Evaluation of Medicine Reverse Logistics Practices in Hospitals
Abstract
1. Introduction
2. Literature Review
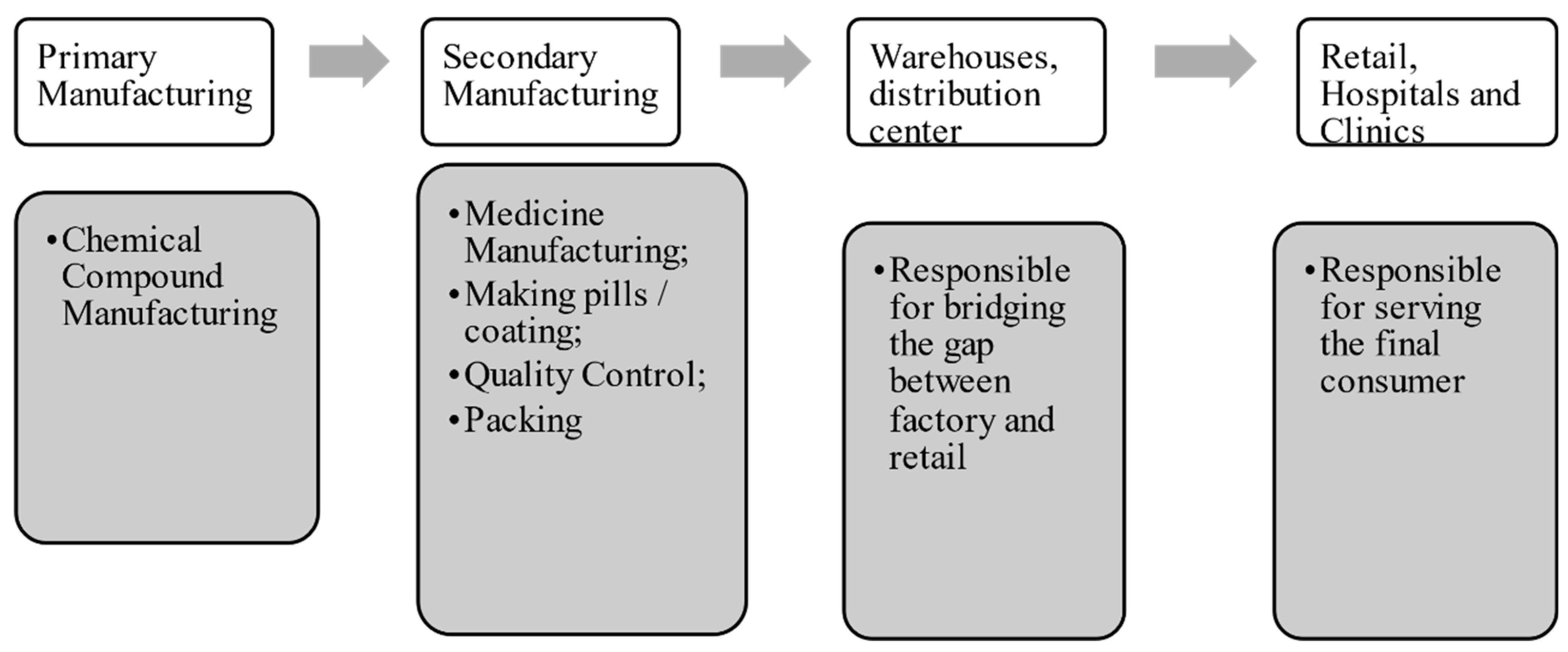
2.1. The Pharmaceutical Supply Chain
2.2. Medicine Reverse Logistics
2.3. Reverse Logistics Practices for the Pharmaceutical Supply Chain
3. Materials and Methods
3.1. Theoretical Research
3.2. Empirical Research
4. Results
4.1. MRL Practices Used
- Most respondents reported that MRL practices for EoL and EoU medicines are inexistent on the part of suppliers, with no cooperation between them and hospital pharmacies. In the interview, only pharmacies alleged being responsible for the waste. In the interviewees’ perception, this fact is due to the lack of specific legislation that makes manufacturers and suppliers responsible for waste;
- All respondents highlighted the lack of communication on the part of hospital professionals to patients regarding the importance of the correct disposal of medication. That is, no patient receives guidance on what to do with the medication if it is not used or if it perishes;
- The interviewees also frequently mentioned the inexistence of government incentives and policies that help the disposal or distribution of medicines in EoU. Resende was the only municipality in which there was help from the municipal government, which will be further discussed in the results.
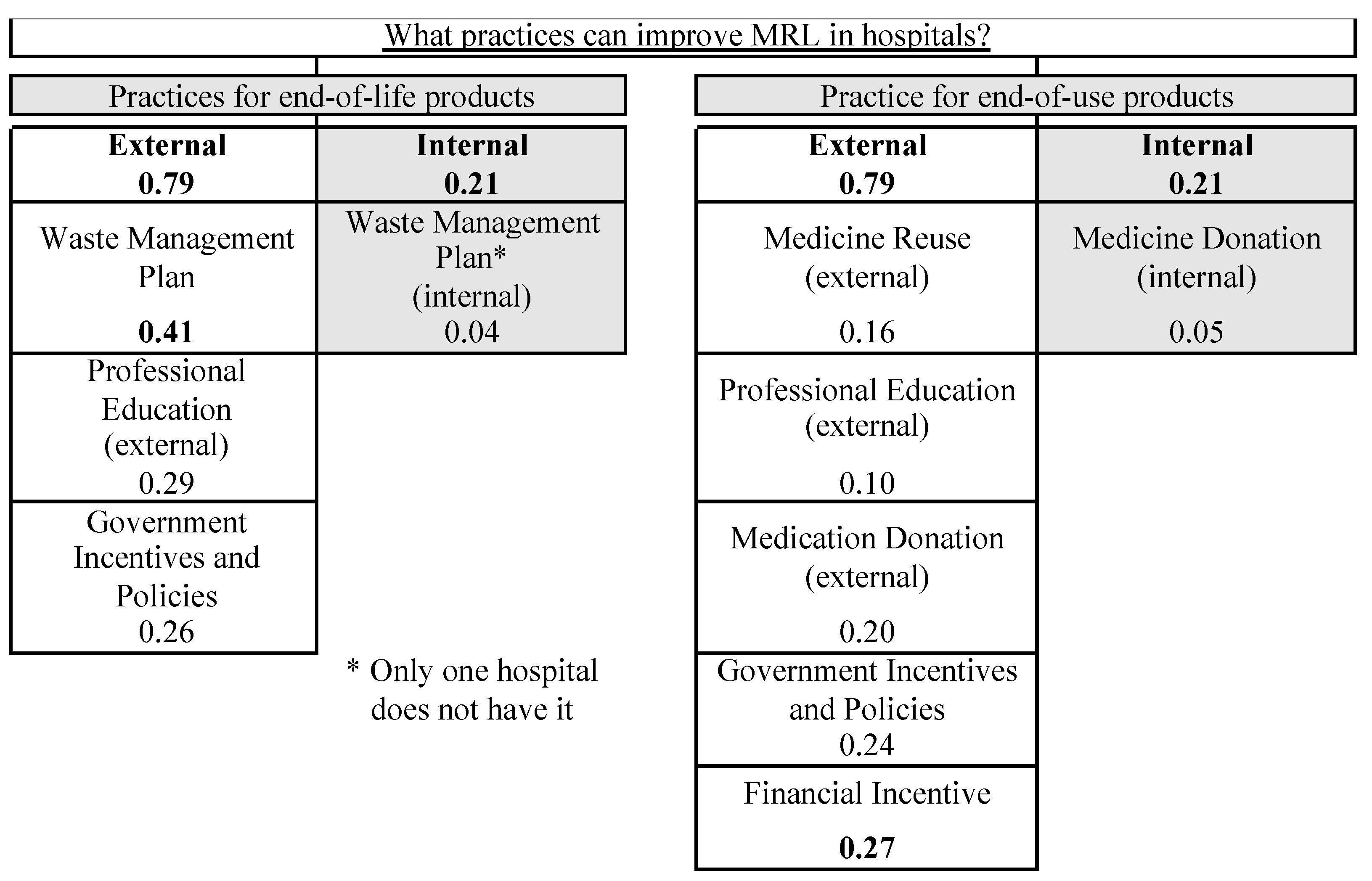
4.2. Practices with the Potential to Improve MRL
5. Discussion
- Separation of tablets from packaging (one report);
- Difficulty with suppliers (three reports);
- Implementation of a standard process for disposal (three reports);
- Medications held by nurses or in satellite pharmacies that are beyond the pharmacist’s control (three reports);
- Free sample medicines that cannot be used (one report).
5.1. Internal Practices
5.2. External Practices
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Susarla, N.; Karimi, I.A. Integrated campaign planning and resource allocation in batch plants. Comput. Chem. Eng. 2012, 35, 2990–3001. [Google Scholar] [CrossRef]
- De Campos, E.A.R.; De Paula, I.C.; Pagani, R.N.; Guarnieri, P. Reverse logistics for the end-of-life and end-of-use products in the pharmaceutical industry: A systematic literature review. Supply Chain Manag. 2017, 22, 375–392. [Google Scholar] [CrossRef]
- Yousefin, N.; Alibabae, A. Information Flow in the Pharmaceutical Supply Chain. Iran. J. Pharm. Res. 2015, 14, 1299–1303. [Google Scholar]
- Parmata, U.M.D.; Rao, S.B.; Rajashekhar, B. Measuring service quality in pharmaceutical supply chain—Distributor’sperspective. Int. J. Pharm. Healthc. Mark. 2016, 10, 258–284. [Google Scholar] [CrossRef]
- Stumpf, M.; Ternes, T.A.; Wilken, R.; Rodrigues, S.V.; Baumann, W. Polar drug residues in sewage and natural waters in the state of Rio de Janeiro, Brazil. Sci. Total Environ. 1999, 225, 135–141. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.T.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and Endocrine Disrupting Compounds in US Drinking Water. Environ. Sci. Technol. 2009, 43, 593–603. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Brasil, 2010. Law No.12.305, of 2 August 2010. Institutes the National Policy on Solid Waste Amends Law No. 9605 of 12 February 1998; and Makes Other Arrangements. 2010. Available online: http://www.planalto.gov.br/ccivil_03/_ato2007-2010/2010/lei/l12305.htm (accessed on 2 May 2019). (In Portuguese)
- Pereira, A.L.; De Vasconcelos, B.R.T.; Pereira, S.R. Pharmacopollution and Household Waste Medicine HWM: How reverse logistics is environmentally important to Brazil. Environ. Sci. Pollut. Res. 2017, 24, 24061–24075. [Google Scholar] [CrossRef]
- Brasil, 2020. Decree No.10.388, of 5 June 2020. Institutes the Reverse Logistics System for Expired or Out-of-Use Medicines; and Makes Other Arrangements. 2020. Available online: https://www.in.gov.br/en/web/dou/-/decreto-n-10.388-de-5-de-junho-de-2020-260391756 (accessed on 17 November 2020). (In Portuguese)
- Schroder, H.F.; Tambosi, J.L.; Sena, R.F.; Moreira, R.F.P.M.; Jose, H.J.; Pinnekamp, J. The removal and degradation of pharmaceutical compounds during membrane bioreactor treatment. Water Sci. Technol. 2012, 65, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhao, H. Mitigating the US Drug Shortages through Pareto-Improving Contracts. Prod. Oper. Manag. 2017, 26, 1463–1480. [Google Scholar] [CrossRef]
- Kumar, S.; Dieveney, E.; Dieveney, A. Reverse logistic process control measures for the pharmaceutical industry supply chain. Int. J. Prod. Perform. Manag. 2009, 58, 188–204. [Google Scholar] [CrossRef]
- Xie, Y.; Breen, L. Greening community pharmaceutical supply chain in UK: A crossboundary approach. Supply Chain Manag. Int. J. 2012, 17, 40–53. [Google Scholar] [CrossRef]
- Xie, Y.; Breen, L. Waste not, want not. What are the drivers of sustainable medicines recycling in National Health Service hospital pharmacies UK? Int. J. Procure Manag. 2015, 8, 82–103. [Google Scholar] [CrossRef]
- Narayana, S.A.; Pati, R.K.; Vrat, P. Managerial research on the pharmaceutical supply chain—A critical review and some insights for future directions. J. Purch. Supply Manag. 2014, 20, 18–40. [Google Scholar] [CrossRef]
- Kwon, I.; Hong, S.-J. Health Care Supply Chain Management in the United States: New Paradigm for Roles of Distributors. Int. J. Health Manag. Inf. 2011, 2, 73–82. [Google Scholar]
- Centobelli, P.; Cerchione, R.; Esposito, E. Pursuing supply chain sustainable development goals through the adoption of green practices and enabling technologies: A cross-country analysis of LSPs. Technol. Forecast. Soc. Chang. 2020, 153. [Google Scholar] [CrossRef]
- Centobelli, P.; Cerchione, R.; Esposito, E.; Shashi. Evaluating environmental sustainability strategies in freight transport and logistics industry. Bus. Strategy Environ. 2020, 29, 1563–1574. [Google Scholar] [CrossRef]
- Drobyazko, S.; Okulich-Kazarin, V.; Rogovyi, A.; Goltvenko, O.; Marova, S. Factors of influence on the sustainable devel-opment in the strategy management of corporations. Acad. Strateg. Manag. J. 2019, 18, 1–5. [Google Scholar]
- Chowdhury, B.; Chowdhury, M.U.; D’souza, C. Challenges relating to RFID implementation within the electronic supply chain management—A practical approach. Softw. Eng. Artif. Intell. Netw. Parallel/Distrib. Comput. 2008, 149, 49–59. [Google Scholar] [CrossRef]
- National Academy of Engineering (US); Institute of Medicine (US); Committee on Engineering and the Health Care System. Building a Better Delivery System: A New Engineering/Health Care Partnership; Reid, P.P., Compton, W.D., Gross-man, J.H., Fanjiang, G., Eds.; National Academies Press (US): Washington, DC, USA, 2005. [Google Scholar] [PubMed]
- Mousazadeh, M.; Torabi, S.A.; Zahiri, B. A robust possibilistic programming approach for pharmaceutical supply chain network design. Comput. Chem. Eng. 2015, 82, 115–128. [Google Scholar] [CrossRef]
- Lambert, D.M.; Enz, M.G. Issues in Supply Chain Management: Progress and potential. Ind. Mark. Manag. 2017, 62, 1–16. [Google Scholar] [CrossRef]
- Kokilam, M.B.; Joshi, H.G.; Kamath, V.G. Strengthening the Pharmaceutical Supply Chain Management with Information Communication Technology Intervention: A Windfall to the Indian Rural Public Healthcare System. J. Health Manag. 2016, 18, 205–217. [Google Scholar] [CrossRef]
- ABDI. Reverse Logistics for the Medicines Sector. 2013. Available online: https://sinir.gov.br/images/sinir/LOGISTICA_REVERSA/EVTE-MEDICAMENTOS (accessed on 2 May 2020). (In Portuguese)
- Zahiri, B.; Zhuang, J.; Mohammadi, M. Toward an integrated sustainable-resilient supply chain: A pharmaceutical case study. Transp. Res. Part E: Logist. Transp. Rev. 2017, 103, 109–142. [Google Scholar] [CrossRef]
- Shah, N. Pharmaceutical supply chains: Key issues and strategies for optimisation. Comput. Chem. Eng. 2004, 28, 929–941. [Google Scholar] [CrossRef]
- Moniveena, M.G.; Kumar, T.M.P.; Venkatesh, M.P. Regulation of Reverse Logistics of Pharmaceutical Products in United States: A Review. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 315–320. [Google Scholar]
- Xie, Y.; Breen, L. Who cares wins? A comparative analysis of household waste medicines and batteries the case of the NHS UK. Supply Chain Manag. 2014, 19, 455–474. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Sarkis, J. An inter-sectoral comparison of green supply chain management in China: Drivers and practices. J. Clean. Prod. 2006, 14, 472–486. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Sarkis, J. Relationships between operational practices and performance among early adopters of green supply chain management practices in Chinese manufacturing enterprises. J. Oper. Manag. 2004, 22, 265–289. [Google Scholar] [CrossRef]
- Hua, M.N.; Tang, H.J.; Wu, Z.L. Analysis of a pharmaceutical reverse supply chain based on unwanted medications categories in household. In Proceedings of the IEEE International Conference on Industrial Engineering and Engineering Management (IEEM), Bali, Indonesia, 4–7 December 2016; pp. 1493–1497. [Google Scholar] [CrossRef]
- Weraikat, D.; Zanjani, M.K.; Lehoux, N. Improving sustainability in a two-level pharmaceutical supply chain through Vendor-Managed Inventory system. Oper. Res. Health Care 2019, 21, 44–55. [Google Scholar] [CrossRef]
- Hua, M.; Tang, H.; Lai, I.K.W. Game theoretic analysis of pricing and cooperative advertising in a reverse supply chain for unwanted medications in households. Sustain. Switz. 2017, 9, 1902. [Google Scholar] [CrossRef]
- Weraikat, D.; Zanjani, M.K.; Lehoux, N. Two-echelon pharmaceutical reverse supply chain coordination with customers incentives. Int. J. Prod. Econ. 2016, 176, 41–52. [Google Scholar] [CrossRef]
- Lainez, J.M.; Schaefer, E.; Reklaitis, G.V. Challenges and opportunities in enterprise-wide optimization in the pharmaceutical industry. Comput. Chem. Eng. 2012, 47, 19–28. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.; Chen, Y.; Zhu, J. Data envelopment analysis application in sustainability: The origins, development and future directions. Eur. J. Oper. Res. 2018, 264, 1–16. [Google Scholar] [CrossRef]
- Abrizah, A.; Zainab, A.N.; Kiran, K.; Raj, R.G. LIS journals scientific impact and subject categorization: A comparison between Web of Science and Scopus. Scientometrics 2012, 9, 721–740. [Google Scholar] [CrossRef]
- Chadegani, A.A.; Salehi, H.; Yunus, M.M.; Farhadi, H.; Fooladi, M.; Farhadi, M.; Ebrahim, N.A. A Comparison between Two Main Academic Literature Collections: Web of Science and Scopus Databases. Asian Soc. Sci. 2013, 9. [Google Scholar] [CrossRef]
- Mongeon, P.; Paul-hus, A. The journal coverage of Web of Science and Scopus: A comparative analysis. Scientometrics 2015, 106, 213–228. [Google Scholar] [CrossRef]
- Elleuch, H.; Hachicha, W.; Chabchoub, H. A combined approach for supply chain risk management: Description and ap-plication to a real hospital pharmaceutical case study. J. Risk Res. 2014, 17, 641–663. [Google Scholar] [CrossRef]
- Jaberidoost, M.; Olfat, L.; Hosseini, A.; Kebriaeezadeh, A.; Abdollahi, M.; Alaeddini, M.; Dinarvand, R. Pharmaceutical supply chain risk assessment in Iran using analytic hierarchy process (AHP) and simple additive weighting (SAW) methods. J. Pharm. Policy Pract. 2015, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raka, C.; Liangrokapart, J. An Analytical Hierarchy Process (AHP) Approach to Risk Analysis: A Case Study of a New Generic Drug Development Process. J. Pharm. Innov. 2017, 12, 319–326. [Google Scholar] [CrossRef]
- Mehralian, G.; Moosivand, A.; Emadi, S.; Asgharian, R. Developing a coordination framework for pharmaceutical supply chain: Using analytical hierarchy process. Int. J. Logist. Syst. Manag. 2017, 26, 277–293. [Google Scholar] [CrossRef]
- Saaty, T.L. Decision making with the analytic hierarchy process. Int. J. Serv. Sci. 2008, 1, 83–98. [Google Scholar] [CrossRef]
- Ribeiro, D.P. A Logística Reversa na Cadeia de Suprimentos Farmacêutica no Setor Hospitalar do Vale do Paraíba Fluminense. Master’s Thesis, Universidade Federal Fluminense, Rio de Janeiro, Brazil, 2019. RIUFF Repository. Available online: https://app.uff.br/riuff/handle/1/13213 (accessed on 18 November 2020).

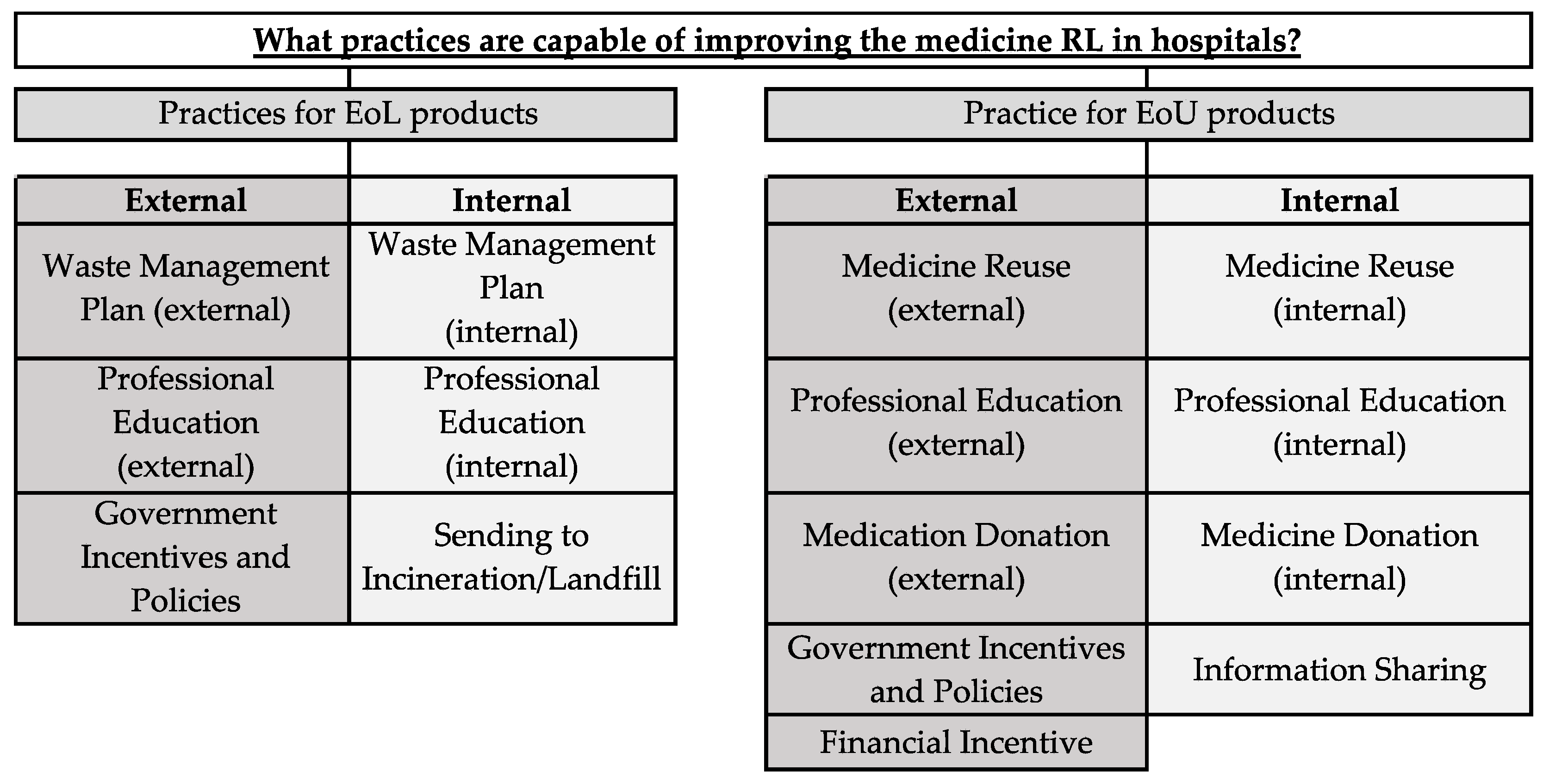
| Practices | Internal/External | EoL or EoU | Researchers | Observations Pointed out by the Researchers |
|---|---|---|---|---|
| Sending to incineration/Landfill | Internal | EoL | De Campos et al. [2]; Hua et al. [36]; Xie and Breen [33]; Narayana [19] | The practice most used and required by Brazilian legislation. |
| Waste Management Plan | Internal/External | EoL | De Campos et al. [2] | It focuses on the collection and treatment of waste that has no other use, which includes the traditional incineration of EoL products. |
| Xie and Breen [33] | Plan to Reduce, Reuse and Properly Dispose of by incineration and in landfill | |||
| Xie and Breen [17] | Goal of Reduction, generation and disposal of waste; Separation of allocated costs; Monitoring of Waste Levels | |||
| Professional Education | Internal/External | EoL or EoU | De Campos et al. [2]; Xie and Breen [33]; Xie and Breen [17] | Awareness and education programs that alert people about the environmental impact generated by medical waste and the correct way to dispose of leftover or expired medicines. |
| Government Incentives and Policies | External | EoL or EoU | De Campos et al. [2]; Narayana et al. [19]; Hua et al. [36] | Regulations and government oversight are crucial for decision-making in a pharmaceutical supply chain. |
| Financial Incentive | External | EoU | Hua et al. [38]; Weraikat et al. [39]. | The producer pays a retailer to collect medicines in customer zones. |
| Medicine Reuse | Internal/External | EoU | De Campos et al. [2] | Operations related to end-of-use products. |
| Hua et al. [36] | Medication from: patients that recover before taking all medications; interrupted or altered therapies; patients’ death; and others. | |||
| Weraikat et al. [39] | If they are returned to the pharmaceutical producer before they expire, they can be sold at subsidized prices. | |||
| Medicine Donation | Internal/External | EoL or EoU | De Campos et al. [2] | Operations related to end-of-use products. |
| Hua et al. [36] | The medicine collected are transported to the recycler and classified according to their expiration dates. | |||
| Weraikat et al. [39] | If they are returned to the pharmaceutical producer before they expire, they can be donated in underdeveloped countries. | |||
| Information Sharing | Internal/External | EoU | Weraikat et al. [39]; Narayana et al. [19]; Lainez [40]; Kumar et al. [16] | Accurate inventory information reduces costs by facilitating the internal and external activities of members involved in the RL process. |
| Hospital | Sector | Municipality | Beds | Appointments per Month | Employees | |
|---|---|---|---|---|---|---|
| 1 | HSJB | Public | Volta Redonda | 180 | 45,000 | 1000 |
| 2 | SCR | Mixed | Resende | 50 | 2500 | 50 |
| 3 | SCBM | Mixed | Barra Mansa | 218 | 7000 | 150 |
| 4 | CSSM | Private | Barra Mansa | 60 | 8000 | 130 |
| 5 | SMR | Private | Resende | 120 | Not reported | 400 |
| 6 | HCL | Private | Volta Redonda | 128 | Not reported | 250 |
| 7 | HRG | Public | Volta Redonda | 80 | 140 (hospitalizations only) | 500 |
| 8 | HUVR | Private | Volta Redonda | 134 | Not reported | 2000 |
| 9 | HUR | Private | Resende | 70 | 4600 | 328 |
| 10 | HEM | Public | Resende | 75 | 1000 | 800 |
| 11 | APM | Mixed | Resende | 60 | 900 | 150 |
| 12 | HML | Public | Barra Mansa | 40 | 120 deliveries | 70 |
| 13 | HMR | Public | Volta Redonda | 70 | Not reported | 500 |
| Practices for End-of-Life Products (EoL) | ||||||||||||||
| # HSJB | SCR | SCBM | CSSM | SMR | HCL | HRG | HUVR | HUR | HEM | APM | HML | HMR | % | |
| Management Plan of Waste (external) | × | 7.6 | ||||||||||||
| Government Incentives and Policies | × | × | × | × | 30.8 | |||||||||
| Waste Management Plan (internal) | × | × | × | × | × | × | × | × | × | × | × | × | 92.3 | |
| Sending to Incineration/Landfill | × | × | × | × | × | × | × | × | × | × | × | × | × | 100 |
| Professional Education (internal) | × | × | × | × | × | × | × | × | × | × | × | × | × | 100 |
| Practices for End-of-Use Products (EoU) | ||||||||||||||
| # HSJB | SCR | SCBM | CSSM | SMR | HCL | HRG | HUVR | HUR | HEM | APM | HML | HMR | % | |
| Government Incentives and Policies | × | × | × | 23.1 | ||||||||||
| Medicine Reuse (external) | × | × | × | × | 30.8 | |||||||||
| Financial Incentive | × | × | × | × | × | × | 46.2 | |||||||
| Medicine Donation (internal) | × | × | × | × | × | × | × | × | × | × | 76.9 | |||
| Medicine Reuse (internal) | × | × | × | × | × | × | × | × | × | × | × | × | × | 100 |
| Professional Education (internal) | × | × | × | × | × | × | × | × | × | × | × | × | × | 100 |
| Information Sharing | × | × | × | × | × | × | × | × | × | × | × | × | × | 100 |
| Hospital | Internal Practices | External Practices |
|---|---|---|
| HSJB | 0.25 | 0.75 |
| SCR | 0.1 | 0.9 |
| SCBM | 0.25 | 0.75 |
| CSSM | 0.25 | 0.75 |
| SMR | 0.13 | 0.88 |
| HCL | 0.25 | 0.75 |
| HRG | 0.17 | 0.83 |
| HUVR | 0.5 | 0.5 |
| HUR | 0.25 | 0.75 |
| HEM | 0.1 | 0.9 |
| APM | 0.17 | 0.83 |
| HML | 0.25 | 0.75 |
| HMR | 0.13 | 0.88 |
| Average | 0.21 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, D.P.; de Oliveira, U.R.; da Silva César, A.; Aprigliano Fernandes, V. Evaluation of Medicine Reverse Logistics Practices in Hospitals. Sustainability 2021, 13, 3496. https://doi.org/10.3390/su13063496
Ribeiro DP, de Oliveira UR, da Silva César A, Aprigliano Fernandes V. Evaluation of Medicine Reverse Logistics Practices in Hospitals. Sustainability. 2021; 13(6):3496. https://doi.org/10.3390/su13063496
Chicago/Turabian StyleRibeiro, Diego Pereira, Ualison Rébula de Oliveira, Aldara da Silva César, and Vicente Aprigliano Fernandes. 2021. "Evaluation of Medicine Reverse Logistics Practices in Hospitals" Sustainability 13, no. 6: 3496. https://doi.org/10.3390/su13063496
APA StyleRibeiro, D. P., de Oliveira, U. R., da Silva César, A., & Aprigliano Fernandes, V. (2021). Evaluation of Medicine Reverse Logistics Practices in Hospitals. Sustainability, 13(6), 3496. https://doi.org/10.3390/su13063496








