fractionation in Soil
3.3. Effect of B on Yield of Sorghum and Cowpea
3.4. Effect of B on Its Accumulation in Sorghum and Cowpea
3.5. Comparison of Shoot Dry Matter Yield in Crops
3.6. Comparison of Different Soil Series
3.7. Role of Boron Geochemical Fractions on its Accumulation in Shoot
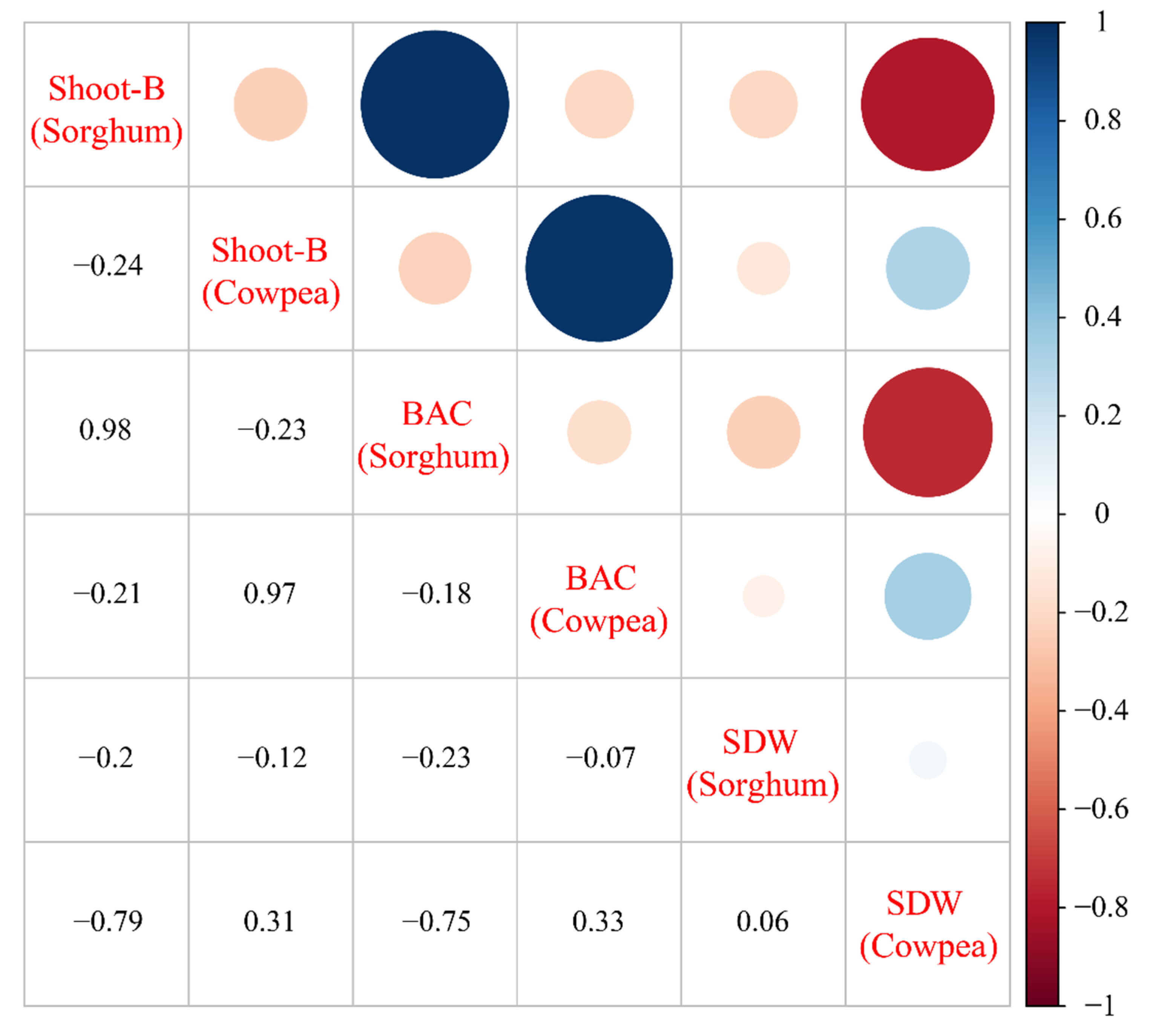
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Sánchez, F.; Simón-Grao, S.; Martínez-Nicolás, J.J.; Alfosea-Simón, M.; Liu, C.; Chatzissavvidis, C.; Pérez-Pérez, J.G.; Cámara-Zapata, J.M. Multiple stresses occurring with boron toxicity and deficiency in plants. J. Hazard. Mater. 2020, 397, 122713. [Google Scholar] [CrossRef]
- Havlin, J.L. Soil: Fertility and Nutrient Management. In Encyclopedia of Natural Resources: Land; Taylor & Francis: Abingdon, UK, 2014; pp. 460–469. [Google Scholar]
- Pandey, N.; Verma, P. Boron Deficiency and Toxicity and Their Tolerance in Plants: A Review. J. Glob. Biosci. 2017, 6, 4958–4965. [Google Scholar]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Rashid, A.; Torrent, J.; Yau, S.K.; Ibrikci, H.; Sommer, R.; Erenoglu, E.B. Micronutrient constraints to crop production in the Middle East–West Asia region: Significance, research, and management. Adv. Agron. 2013, 122, 1–84. [Google Scholar]
- Bhupenchandra, I.; Basumatary, A.; Dutta, S.; Singh, L.K.; Datta, N. Impact of boron fertilization on boron fractions at different crop growth stages in cauliflower_cowpea_okra sequence in an inceptisols of North East India. J. Plant Nutr. 2020, 43, 1175–1188. [Google Scholar] [CrossRef]
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Lehto, T.; Ruuhola, T.; Dell, B. Boron in forest trees and forest ecosystems. For. Ecol. Manag. 2010, 260, 2053–2069. [Google Scholar] [CrossRef]
- Sarkar, D.; Batabyal, K.; Das, R.; Datta, A.; Hazra, G.C.; Mandal, B.; Saha, J.K. Boron in soils and crops of West Bengal. Dir. Res. 2012, 1–24. [Google Scholar]
- Camacho-cristóbal, J.J.; Rexach, J.; González-Fontes, A. Boron in plants: Deficiency and toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.Z.; Yaseen, M.; Abbas, T.; Naveed, M.; Mustafa, A.; Hamid, Y.; Saeed, Q.; XU, M. Foliar application of micronutrients enhances crop stand, yield and the biofortification essential for human health of different wheat cultivars. J. Integr. Agric. 2019, 18, 1369–1378. [Google Scholar] [CrossRef]
- Aboyeji, C.; Dunsin, O.; Adekiya, A.O.; Chinedum, C.; Suleiman, K.O.; Okunlola, F.O.; Aremu, C.O.; Owolabi, I.O.; Olofintoye, T.A.J. Zinc Sulphate and Boron-Based Foliar Fertilizer Effect on Growth, Yield, Minerals, and Heavy Metal Composition of Groundnut (Arachis hypogaea L) Grown on an Alfisol. Int. J. Agron. 2019, 2019. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M. Cotton carbon exchange, nonstructural carbohydrates, and boron distribution in tissues during development of boron deficiency. Field Crop. Res. 2002, 78, 75–87. [Google Scholar] [CrossRef]
- Saleem, M.; Khanif, Y.M.; Fauziah, I.; Samsuri, A.W.; Hafeez, B. Importance of Boron for Agriculture Productivity: A Review. Int. Res. J. Agric. Sci. Soil Sci. 2011, 1, 2251–44293. [Google Scholar]
- Simón, I.; Díaz-López, L.; Gimeno, V.; Nieves, M.; Pereira, W.E.; Martínez, V.; Lidon, V.; García-Sánchez, F. Effects of boron excess in nutrient solution on growth, mineral nutrition, and physiological parameters of Jatropha curcas seedlings. J. Plant Nutr. Soil Sci. 2013, 176, 165–174. [Google Scholar] [CrossRef]
- Woods, W.G. An introduction to boron: History, sources, uses, and chemistry. Environ. Health Perspect. 1994, 102, 5–11. [Google Scholar]
- Wimmer, M.A.; Eichert, T. Review: Mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci. 2013, 203–204, 25–32. [Google Scholar] [CrossRef]
- Faostat Agriculture Organization Corporate Statistical Database. 2018. Available online: http://www.fao.org/faostat/en/#home (accessed on 14 April 2020).
- Kamtchoum, S.M.; Nchoutnji, I.; Temegne, C.N.; Tonfack, L.B.; Fofe, L.; Seutchueng, T.G.T.; Kemayou, C.M.; Notche, F.K.; Suh, C.; Youmbi, E. Comparative effect of biological fixation of nitrogen and chemical fertilizer on yield optimization of two sorghum varieties in the Western Highlands. Asian J. Agric. Hortic. Res. 2019, 1–10. [Google Scholar] [CrossRef]
- Sahrawat, K.L.; Rego, T.J.; Wani, S.P.; Pardhasaradhi, G. Sulfur, boron, and zinc fertilization effects on grain and straw quality of maize and sorghum grown in semi-arid tropical region of India. J. Plant Nutr. 2008, 31, 1578–1584. [Google Scholar] [CrossRef]
- Sahrawat, K.L.; Wani, S.P.; Rego, T.J.; Pardhasaradhi, G.; Murthy, K.V.S. Widespread deficiencies of sulphur, boron and zinc in dryland soils of the Indian semi-arid tropics. Curr. Sci. 2007, 93, 1428–1432. [Google Scholar]
- Boukar, O.; Fatokun, C.A.; Huynh, B.L.; Roberts, P.A.; Close, T.J. Genomic tools in cowpea breeding programs: Status and perspectives. Front. Plant Sci. 2016, 7, 757. [Google Scholar] [CrossRef]
- Kebede, E.; Bekeko, Z. Expounding the production and importance of cowpea (Vigna unguiculata (L.) Walp.) in Ethiopia. Cogent Food Agric. 2020, 6, 1769805. [Google Scholar] [CrossRef]
- Agbenin, J.O.; Lombin, G.; Owonubi, J.J. Effect of boron and nitrogen fertilization on cowpea nodulation, mineral nutrition and grain yield. Fertil. Res. 1990, 22, 71–78. [Google Scholar] [CrossRef]
- Shahid, M.; Nayak, A.K.; Tripathi, R.; Katara, J.L.; Bihari, P.; Lal, B.; Gautam, P. Boron application improves yield of rice cultivars under high temperature stress during vegetative and reproductive stages. Int. J. Biometeorol. 2018, 62, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Motagally, F.M.F.; El-Zohri, M. Improvement of wheat yield grown under drought stress by boron foliar application at different growth stages. J. Saudi Soc. Agric. Sci. 2018, 17, 178–185. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Basumatary, A.; Datta, S.; Kanta Singh, L.; Bora, S.S.; Helena Devi, S.; Gogoi, B.; Kalita, P.; Das, J.; Tamuli, B. Micronutrient Content in Plant, Its Uptake, and Crop Yield in Cauliflower–cowpea-Okra Cropping Sequence as Impacted by Graded Level of Boron Fertilization. Commun. Soil Sci. Plant Anal. 2020, 51, 1870–1887. [Google Scholar] [CrossRef]
- Chatterjee, R.; Bandyopadhyay, S. Effect of boron, molybdenum and biofertilizers on growth and yield of cowpea (Vigna unguiculata L. Walp.) in acid soil of eastern Himalayan region. J. Saudi Soc. Agric. Sci. 2017, 16, 332–336. [Google Scholar] [CrossRef]
- Anjum, S.A.; Saleem, M.F.; Shahid, M.; Shakoor, A.; Safeer, M.; Khan, I.; Farooq, A.; Ali, I.; Nazir, U. Dynamics of Soil and Foliar Applied Boron and Zinc to Improve Maize Productivity and Profitability. Pakistan J. Agric. Res. 2017, 30. [Google Scholar] [CrossRef]
- Dursun, A.; Turan, M.; Ekinci, M.; Gunes, A.; Ataoglu, N.; Aslihan, E.; Yildirim, E. Effects of boron fertilizer on tomato, pepper, and cucumber yields and chemical composition. Commun. Soil Sci. Plant Anal. 2010, 41, 1576–1593. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Basumatary, A.; Singh, L.K.; Khwairakpam, R. Assessment of the complex relationship of boron fractions with available soil nutrient status as influenced by boron fertilization in cauliflower. IJCS 2019, 7, 4253–4256. [Google Scholar]
- Javed, M.B.; Shotyk, W. Estimating bioaccessibility of trace elements in particles suspended in the Athabasca River using sequential extraction. Environ. Pollut. 2018, 240, 466–474. [Google Scholar] [CrossRef]
- Boparai, A.K.; Manchanda, J.S. Extractability of available boron and its sequential fractionation in alkaline calcareous soils of India. Commun. Soil Sci. Plant Anal. 2018, 49, 2197–2208. [Google Scholar] [CrossRef]
- Javed, M.B. Distribution of Arsenic in Surficial Deposits in the Cold Lake Area of Alberta, Canada. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2015. [Google Scholar]
- Javed, M.B.; Kachanoski, G.; Siddique, T. Arsenic fractionation and mineralogical characterization of sediments in the Cold Lake area of Alberta, Canada. Sci. Total Environ. 2014, 500, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Evans, L.J.; Spiers, G.A. Boron fractionation in soils. Commun. Soil Sci. Plant Anal. 1994, 25, 1841–1853. [Google Scholar] [CrossRef]
- Ahmad, M.; Shabbir Baig, M.S.B.; Akram, M.; Yasin Javed, M.Y.J.; Riaz-ul-Amin, R.A. Xii International Forum on Soil Taxonomy and Agrotechnology Transfer, 9–23 October Soil Survey of Pakistan; Soil Management Support Services U.S.A: Lahore, Pakistan, 1986.
- Day, P.R. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; American Society of Agronomy, Inc.: Madison WI, USA, 2015. [Google Scholar]
- Allison, L.E.; Moodie, C.D. Carbonate. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American So-ciety of Agronomy, Inc.: Madison, WI, USA, 1965; Volume 9, pp. 1379–1396. [Google Scholar]
- Allison, L. Organic carbon. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; Volume 9, pp. 1367–1378. [Google Scholar]
- Parker, D.R.; Gardner, E.H. The Determination of Hot-Water-Soluble Boron in Some Acid Oregon Soils Using a Modified Azomethine-H Procedure. Commun. Soil Sci. Plant Anal. 1981, 12, 1311–1322. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill Book Co. Inc.: New York, NY, USA, 1980; p. 481. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Gaines, T.P.; Mitchell, G.A. Boron determination in plant tissue by the azomethine h method. Commun. Soil Sci. Plant Anal. 1979, 10, 1099–1108. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis. J. Agric. Food Chem. 1959. [Google Scholar] [CrossRef]
- Wu, X.; Lu, X.; Riaz, M.; Yan, L.; Jiang, C. Boron toxicity induced specific changes of cell ultrastructure and architecture of components in leaf center and tip of trifoliate orange [Poncirus trifoliata (L.) Raf.]. J. Environ. Manag. 2019, 246, 426–433. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Boron deprivation induced inhibition of root elongation is provoked by oxidative damage, root injuries and changes in cell wall structure. Environ. Exp. Bot. 2018, 156, 74–85. [Google Scholar] [CrossRef]
- Wang, G.; DiTusa, S.F.; Oh, D.H.; Herrmann, A.D.; Mendoza-Cozatl, D.G.; O’Neill, M.A.; Smith, A.P.; Dassanayake, M. Cross species multi-omics reveals cell wall sequestration and elevated global transcription as mechanisms of boron tolerance in plants. bioRxiv 2020. [Google Scholar] [CrossRef]
- Turhan, A. Interactive effects of boron stress and mycorrhizal (AMF) treatments on tomato growth, yield, leaf chlorophyll and boron accumulation, and fruit characteristics. Arch. Agron. Soil Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Reid, R. Can we really increase yields by making crop plants tolerant to boron toxicity? Plant Sci. 2010, 178, 9–11. [Google Scholar] [CrossRef]
- Sinha, P.; Chatterjee, C.; Sharma, C.P.; Sinha, P. Changes in physiology and quality of pea by boron stress. Ann. Agric. Res 1999, 20, 304–307. [Google Scholar]
- Gill, M.A.; Kanwal, S.; Aziz, T. Differences in phosphorus-zinc interaction among sunflower (Helianthus annuus L.) brassica (Brassica napus L.) and maize (Zea mays L.). Pakistan J. Agric. Sci. 2004, 41, 29–34. [Google Scholar]
- Metwally, A.M.; Radi, A.A.; El-Shazoly, R.M.; Hamada, A.M. The role of calcium, silicon and salicylic acid treatment in protection of canola plants against boron toxicity stress. J. Plant Res. 2018, 131, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Marcar, N.E.; Guo, J.; Crawford, D.F. Response of Eucalyptus camaldulensis Dehnh., E. globulus Labill. ssp. globulus and E. grandis W. Hill to excess boron and sodium chloride. Plant Soil 1999, 208, 251–257. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.A. Wheat response to interactive effects of boron and salinity. J. Plant Nutr. 2000, 23, 1217–1226. [Google Scholar] [CrossRef]
- Ismail, A.M. Response of maize and sorghum to excess boron and salinity. Biol. Plant. 2003, 47, 313–316. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Nutrient Management: An Introduction to Nutrient Management; Pearson: London, UK, 2013; ISBN 0138618992. [Google Scholar]
- Hu, H.; Brown, P.H. Absorption of boron by plant roots. Plant Soil 1997, 193, 49–58. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Inal, A.; Tarakcioglu, C. Effects of nitrogen forms on growth, nitrate accumulation, membrane permeability, and nitrogen use efficiency of hydroponically grown bunch onion under boron deficiency and toxicity. J. Plant Nutr. 2001, 24, 1521–1534. [Google Scholar] [CrossRef]
- Bogiani, J.C.; Sampaio, T.F.; Abreu-Junior, C.H.; Rosolem, C.A. Boron uptake and translocation in some cotton cultivars. Plant Soil 2014, 375, 241–253. [Google Scholar] [CrossRef]
- Oliveira, K.R.; Souza Junior, J.P.; Bennett, S.J.; Checchio, M.V.; Alves, R.; Felisberto, G.; Prado, R.; Gratão, P.L. Exogenous silicon and salicylic acid applications improve tolerance to boron toxicity in field pea cultivars by intensifying antioxidant defence systems. Ecotoxicol. Environ. Saf. 2020, 201, 110778. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, M.; Xian, Y.; Ferré, T.P.A. Combined effect of sodium chloride and boron in irrigation water on cotton growth. Agron. J. 2017, 109, 1388–1396. [Google Scholar] [CrossRef]
- Shah, A.; Wu, X.; Ullah, A.; Fahad, S.; Muhammad, R.; Yan, L.; Jiang, C. Deficiency and toxicity of boron: Alterations in growth, oxidative damage and uptake by citrange orange plants. Ecotoxicol. Environ. Saf. 2017, 145, 575–582. [Google Scholar] [CrossRef]
- Chatterjee, C.; Sinha, P.; Agarwala, S.C. Boron nutrition of cowpea. Proc. Plant Sci. 1990, 100, 311–318. [Google Scholar] [CrossRef]
- Wang, B.L.; Shi, L.; Li, Y.X.; Zhang, W.H. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta 2010, 231, 1301–1309. [Google Scholar] [CrossRef]
- Nable, R.O. Resistance to boron toxicity amongst several barley and wheat cultivars: A preliminary examination of the resistance mechanism. Plant Soil 1988, 112, 45–52. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M. Melatonin-Induced Salinity Tolerance by Ameliorating Osmotic and Oxidative Stress in the Seedlings of Two Tomato (Solanum lycopersicum L.) Cultivars. J. Plant Growth Regul. 2020, 1–13. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Shahbaz, M.; Ahsan, M.; Nafees, M.; Nadeem, H.; Akram, M.; Maqsood, A.; Ahmar, S.; Kamran, M.; Alamri, S.; et al. Strigolactone (GR24) Induced Salinity Tolerance in Sunflower (Helianthus annuus L.) by Ameliorating Morpho-Physiological and Biochemical Attributes Under In Vitro Conditions. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Ferreyra, R.E.; Aljaro, A.U.; Ruiz, R.S.; Rojas, L.P.; Oster, J.D. Behavior of 42 crop species grown in saline soils with high boron concentrations. Agric. Water Manag. 1997, 34, 111–124. [Google Scholar] [CrossRef]
- Centofanti, T.; Bañuelos, G. Evaluation of the halophyte Salsola soda as an alternative crop for saline soils high in selenium and boron. J. Environ. Manag. 2015, 157, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Shani, U.; Hanks, R.J. Model of Integrated Effects of Boron, Inert Salt, and Water Flow on Crop Yield. Agron. J. 1993, 85, 713–717. [Google Scholar] [CrossRef]
- Esteban, W.; Pacheco, P.; Tapia, L.; Bastías, E. Remediation of salt and boron-affected soil by addition of organic matter: An investigation into improving tomato plant productivity. Idesia (Arica) 2016, 34, 25–32. [Google Scholar] [CrossRef]
- Reid, R. Understanding the boron transport network in plants. Plant Soil 2014, 385, 1–13. [Google Scholar] [CrossRef]
- Gupta, U.C.; Jame, Y.W.; Campbell, C.A.; Leyshon, A.J.; Nicholaichuk, W. Boron toxicity and deficiency: A review. Can. J. Soil Sci. 1985, 65, 381–409. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Shaaban, A. Integrative applications of nitrogen, zinc, and boron to nutrients-deficient soil improves sugar beet productivity and technological sugar contents under semi-arid conditions. J. Plant Nutr. 2020, 43, 1935–1950. [Google Scholar] [CrossRef]
- Arunkumar, B.; Thippeshappa, G.; Anjali, M.; Prashanth, K. Boron: A critical micronutrient for crop growth and productivity. J. Pharmacogn. Phytochem. 2018, 7, 2738–2741. [Google Scholar]
- Grieve, C.M.; Poss, J.A.; Grattan, S.R.; Suarez, D.L.; Smith, T.E. The combined effects of salinity and excess boron on mineral ion relations in broccoli. Sci. Hortic. 2010, 125, 179–187. [Google Scholar] [CrossRef]
- dos Santos, A.R.; de Mattos, W.T.; Almeida, A.A.S.; Monteiro, F.A.; Corrêa, B.D.; Gupta, U.C. Boron nutrition and yield of alfalfa cultivar crioula in relation to boron supply. Sci. Agric. 2004, 61, 496–500. [Google Scholar] [CrossRef]
- Smallwood, C.L.; Lipscomb, J.; Swartout, J.; Teuschler, L. Toxicological Report of Boron and Compounds; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.




| Soil Characteristic | Unit | Soil Series and Regions | |||
|---|---|---|---|---|---|
| Udic Haplustalf | Typic Torrifluvent | Halic Camborthid | Udic Haplustalf | ||
| Texture | Sandy loam | Silty clay loam | Clay loam | Clay loam | |
| Parent material | alluvial | alluvial | loess | alluvial | |
| Sand | % | 86.8 | 46.8 | 62.8 | 60.8 |
| Silt | % | 3.3 | 35.3 | 20.0 | 20.7 |
| Clay | % | 9.9 | 17.9 | 17.2 | 19.2 |
| pH 1:1 a | 7.4 | 7.8 | 7.7 | 7.7 | |
| EC 1:1 a | (dSm−1) | 1.1 | 1.8 | 7.8 | 0.8 |
| SAR | (mmol L−1)1/2 | 0.9 | 5.9 | 40.0 | 1.1 |
| Organic matter | % | 1.4 | 1.5 | 1.5 | 1.5 |
| CaCO3 equal. b | % | 1.5 | 3.5 | 3.5 | 6.0 |
| Total B | µg g−1 | 26.9 | 30.1 | 34.9 | 39.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, M.B.; Malik, Z.; Kamran, M.; Abbasi, G.H.; Majeed, A.; Riaz, M.; Bukhari, M.A.; Mustafa, A.; Ahmar, S.; Mora-Poblete, F.; et al. Assessing Yield Response and Relationship of Soil Boron Fractions with Its Accumulation in Sorghum and Cowpea under Boron Fertilization in Different Soil Series. Sustainability 2021, 13, 4192. https://doi.org/10.3390/su13084192
Javed MB, Malik Z, Kamran M, Abbasi GH, Majeed A, Riaz M, Bukhari MA, Mustafa A, Ahmar S, Mora-Poblete F, et al. Assessing Yield Response and Relationship of Soil Boron Fractions with Its Accumulation in Sorghum and Cowpea under Boron Fertilization in Different Soil Series. Sustainability. 2021; 13(8):4192. https://doi.org/10.3390/su13084192
Chicago/Turabian StyleJaved, Muhammad Babar, Zaffar Malik, Muhammad Kamran, Ghulam Hassan Abbasi, Asma Majeed, Muhammad Riaz, Muhammad Adnan Bukhari, Adnan Mustafa, Sunny Ahmar, Freddy Mora-Poblete, and et al. 2021. "Assessing Yield Response and Relationship of Soil Boron Fractions with Its Accumulation in Sorghum and Cowpea under Boron Fertilization in Different Soil Series" Sustainability 13, no. 8: 4192. https://doi.org/10.3390/su13084192
APA StyleJaved, M. B., Malik, Z., Kamran, M., Abbasi, G. H., Majeed, A., Riaz, M., Bukhari, M. A., Mustafa, A., Ahmar, S., Mora-Poblete, F., Rafay, M., & Bukhari, S. A. H. (2021). Assessing Yield Response and Relationship of Soil Boron Fractions with Its Accumulation in Sorghum and Cowpea under Boron Fertilization in Different Soil Series. Sustainability, 13(8), 4192. https://doi.org/10.3390/su13084192











