Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and Pb Uptake
Abstract
1. Introduction
2. Material and Method
2.1. Pot Experiment
2.2. Experimental Design
2.3. Plants Harvesting
2.4. Chlorophyll Contents and Gas Exchange Parameters
2.5. Estimation of MDA, EL, H2O2 and Antioxidants Enzymes
2.6. Estimation of Pb Contents
2.7. Statistical Analysis
3. Results
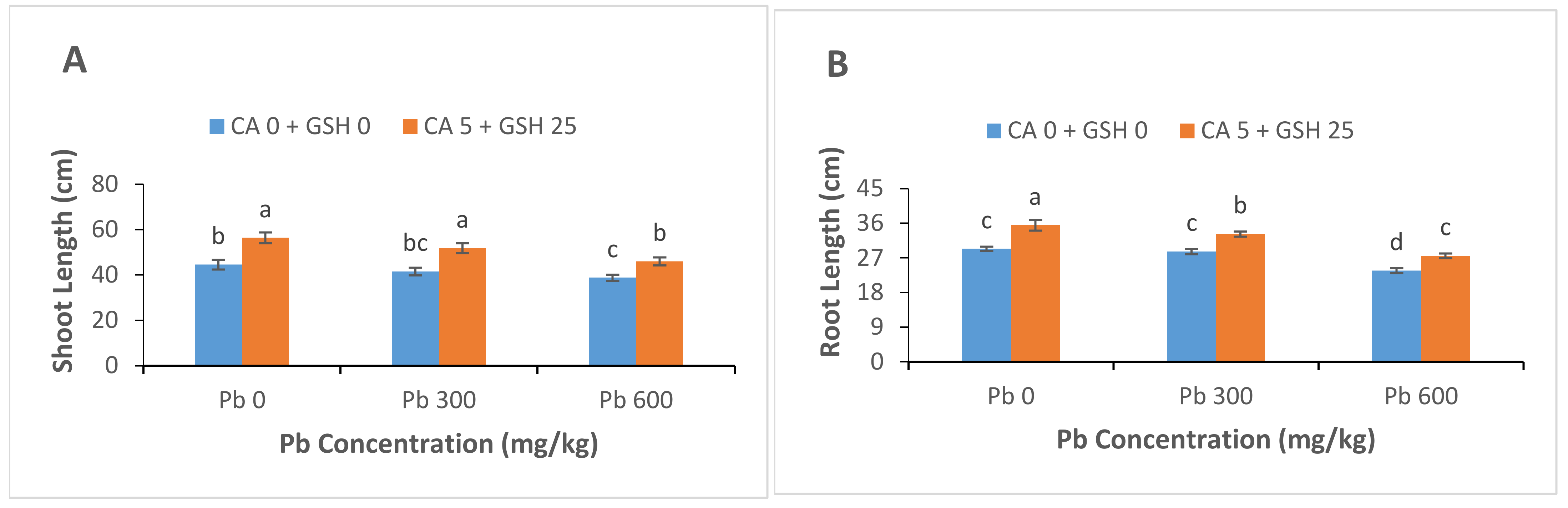
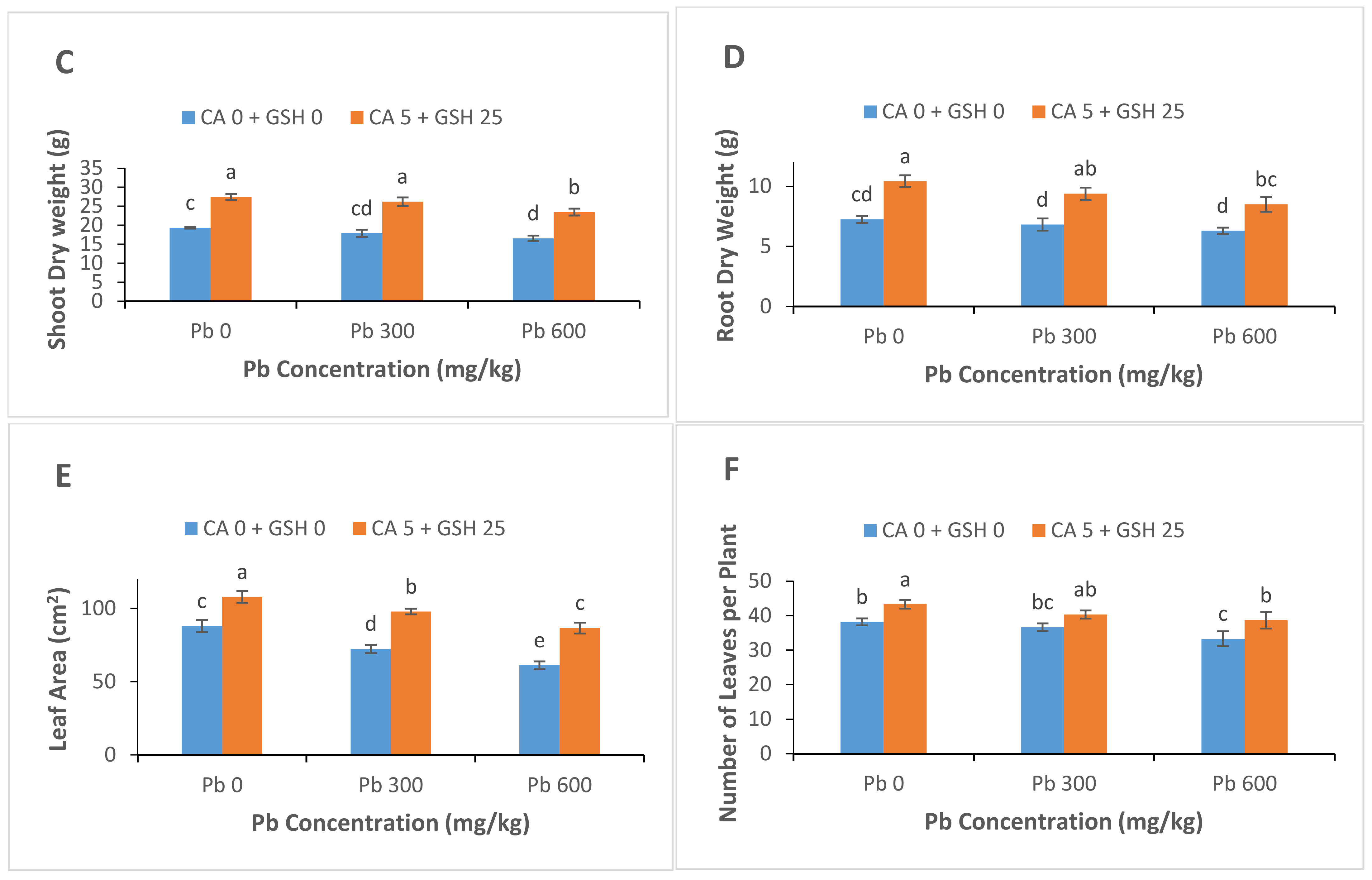
3.1. Plant Growth and Biomass
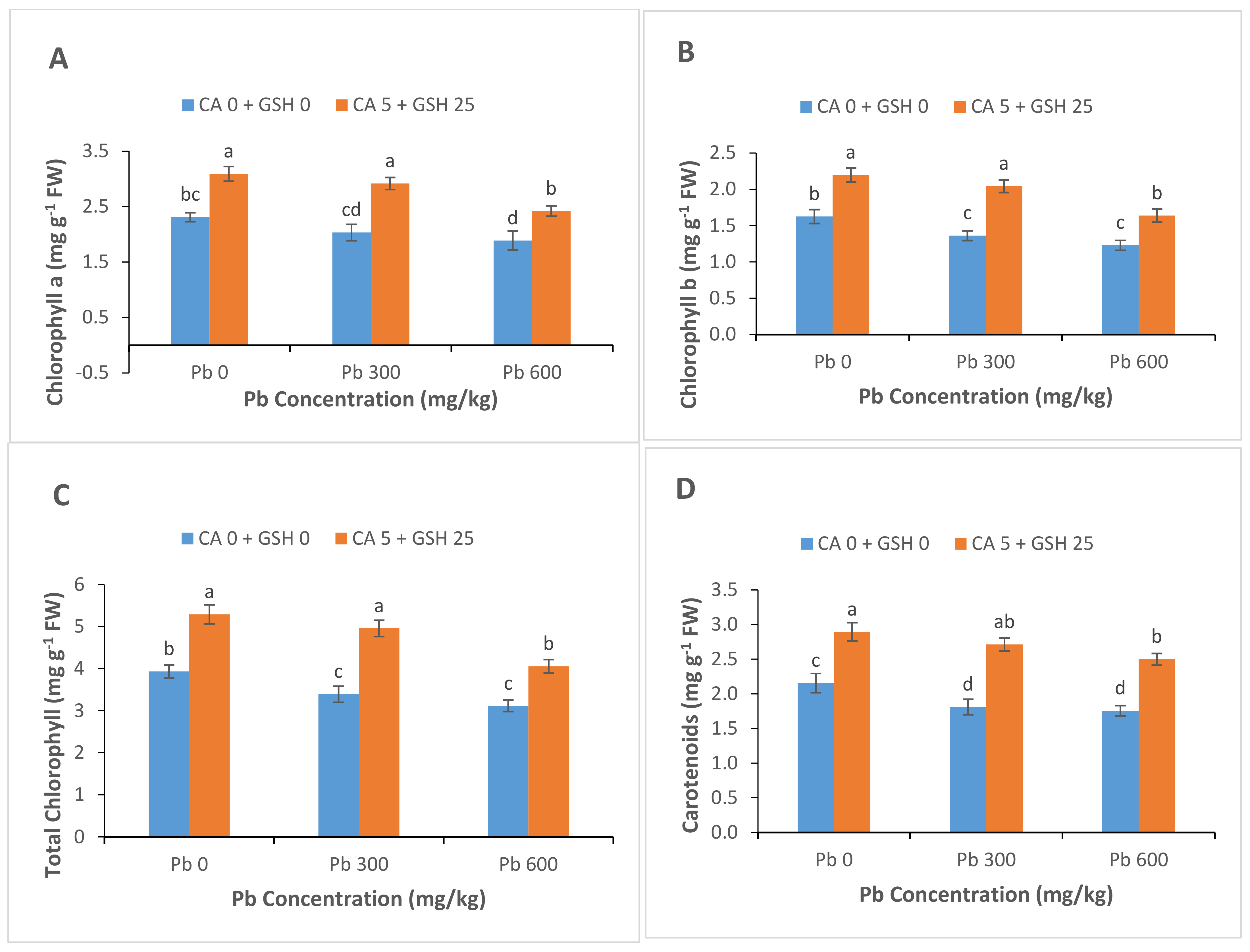
3.2. Light-Harvesting Pigments
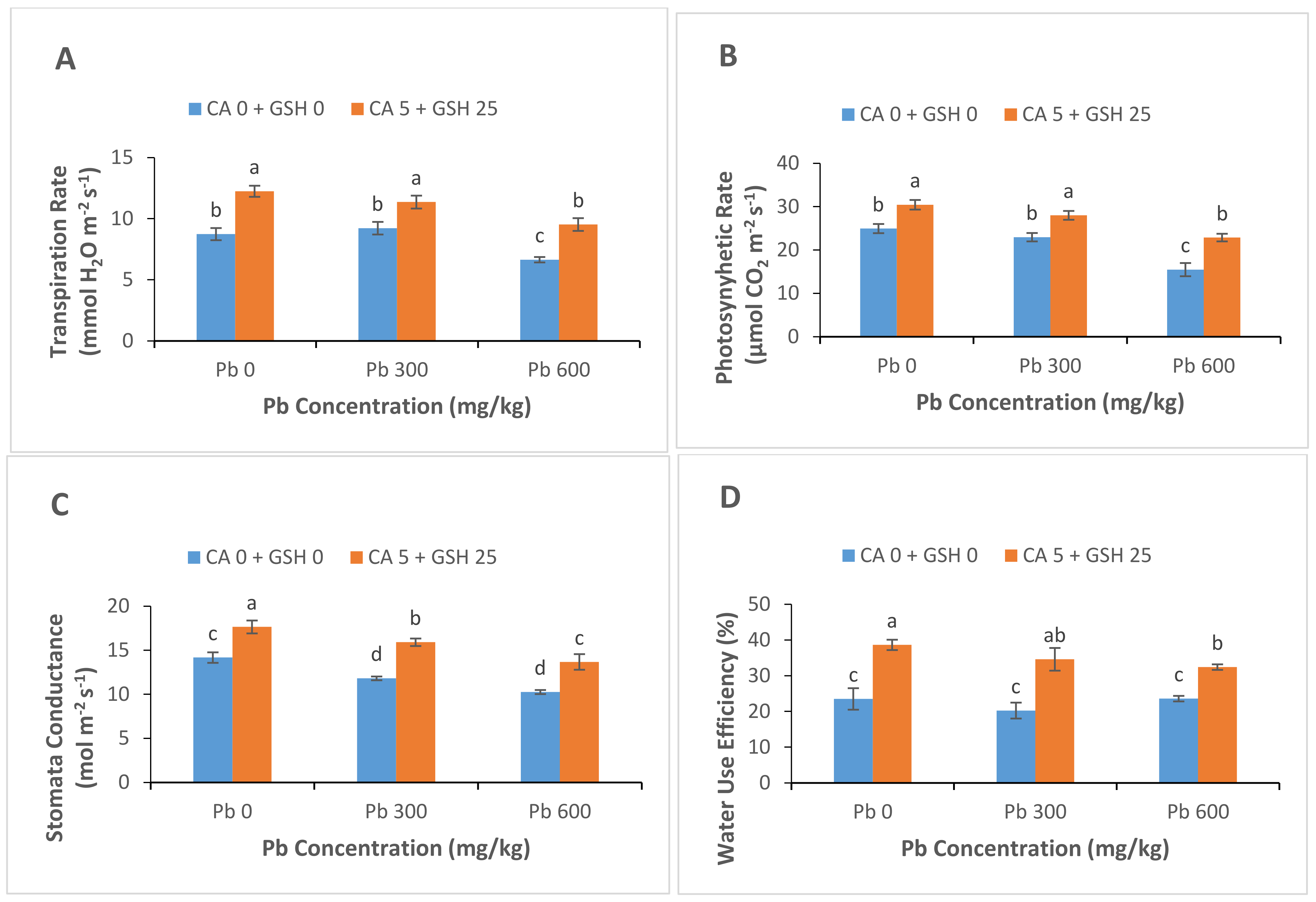
3.3. Gas Exchange Attributes
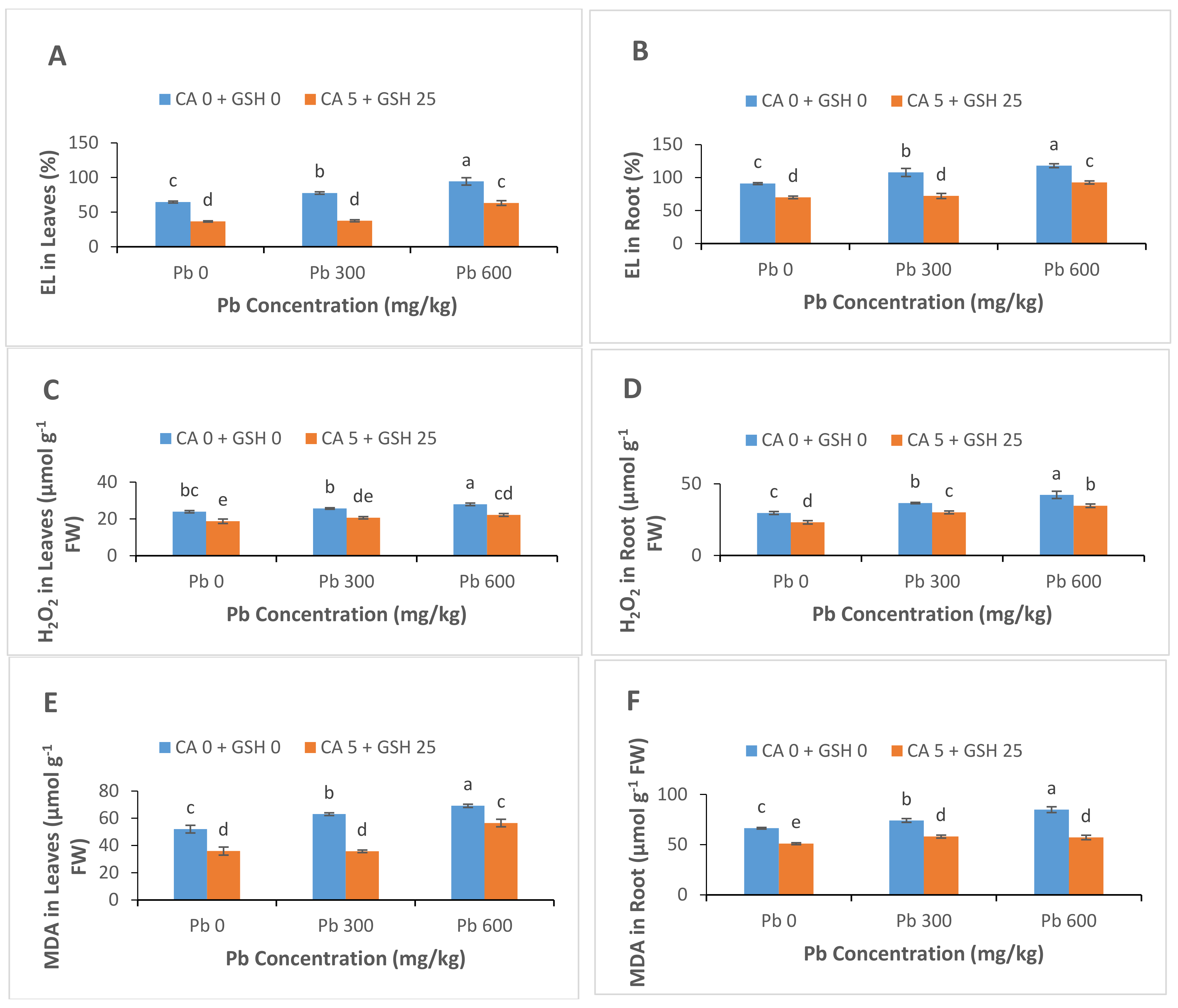
3.4. Electrolyte Leakage, Hydrogen Peroxide and Malondialdehyde Contents
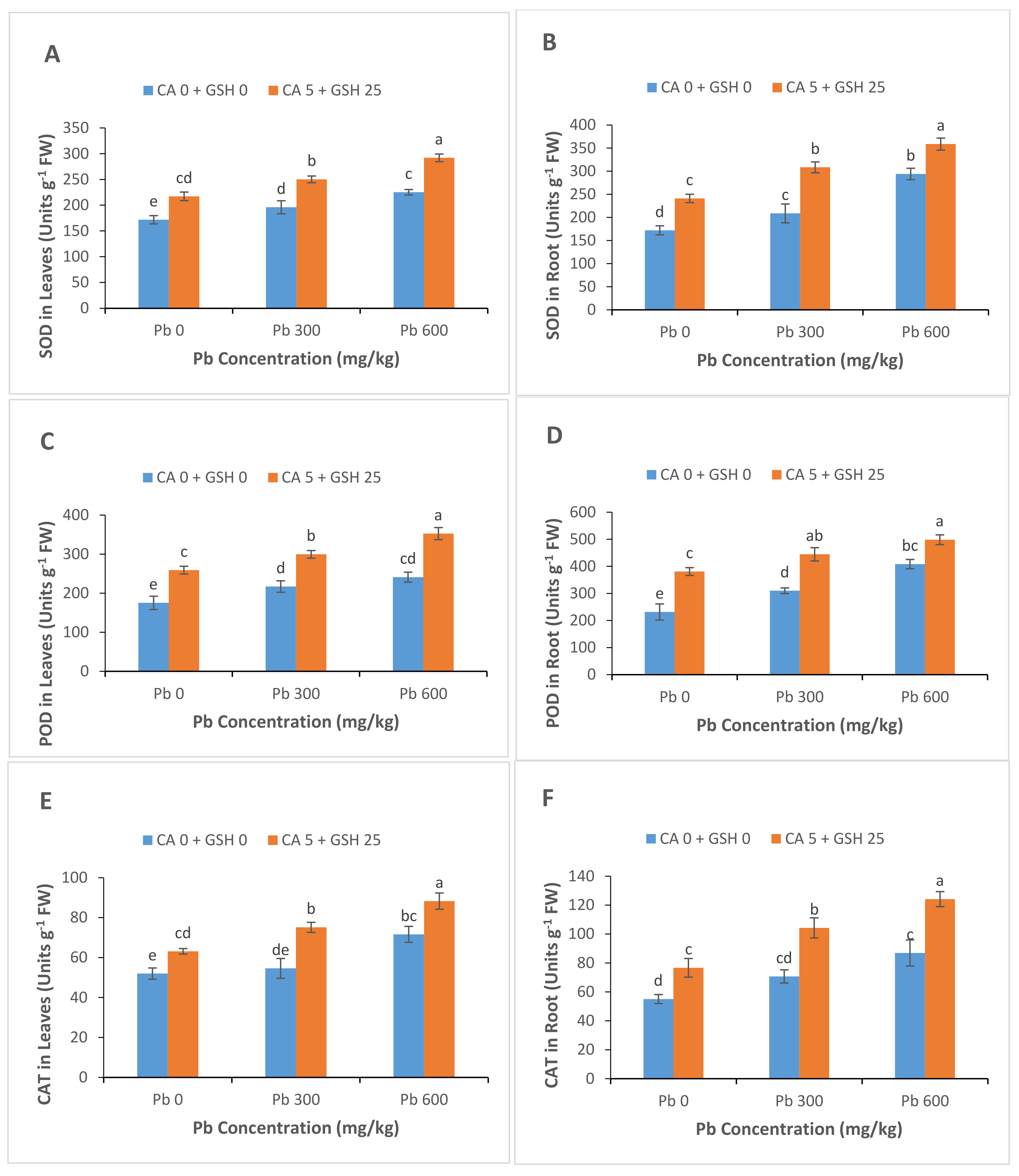
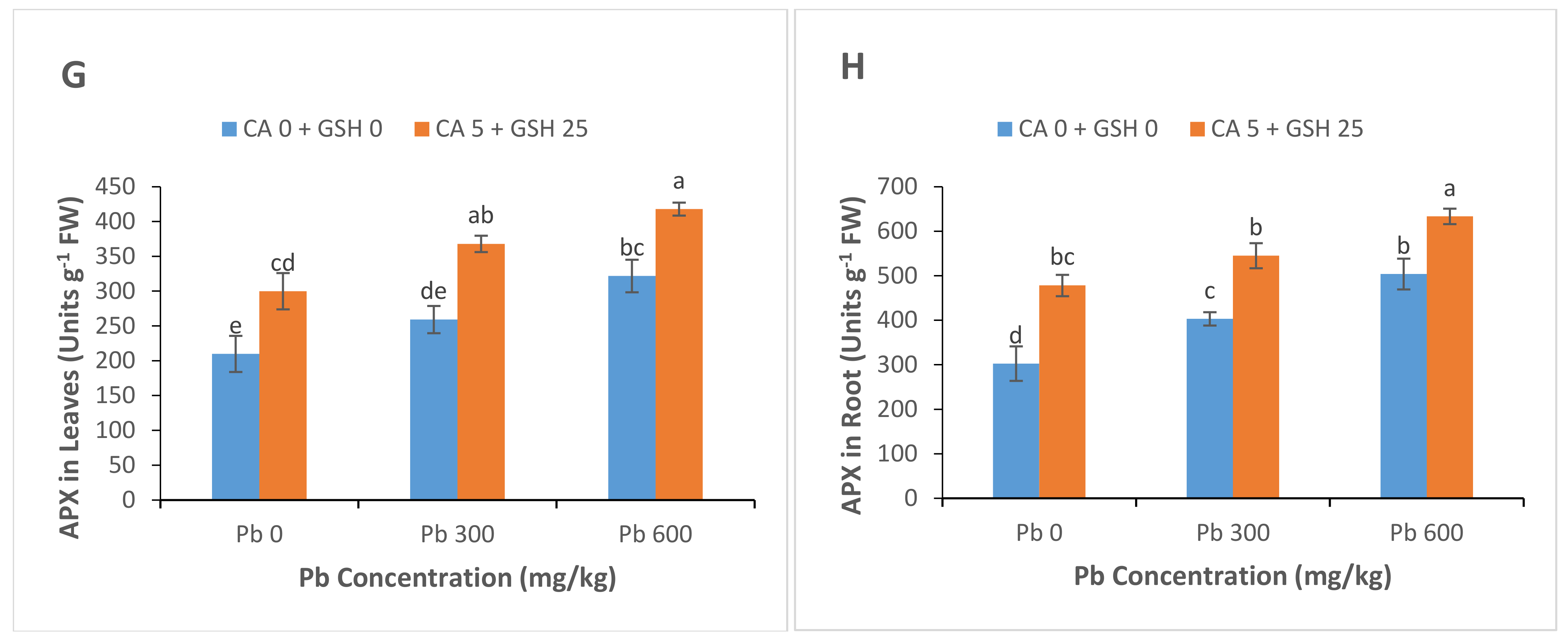
3.5. Antioxidant Enzyme Activities
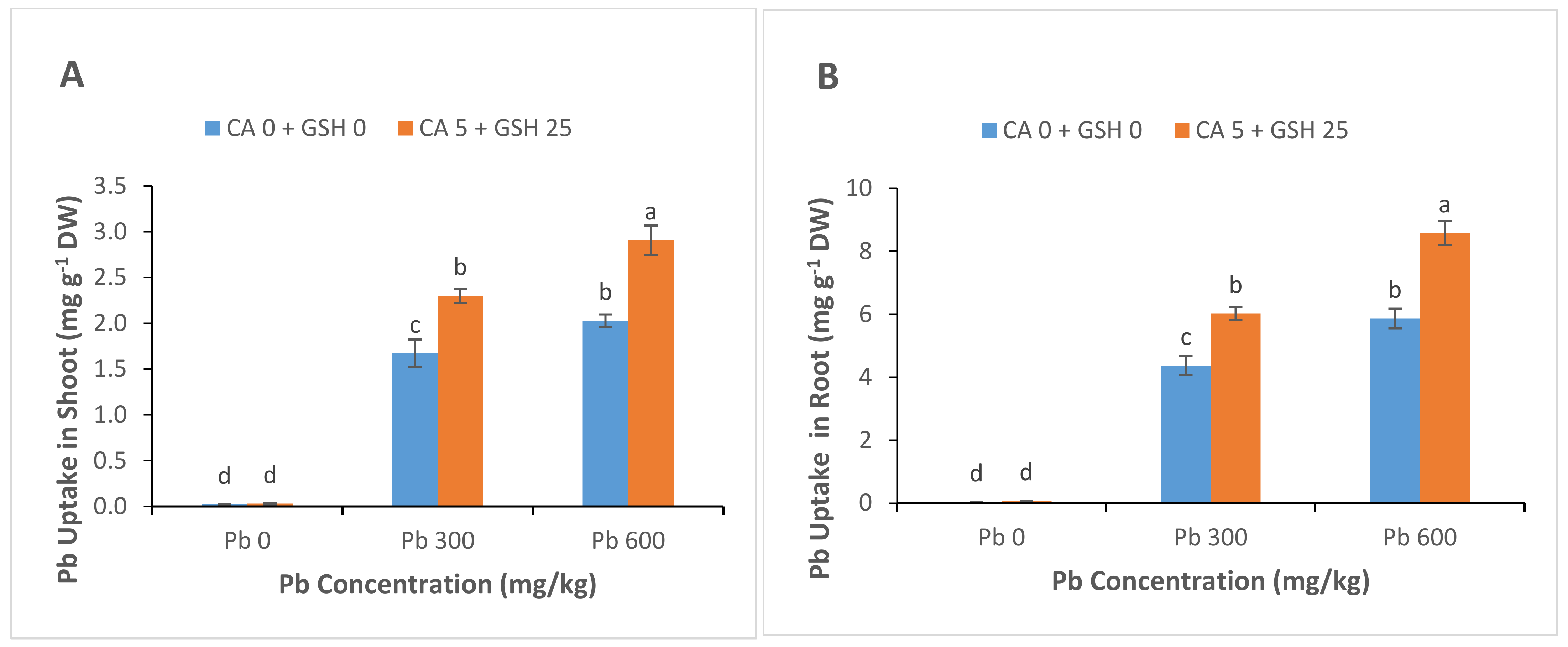
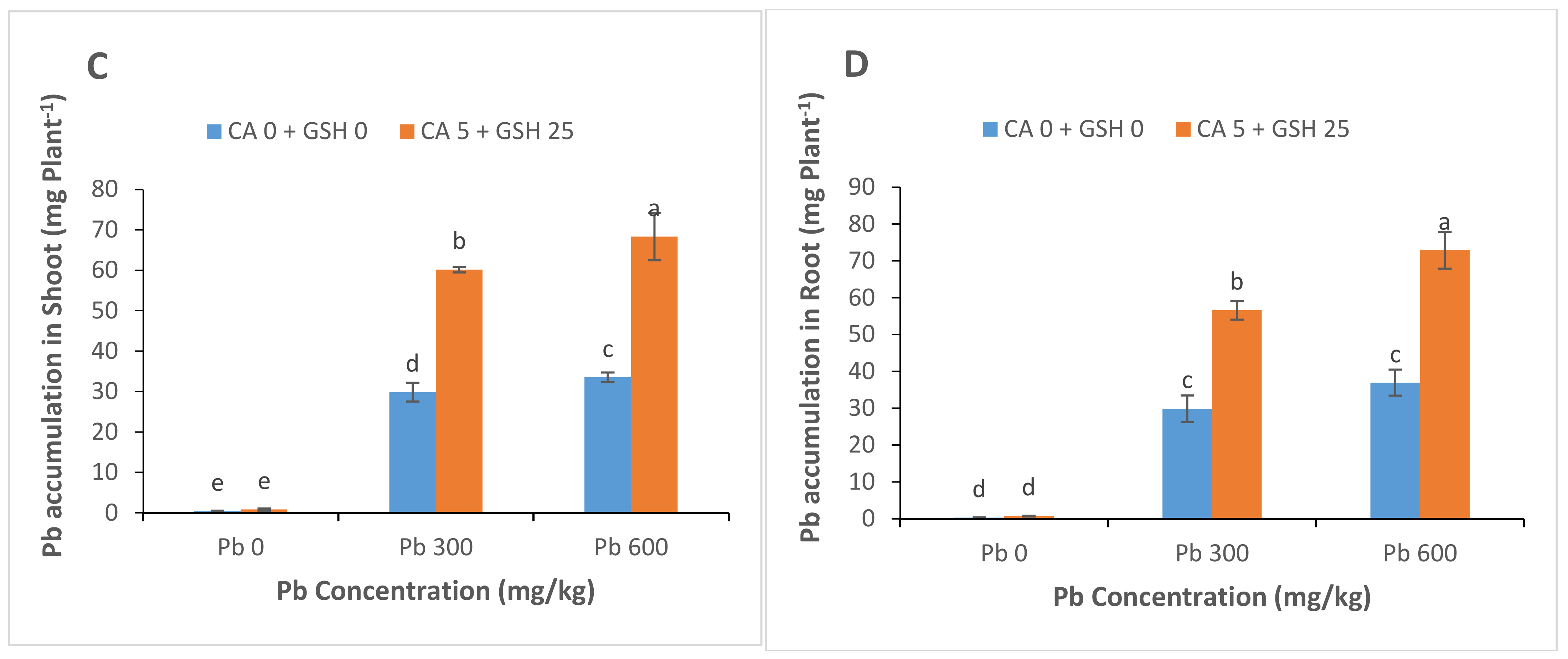
3.6. Lead Uptake and Accumulation
4. Discussion
4.1. H2O2, MDA and Electrolyte Leakage (EL) Contents
4.2. Deposition of Pb in Roots and Leaves
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hengstler, J.G.; Bolm-Audorff, U.; Faldum, A.; Janssen, K.; Reifenrath, M.; Götte, W.; Jung, D.; Mayer-Popken, O.; Fuchs, J.; Gebhard, S.; et al. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 2003, 24, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gottesfeld, P.; Were, F.H.; Adogame, L.; Gharbi, S.; San, D.; Nota, M.M.; Kuepouo, G. Soil contamination from lead battery manufacturing and recycling in seven African countries. Environ. Res. 2018, 161, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Jamwal, V.L.; Kohli, S.K.; Kaur, P.; Tejpal, R.; Bhalla, V.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; et al. Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 2019, 235, 734–748. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef]
- Kumar, A.; Schreiter, I.; Wefer-Roehl, A.; Tsechansky, L.; Schüth, C.; Graber, E. Production and utilization of biochar from organic wastes for pollutant control on contaminated sites. In Environmental Materials and Waste; Elsevier: Amsterdam, The Netherlands, 2016; pp. 91–116. [Google Scholar]
- Peng, W.; Li, X.; Xiao, S.; Fan, W. Review of remediation technologies for sediments contaminated by heavy metals. J. Soils Sediments 2018, 18, 1701–1719. [Google Scholar] [CrossRef]
- Laghlimi, M.; Baghdad, B.; Hadi, H.E.; Bouabdli, A. Phytoremediation mechanisms of heavy metal contaminated soils: A review. Open J. Ecol. 2015, 5, 375. [Google Scholar] [CrossRef]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 2015, 10, e0123328. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Sharma, A.; Thukral, A.K.; Kumar, V.; Kohli, S.K.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Smolinska, B.; Szczodrowska, A. Antioxidative response of Lepidium sativum L. during assisted phytoremediation of Hg contaminated soil. New Biotechnol. 2017, 38, 74–83. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, R.; Handa, N.; Gautam, V.; Kohli, S.K.; Bali, S.; Kaur, P.; Thukral, A.K.; Arora, S.; Ohri, P.; et al. Responses of phytochelatins and metallothioneins in alleviation of heavy metal stress in plants: An overview. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 263–283. [Google Scholar]
- Freitas, E.V.; Nascimento, C.W.; Souza, A.; Silva, F.B. Citric acid-assisted phytoextraction of lead: A field experiment. Chemosphere 2013, 92, 213–217. [Google Scholar] [CrossRef]
- Kolbas, A.; Marchand, L.; Herzig, R.; Nehnevajova, E.; Mench, M. Phenotypic seedling responses of a metal-tolerant mutant line of sunflower growing on a Cu-contaminated soil series: Potential uses for biomonitoring of Cu exposure and phytoremediation. Plant Soil 2014, 376, 377–397. [Google Scholar] [CrossRef]
- Cornu, J.; Bakoto, R.; Bonnard, O.; Bussière, S.; Coriou, C.; Sirguey, C.; Sterckeman, T.; Thunot, S.; Visse, M.; Nguyen, C. Cadmium uptake and partitioning during the vegetative growth of sunflower exposed to low Cd 2+ concentrations in hydroponics. Plant Soil 2016, 404, 263–275. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Saeed, R.; Tauqeer, H.M.; Sallah-Ud-Din, R.; Azam, A.; Raza, N. Microwave irradiation and citric acid assisted seed germination and phytoextraction of nickel (Ni) by Brassica napus L.: Morpho-physiological and biochemical alterations under Ni stress. Environ. Sci. Pollut. Res. 2017, 24, 21050–21064. [Google Scholar] [CrossRef]
- Ou, X.; Zhang, Y.; Xu, C.; Lin, X.; Zang, Q.; Zhuang, T.; Jiang, L.; von Wettstein, D.; Liu, B. Transgenerational inheritance of modified DNA methylation patterns and enhanced tolerance induced by heavy metal stress in rice (Oryza sativa L.). PLoS ONE 2012, 7, e41143. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytoremediation of lead by a wild, non-edible Pb accumulator Coronopus didymus (L.) Brassicaceae. Int. J. Phytorem. 2018, 20, 483–489. [Google Scholar] [CrossRef]
- Gul, I.; Manzoor, M.; Silvestre, J.; Rizwan, M.; Hina, K.; Kallerhoff, J.; Arshad, M. EDTA-assisted phytoextraction of lead and cadmium by Pelargonium cultivars grown on spiked soil. Int. J. Phytorem. 2019, 21, 101–110. [Google Scholar] [CrossRef]
- Qin, F.; Liu, G.; Huang, G.; Dong, T.; Liao, Y.; Xu, X. Zinc application alleviates the adverse effects of lead stress more in female Morus alba than in males. Environ. Exp. Bot. 2018, 146, 68–76. [Google Scholar] [CrossRef]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Gu, J.; Zhao, J.; Fu, J. Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int. Biodeterior. Biodegrad. 2018, 128, 15–21. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ishaque, W.; Khan, M.; Ashraf, U.; Riaz, M.A.; Ghulam, S.; Ahmad, A.; Rizwan, M.; Ali, S.; Alkahtani, S.; et al. Relief role of lysine chelated zinc (Zn) on 6-week-old maize plants under tannery wastewater irrigation stress. Int. J. Environ. Res. Public Health 2020, 17, 5161. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Anjum, S.; Khan, M.I.R.; Masood, A.; Verma, S.; Khan, N.A. Contribution of glutathione in heavy metal stress tolerance in plants. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2017; pp. 297–313. [Google Scholar]
- Zeng, F.; Zahoor, M.; Waseem, M.; Anayat, A.; Rizwan, M.; Ahmad, A.; Yasmeen, T.; Ali, S.; El-Sheikh, M.A.; Alyemeni, M.N.; et al. Influence of metal-resistant staphylococcus aureus strain K1 on the alleviation of chromium stress in wheat. Agronomy 2020, 10, 1354. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Rai, R.; Agrawal, M.; Agrawal, S. Impact of heavy metals on physiological processes of plants: With special reference to photosynthetic system. In Plant Responses to Xenobiotics; Springer: Berlin/Heidelberg, Germany, 2016; pp. 127–140. [Google Scholar]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef]
- Bai, X.; Dong, Y.; Wang, Q.; Xu, L.; Kong, J.; Liu, S. Effects of lead and nitric oxide on photosynthesis, antioxidative ability, and mineral element content of perennial ryegrass. Biol. Plant. 2015, 59, 163–170. [Google Scholar] [CrossRef]
- Dao, L.H.; Beardall, J. Effects of lead on growth, photosynthetic characteristics and production of reactive oxygen species of two freshwater green algae. Chemosphere 2016, 147, 420–429. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Talarek, M.; Bralska, M.; Zambrzycka, E. The effect of lead on the growth, content of primary metabolites, and antioxidant response of green alga Acutodesmus obliquus (Chlorophyceae). Environ. Sci. Pollut. Res. 2015, 22, 19112–19123. [Google Scholar] [CrossRef]
- Daud, M.; Mei, L.; Azizullah, A.; Dawood, M.; Ali, I.; Mahmood, Q.; Ullah, W.; Jamil, M.; Zhu, S. Leaf-based physiological, metabolic, and ultrastructural changes in cultivated cotton cultivars under cadmium stress mediated by glutathione. Environ. Sci. Pollut. Res. 2016, 23, 15551–15564. [Google Scholar] [CrossRef]
- Andresen, E.; Kappel, S.; Stärk, H.J.; Riegger, U.; Borovec, J.; Mattusch, J.; Heinz, A.; Schmelzer, C.E.; Matoušková, Š.; Dickinson, B.; et al. Cadmium toxicity investigated at the physiological and biophysical levels under environmentally relevant conditions using the aquatic model plant Ceratophyllum demersum. New Phytol. 2016, 210, 1244–1258. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytorem. 2019, 21, 760–767. [Google Scholar] [CrossRef]
- Arsenov, D.; Zupunski, M.; Borisev, M.; Nikolic, N.; Orlovic, S.; Pilipovic, A.; Pajevic, S. Exogenously applied citric acid enhances antioxidant defense and phytoextraction of cadmium by willows (Salix spp.). Water Air Soil Pollut. 2017, 228, 221. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, H.; Wang, X.; Guo, Y.; Yuan, X.; Yang, L.; Liang, D. Effects of exogenous melatonin on antioxidant properties of kiwifruit seedling leaves under copper stress. In 2017 6th International Conference on Energy, Environment and Sustainable Development (ICEESD 2017); Atlantis Press: Zhuhai, China, 2017. [Google Scholar]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Marques, D.N.; Carvalho, M.E.A.; Piotto, F.A.; Batagin-Piotto, K.D.; Nogueira, M.L.; Gaziola, S.A.; Azevedo, R.A. Antioxidant Defense Response in Plants to Cadmium Stress. In Cadmium Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 423–461. [Google Scholar]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. Res. 2014, 21, 9899–9910. [Google Scholar] [CrossRef]
- Sallah-Ud-Din, R.; Farid, M.; Saeed, R.; Ali, S.; Rizwan, M.; Tauqeer, H.M.; Bukhari, S.A.H. Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. Environ. Sci. Pollut. Res. 2017, 24, 17669–17678. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J. Plant Growth Regul. 2019, 39, 641–655. [Google Scholar] [CrossRef]
- Kumar, A.; Pal, L.; Agrawal, V. Glutathione and citric acid modulates lead-and arsenic-induced phytotoxicity and genotoxicity responses in two cultivars of Solanum lycopersicum L. Acta Physiol. Plant. 2017, 39, 151. [Google Scholar] [CrossRef]
- Iqbal, A.; Iqbal, K.; Xu, L.; Li, B.; Gong, D.; Liu, X.; Guo, Y.; Liu, W.; Qin, W.; Guo, H. Heterogeneous synthesis of nitrogen-doped carbon dots prepared via anhydrous citric acid and melamine for selective and sensitive turn on-off-on detection of Hg (II), glutathione and its cellular imaging. Sens. Actuators B Chem. 2018, 255, 1130–1138. [Google Scholar] [CrossRef]
- Gupta, D.; Pena, L.B.; Romero-Puertas, M.; Hernández, A.; Inouhe, M.; Sandalio, L. NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity. Plant Cell Environ. 2017, 40, 509–526. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Zularisam, A.; Sakinah, M.; Din, M. Lead induced oxidative stress and alteration in the activities of antioxidative enzymes in rice shoots. Biol. Plant. 2017, 61, 595–598. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, S.; Noman, A.; Ali, Q.; Rizwan, M.; Farid, M.; Irshad, M.K. Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron. Soil Sci. 2016, 62, 533–546. [Google Scholar] [CrossRef]
- Khaliq, A.; Ali, S.; Hameed, A.; Farooq, M.A.; Farid, M.; Shakoor, M.B.; Mahmood, K.; Ishaque, W.; Rizwan, M. Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis, and suppressing Ni uptake and oxidative stress. Arch. Agron. Soil Sci. 2016, 62, 633–647. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Ullah, N.; Bharwana, S.A.; Waseem, M.; Farooq, M.A.; Abbasi, G.H.; Farid, M. Physiological and biochemical mechanisms of silicon-induced copper stress tolerance in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38, 262. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Prasad, M.N.V. Responses of Ricinus communis L.(castor bean, phytoremediation crop) seedlings to lead (Pb) toxicity in hydroponics. Selcuk J. Agric. Food Sci. 2017, 31, 73–80. [Google Scholar]
- Kumar, B.; Smita, K.; Flores, L.C. Plant mediated detoxification of mercury and lead. Arab. J. Chem. 2017, 10, S2335–S2342. [Google Scholar] [CrossRef]
- Puga, A.; Abreu, C.; Melo, L.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Effect of lead on oxidative status, antioxidative response and metal accumulation in Coronopus didymus. Plant Physiol. Biochem. 2016, 105, 290–296. [Google Scholar] [CrossRef]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—a mini review. Environ. Sci. Pollut. Res. 2015, 22, 18318–18332. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.; Liu, X.; Hashmi, M.Z.; Xu, J.; Brookes, P.C. Effects of inorganic and organic amendments on the uptake of lead and trace elements by Brassica chinensis grown in an acidic red soil. Chemosphere 2015, 119, 177–183. [Google Scholar] [CrossRef]
| Texture | Sandy Loam |
|---|---|
| Silt | 15.0% |
| Sand | 67.9% |
| Clay | 17.1% |
| EC | 1.96 dS m−1 |
| pH | 7.61 |
| Sodium adsorption ratio (SAR) | 1.89 (mmol L−1)1/2 |
| Available P | 2.11 mg kg−1 |
| Organic matter | 0.59% |
| HCO3 | 2.51 mmol L−1 |
| SO4−2 | 11.44 mmol L−1 |
| Cl− | 5.45 mmol L−1 |
| Ca2+ + Mg2+ | 13.98 mmol L−1 |
| K+ | 0.04 mmol L−1 |
| Na2 | 5.23 mmol L−1 |
| Available Zn2 | 0.77 mg kg−1 |
| Available Cu2+ | 0.31 mg kg−1 |
| Available Cr +6 | 0.16 mg kg−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Mallhi, Z.I.; Khan, N.; Rizwan, M.; Ali, S.; Ahmad, A.; Hussain, A.; Alsahli, A.A.; Alyemeni, M.N. Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and Pb Uptake. Sustainability 2021, 13, 4073. https://doi.org/10.3390/su13074073
Zeng F, Mallhi ZI, Khan N, Rizwan M, Ali S, Ahmad A, Hussain A, Alsahli AA, Alyemeni MN. Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and Pb Uptake. Sustainability. 2021; 13(7):4073. https://doi.org/10.3390/su13074073
Chicago/Turabian StyleZeng, Fanrong, Zahid Imran Mallhi, Naeem Khan, Muhammad Rizwan, Shafaqat Ali, Awais Ahmad, Afzal Hussain, Abdulaziz Abdullah Alsahli, and Mohammed Nasser Alyemeni. 2021. "Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and Pb Uptake" Sustainability 13, no. 7: 4073. https://doi.org/10.3390/su13074073
APA StyleZeng, F., Mallhi, Z. I., Khan, N., Rizwan, M., Ali, S., Ahmad, A., Hussain, A., Alsahli, A. A., & Alyemeni, M. N. (2021). Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and Pb Uptake. Sustainability, 13(7), 4073. https://doi.org/10.3390/su13074073










