Vegetation Cover and Tumuli’s Shape as Affecting Factors of Microclimate and Biodeterioration Risk for the Conservation of Etruscan Tombs (Tarquinia, Italy)
Abstract
1. Introduction
2. Materials and Methods
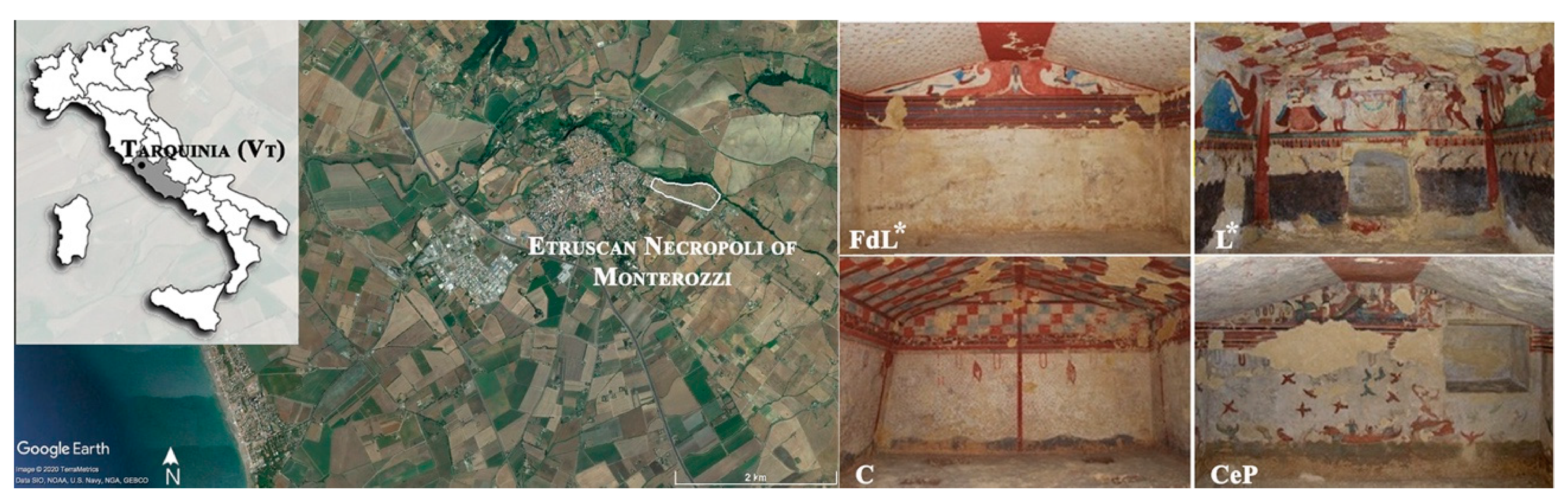
2.1. Study Area
2.2. Analysis of Vegetation Changes in Composition and Cover during the Seasons
2.3. Analysis of Tumuli’s Shapes and the Solar Radiation Inputs as Related to Microclimatic Influences
2.4. Collection and Analysis of Microclimatic Data
3. Results
3.1. Changes in Vegetation Cover during the Analysed Seasons
3.2. Seasonal Trends of Microclimatic Data and Related Risks for Weathering and Biodeterioration Phenomena
3.3. Relationships among External Factors and Underground Microclimatic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paribeni, M. Cause di Deperimento e Metodi di Conservazione Delle Pitture Murali Delle Tombe Sotterranee di Tarquinia; Rapporto Scientifico C.N.R.—Istituto di Fisica Tecnica di Roma, Edizioni Sistema: Rome, Italy, 1970. [Google Scholar]
- Massa, S.; Nichi, D. Conservazione e fruizione: Analisi ambientale sulle tombe dipinte di Tarquinia. In Etruria Meridionale: Conoscenza, Conservazione, Fruizione, Proceedings of Convegno Viterbo, Italy, 29 November–1 December 1985; Bettini, C., Colonna, G., Staccioli, R., Eds.; Casa Editrice Quasar: Rome, Italy, 1988; pp. 139–143. [Google Scholar]
- Bracci, S.; Cuzman, O.A.; Ignesti, A.; Del Fa, R.M.; Olmi, R.; Pallecchi, P.; Riminesi, P.; Tiano, P. Multidisciplinary approach for the conservation of an Etruscan hypogean monument. Eur. J. Sci. Theol. 2013, 9, 91–106. [Google Scholar]
- Saiz-Jimenez, C. The Conservation of Subterranean Cultural Heritage; CRC Press Taylor & Francis Group: London, UK, 2014; ISBN 9781138026940. [Google Scholar]
- Chung, Y.J. Conservation environment of Korean mural tombs in the period of the Three Kingdoms. In Proceedings of the International Conference on Conservation of Stone and Earthen Architectural Heritage, Gongju, Korea, 20–23 May 2014; Lee, C.H., Kim, J., Kim, R.H., Eds.; Kongju National University: Gongju, Korea, 2014; ISBN 979-11-953029-0-1. [Google Scholar]
- Caneva, G.; Isola, D.; Lee, H.J.; Chung, Y.J. Biological Risk for Hypogea: Shared Data from Etruscan Tombs in Italy and Ancient Tombs of the Baekje Dynasty in Republic of Korea. Appl. Sci. 2020, 10, 6104. [Google Scholar] [CrossRef]
- Albertano, P.; Urzì, C.; Caneva, G. Tombs, catacombs, and other hypogean environments. In Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; Getty Con-servation Institute: Los Angeles, LA, USA, 2008; pp. 77–81. [Google Scholar]
- Jeong, S.H.; Lee, H.J.; Lee, M.Y.; Chung, Y.J. Conservation Environment for Mural Tomb in Goa-ri, Goryeong. J. Conserv. Sci. 2017, 33, 189–201. [Google Scholar] [CrossRef][Green Version]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Getty Publications: Los Angeles, LA, USA, 2008. [Google Scholar]
- He, X.; Xu, M.; Zhang, H.; Zhang, B.; Su, B. An exploratory study of the deterioration mechanism of ancient wall-paintings based on thermal and moisture expansion property analysis. J. Archaeol. Sci. 2014, 42, 194–200. [Google Scholar] [CrossRef]
- Li, H.; Wang, W.; Zhan, H.; Qiu, F.; Guo, Q.; Sun, S.; Zhang, G. The effects of atmospheric moisture on the mural paintings of the Mogao Grottoes. Stud. Conserv. 2017, 62, 229–239. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Petropoulos, E.; Ma, Y.; Shen, Y. Humidity governs the wall-inhabiting fungal community composition in a 1600-year tomb of Emperor Yang. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Bettini, G.; Massa, S. Preservation problems, visitors and deterioration on the painted Etruscan tomb. In Science, Technology and European Cultural Heritage, Proceedings of the European Symposium, Bologna, Italy, 13–16 June 1989; Baer, N.S., Sabbioni, C., Sors, A.I., Eds.; Butterworth-Heinemann Publishers: Guildford, UK, 1991; pp. 761–769. ISBN 978-0-7506-0237-2. [Google Scholar]
- Pantazidou, A.; Roussomoustakaki, M.; Urzì, C. The microflora of Milos Catacombs. In Archaeological Sciences 1995, 1st ed.; Sinclair, A., Gowlett, J.A.J., Slater, E.A.S., Eds.; Owbow Books: Oxford, UK, 1997; pp. 321–325. ISBN 9781785708053. [Google Scholar]
- Scatigno, C.; Gaudenzi, S.; Sammartino, M.; Visco, G. A microclimate study on hypogea environments of ancient roman building. Sci. Total. Environ. 2016, 566, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Mora, P.; Mora, L.; Philippot, P. The Conservation of Wall Paintings; Butterworths: London, UK, 1984; ISBN 0408108126. [Google Scholar]
- Oligschläger, D.; Waldow, S.; Haber, A.; Zia, W.; Blumich, B. Moisture dynamics in wall paintings monitored by single-sided NMR. Magn. Reson. Chem. 2015, 53, 48–57. [Google Scholar] [CrossRef]
- Monte, M.; Ferrari, R. Biodeterioration in subterranean environments. Aerobiologia 1993, 9, 141–148. [Google Scholar] [CrossRef]
- Ariño, X.; Hernandez-Marine, M.; Saiz-Jimenez, C. Colonization of Roman tombs by calcifying cyanobacteria. Phycologia 1997, 36, 366–373. [Google Scholar] [CrossRef]
- Saiz-Jimenez, I.G.C. Actinomycetes in Hypogean Environments. Geomicrobiol. J. 1999, 16, 1–8. [Google Scholar] [CrossRef]
- Nugari, M.; Pietrini, A.; Caneva, G.; Imperi, F.; Visca, P. Biodeterioration of mural paintings in a rocky habitat: The Crypt of the Original Sin (Matera, Italy). Int. Biodeterior. Biodegrad. 2009, 63, 705–711. [Google Scholar] [CrossRef]
- Albertano, P. Epilithic algal communities in hypogean environments. G. Bot. Ital. 1993, 127, 386–392. [Google Scholar] [CrossRef]
- Bruno, L.; Belleza, S.; Urzì, C.; De Leo, F. A study for monitoring and conservation in the Roman Catacombs of St. Callistus and Domitilla, Rome (Italy). In The Conservation of Subterranean Cultural Heritage; Apple Academic Press: Palm Bay, FL, USA, 2014; pp. 37–44. [Google Scholar]
- Capitani, D.; Proietti, N.; Gobbino, M.; Soroldoni, L.; Casellato, U.; Valentini, M.; Rosina, E. An integrated study for mapping the moisture distribution in an ancient damaged wall painting. Anal. Bioanal. Chem. 2009, 395, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Cultural Heritage and Aerobiology. In Cultural Heritage and Aerobiology; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2003; pp. 207–224.
- Ruga, L.; Orlandi, F.; Romano, B.; Fornaciari, M. The assessment of fungal bioaerosols in the crypt of St. Peter in Perugia (Italy). Int. Biodeterior. Biodegrad. 2015, 98, 121–130. [Google Scholar] [CrossRef]
- Di Carlo, E.; Chisesi, R.; Barresi, G.; Barbaro, S.; Lombardo, G.; Rotolo, V.; Sebastianelli, M.; Travagliato, G.; Palla, F. Fungi and Bacteria in Indoor Cultural Heritage Environments: Microbial-related Risks for Artworks and Human Health. Environ. Ecol. Res. 2016, 4, 257–264. [Google Scholar] [CrossRef]
- Bruno, L.; Rugnini, L.; Spizzichino, V.; Caneve, L.; Canini, A.; Ellwood, N.T.W. Biodeterioration of Roman hypogea: The case study of the Catacombs of SS. Marcellino and Pietro (Rome, Italy). Ann. Microbiol. 2019, 69, 1023–1032. [Google Scholar] [CrossRef]
- KNUCH. Study on the Preservation Methods of Mural Painting in the Ancient Tombs in Songsan-ri; Gongju: Gongju City, Korea, 2012; pp. 108–156. [Google Scholar]
- Chung, Y.J.; Lee, H.J.; Kim, H.M.; Kim, D.W. Study on the microbe growth characteristics by environmental condition of ancient tomb. In Proceedings of the 41th Conference of the Korean Society of Conservation Science for Cultural Heritage, Seoul, Korea, 27 March 2015; pp. 27–30. [Google Scholar]
- Sprocati, A.R.; Alisi, C.; Tasso, F.; Vedovato, E.; Barbabietola, N.; Cremisini, C. A microbiological survey of the Etruscan Mercareccia tomb (Italy): Contribution of microorganisms to deterioration and restoration. In Proceedings of the 9th International Conference on NDT of Art 2008, Jerusalem, Israel, 25–30 May 2008; NDT of Art: Jerusalem, Israel, 2008; p. 9. Available online: www.ndt.net/search/docs.php3?MainSource=65 (accessed on 26 January 2021).
- Tomassetti, M.C.; Cirigliano, A.; Arrighi, C.; Negri, R.; Mura, F.; Maneschi, M.L.; Gentili, M.D.; Stirpe, M.; Mazzoni, C.; Rinaldi, T. A role for microbial selection in frescoes’ deterioration in Tomba degli Scudi in Tarquinia, Italy. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Cirigliano, A.; Tomassetti, M.C.; Di Pietro, M.; Mura, F.; Maneschi, M.L.; Gentili, M.D.; Cardazzo, B.; Arrighi, C.; Mazzoni, C.; Negri, R.; et al. Calcite moonmilk of microbial origin in the Etruscan Tomba degli Scudi in Tarquinia, Italy. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Isola, D.; Zucconi, L.; Cecchini, A.; Caneva, G. Dark-pigmented biodeteriogenic fungi in Etruscan hypogeal tombs: New data on their diversity and favouring conditions. Fun. Biol. 2021, in press. [Google Scholar]
- Isola, D.; Bartoli, F.; Langone, S.; Ceschin, S.; Zucconi, D.; Caneva, G. Plant DNA barcode as tool for root identification in hypogeans: The case of the Etruscan tombs of Tarquinia (Italy). PLoS ONE 2021, in press. [Google Scholar]
- Pallottino, M. La Peinture Étrusque; Flammarion: Geneva, Switzerland, 1952. [Google Scholar]
- Marzullo, M. Grotte Cornetane. Materiale e Apparato Critico per lo Studio Delle Tombe Dipinte di Tarquinia. Tarchna Suppl. 6, 1st ed.; Ledizioni Ledi Publishing: Milano, Italy, 2016; ISBN 978-88-6705-484-8. [Google Scholar]
- Cecchini, A. Le Tombe Dipinte di Tarquinia. Vicenda Conservativa, Restauri e Tecnica di Esecuzione, 2nd ed.; Nardini Editore: Firenze, Italy, 2012; pp. 15–102. ISBN 978-88-404-4347-8. [Google Scholar]
- Kyriakou, V.T.; Panoskaltsis, V.P. Impact of the tumulus on the stability of microclimate in underground heritage structures. Int. J. Sci. Eng. Technol. 2019, 4. Available online: http://www.ijstre.com/v4i5.php (accessed on 26 January 2021).
- Massa, S. Esigenze conservative di un ipogeo interessato da vegetazione sovrastante. In Ruderi & Vegetazione. Questioni di Restauro, 1st ed.; Mancini, R., Rossi Doria, I., Eds.; Ginevra Bentivoglio Editoria: Rome, Italy, 2017; pp. 265–289. ISBN 8899618577. [Google Scholar]
- Mitrakos, K. A theory for Mediterranean plant life. Acta Oecol. Sér. Oecol. Plant. Montreuil 1980, 1, 245–252. [Google Scholar]
- Raunkiaer, C. Plant Life Forms; The Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Potenza, G.; Fascetti, S.; Castronuovo, D.; Lovelli, S.; Perniola, M.; Viggiani, R.; Rossi, R.; Marchione, V.; Can-dido, V. Collection and preliminary characterization of native turfgrass accessions of Cynodon dactylon L. in the Mediterranean area. J. Food Agric. Environ. 2014, 12, 770–774. [Google Scholar] [CrossRef]
- Croce, P.; De Luca, A.; Mocioni, M.; Volterrani, M.; Beard, J.B. Warm season turfgrass species and cultivar characterizations for a Mediterranean climate. Int. Turfgrass Soc. Res. J. 2001, 9, 855–859. [Google Scholar]
- Parson, D.L.P.; Lehman, V.G. Bermudagrass plant named “Premier”. U.S. Patent PP18,247 P3, 27 November 2007. [Google Scholar]
- Pesaresi, S.; Galdenzi, D.; Biondi, E.; Casavecchia, S. Bioclimate of Italy: Application of the worldwide bioclimatic classification system. J. Maps 2014, 10, 538–553. [Google Scholar] [CrossRef]
- Mattias, P.P.; Ventriglia, U. La regione vulcanica dei Monti Sabatini e Cimini. Mem. Soc. Geol. Ital. 1970, 9, 331–384. [Google Scholar]
- D’Agostino, S.; Lombardi, G.; Russo, G.; Viggiani, C. Structural engineering and geology applied to the static problems of the Etruscan “Tomba dell’Orco” (Tarquinia, Central Italy). J. Cult. Herit. 2010, 11, 107–112. [Google Scholar] [CrossRef]
- Spizzichino, D.; Leoni, G.; Guarino, P.M.; Boldini, D.; Mengoni, S.; Marino, E.; Cecchini, A.; Trucco, F.; Casocavallo, B. Influenza delle condizioni climatiche sulla stabilità delle tombe etrusche della necropoli di Monterozzi a Tarquinia. In Monitoraggio e Manutenzione delle Aree Archeologiche. Cambiamenti Climatici, Dissesto Idrogeologico, Degrado Chimico-Ambientale, 1st ed.; Russo, A., Della Giovampaola, I., Eds.; «L’Erma» di Bretschneider: Roma, Italy, 2020; pp. 227–230. ISBN 9788891319470. [Google Scholar]
- Braun-Blanquet, J. Plant Sociology. In The Study of Plant Communities; McGraw-Hill Book Co., Inc.: New York, NY, USA; London, UK, 1932. [Google Scholar]
- Pignatti, S. La Flora d’Italia; Ed. Edagricole: Milano, Italy, 2017. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Blasi, C.; Facioni, L.; Burrascano, S.; Del Vico, E.; Tilia, A.; Rosati, L. Submediterranean dry grasslands along the Tyrrhenian sector of central Italy: Synecology, syndynamics and syntaxonomy. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2012, 146, 266–290. [Google Scholar] [CrossRef]
- Diaz-Herraiz, M.; Jurado, V.; Cuezva, S.; Laiz, L.; Pallecchi, P.; Tiano, P.; Sanchez-Moral, S.; Saiz-Jimenez, C. The Actinobacterial Colonization of Etruscan Paintings. Sci. Rep. 2013, 3, srep01440. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Herraiz, M.; Jurado, V.; Cuezva, S.; Laiz, L.; Pallecchi, P.; Tiano, P.; Sanchez-Moral, S.; Saiz-Jimenez, C. Deterioration of an Etruscan tomb by bacteria from the order Rhizobiales. Sci. Rep. 2015, 4, 3610. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G. A botanical approach to the planning of archaeological, parks in Italy. Conserv. Manag. Archaeol. Sites 1999, 3, 127–134. [Google Scholar] [CrossRef]
- Caneva, G.; Galotta, G.; Cancellieri, L.; Savo, V. Tree roots and damages in the Jewish catacombs of Villa Torlonia (Roma). J. Cult. Herit. 2009, 10, 53–62. [Google Scholar] [CrossRef]
- Cicinelli, E.; Salerno, G.; Caneva, G. An assessment methodology to combine the preservation of biodiversity and cultural heritage: The San Vincenzo al Volturno historical site (Molise, Italy). Biodivers. Conserv. 2017, 27, 1073–1093. [Google Scholar] [CrossRef]
- Caneva, G.; Benelli, F.; Bartoli, F.; Cicinelli, E. Safeguarding natural and cultural heritage on Etruscan tombs (La Banditaccia, Cerveteri, Italy). Rend. Lincei Sci. Fis. Nat. 2018, 29, 891–907. [Google Scholar] [CrossRef]
- Caneva, G.; Cicinelli, E.; Scolastri, A.; Bartoli, F. Guidelines for urban community gardening: Proposal of preliminary indicators for several ecosystem services (Rome, Italy). Urban For. Urban Green. 2020, 56, 126866. [Google Scholar] [CrossRef]
- Agarossi, G.; Ferrari, R.; Monte, M.; Gugliandolo, C.; Maugeri, T. Changes of microbial system in an Etruscan tomb after biocidal treatments. In Proceedings of the Vlth International Congress on Deterioration and Conservation of Stone, Torun, Poland, 12–14 September 1988; Nicolaus Copernicus University Press: Torun, Poland, 1988; pp. 82–91. [Google Scholar]
- Agarossi, G. Biodeterioramento in ambienti ipogei: Esperienze e considerazioni. In Studi e Ricerche sulla Conservazione delle Opere d’Arte Dedicati alla Memoria di Marcello Paribeni; Guidobaldi, F., Ed.; CNR: Rome, Italy, 1994; pp. 1–18. [Google Scholar]
- Ma, Y.; Zhang, H.; Du, Y.; Tian, T.; Xiang, T.; Liu, X.; Wu, F.; An, L.; Wang, W.; Gu, J.-D.; et al. The community distribution of bacteria and fungi on ancient wall paintings of the Mogao Grottoes. Sci. Rep. 2015, 5, 7752. [Google Scholar] [CrossRef]
- Ma, W.; Wu, F.; Tian, T.; He, D.; Zhang, Q.; Gu, J.-D.; Duan, Y.; Ma, D.; Wang, W.; Feng, H. Fungal diversity and its contribution to the biodeterioration of mural paintings in two 1700-year-old tombs of China. Int. Biodeterior. Biodegrad. 2020, 152, 104972. [Google Scholar] [CrossRef]
- Nugari, M.P.; Realini, M.; Roccardi, A. Contamination of mural paintings by indoor airborne fungal spores. Aerobiologia 1993, 9, 131–139. [Google Scholar] [CrossRef]
- Monte, M.; Ferrari, R.; Massa, S. Biodeterioration of Etruscan tombs: Aerobiology and microclimate. In Proceedings of the 5th International Conference, Bangalore, India, 1994; Agashe, S.N., Ed.; Science Publishers: Norwood, NJ, USA, 1997; pp. 333–346. ISBN 9781886106840. [Google Scholar]
- Caneva, G.; De Nuntiis, P.; Fornaciari, M.; Ruga, L.; Valenti, P.; Pasquariello, G. Aerobiology applied to the preventive conservation of cultural heritage. Aerobiologia 2019, 36, 99–103. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeong, S.H.; Lee, M.Y.; Chung, Y.J. Thermal Environment Analysis for Preserving Ancient Mural Painting in Songsan-ri Tomb No. 6, Gongju, Korea. J. Conserv. Sci. 2016, 32, 521–534. [Google Scholar] [CrossRef][Green Version]





| Family | Chorotype | Species | Life Form | Cover Values Summer | Cover Values Autumn | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FdL* | L* | C | CeP | FdL* | L* | C | CeP | ||||
| Lamiaceae | Steno-medit. | Clinopodium nepeta (L.) Kuntze | Ch suffr | + | 1 | ||||||
| Lamiaceae | Euri-Medit. | Teucrium chamaedrys L. | Ch suffr | + | |||||||
| Asphodelaceae | Steno-medit. | Asphodelus ramosus L. | G rhiz | 1 | 1 | ||||||
| Poaceae | Silv-cult | Cynodon dactylon (L.) Pers. var. Premier | G rhiz | 5 | 5 | 4 | 4 | ||||
| Asteraceae | Submedit | Crepis vesicaria L. | H bienn | 1 | 1 | ||||||
| Boraginaceae | Euri-medit. | Echium plantagineum L. | H bienn | + | + | ||||||
| Apiaceae | Steno-medit. | Seseli tortuosum L. | H bienn | + | + | + | + | 1 | 1 | 1 | 1 |
| Caprifoliaceae | Steno-medit. | Sixalix atropurpurea (L.) Greuter & Burdet | H bienn | + | |||||||
| Scrophulariaceae | Euri-medit. | Verbascum sinuatum L. | H bienn | 1 | + | 1 | + | ||||
| Poaceae | Euroasiat. | Dactylis glomerata L. | H caesp | + | 2 | ||||||
| Asteraceae | Euro-Cauc. | Bellis perennis L. | H ros | + | |||||||
| Asteraceae | Steno-medit. | Hyoseris radiata L. | H ros | + | + | + | + | 1 | 1 | 2 | 2 |
| Plantaginaceae | Euroasiat. | Plantago lanceolata L. | H ros | + | + | 1 | 1 | + | |||
| Asteraceae | Steno-medit. | Centaurea aspera L. | H scap | + | + | + | 1 | + | |||
| Convolvulaceae | Euri-medit. | Convolvulus cantabrica L. | H scap | 1 | 2 | + | + | + | 1 | 1 | 1 |
| Asteraceae | Euri-medit. | Crepis bursifolia L. | H scap | + | |||||||
| Brassicaceae | Euri-medit. | Diplotaxis tenuifolia (L.) DC. | H scap | + | + | + | 1 | 1 | 1 | ||
| Apiaceae | Euri-medit. | Ferula communis L. | H scap | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Apiaceae | Steno-medit. | Foeniculum vulgare Mill. | H scap | 2 | 1 | 1 | 5 | 1 | 1 | + | 2 |
| Lamiaceae | Eurasiat. | Lamium maculatum L. | H scap | + | + | ||||||
| Plantaginaceae | Endem. | Linaria purpurea (L.) Mill. | H scap | + | + | ||||||
| Malvaceae | Wide distr. | Malva sylvestris L. | H scap | + | 1 | + | 2 | + | 2 | ||
| Fabaceae | Subcosmop. | Medicago sativa L. | H scap | 1 | |||||||
| Asteraceae | Eurosiber. | Picris hieracioides L. | H scap | 1 | 1 | 1 | |||||
| Asteraceae | Steno-medit. | Reichardia picroides (L.) Roth | H scap | + | + | + | + | 2 | 2 | 2 | 3 |
| Polygonaceae | Euroasiat. | Rumex acetosa L. | H scap | + | + | 2 | + | + | |||
| Lamiaceae | Euri-medit. | Salvia verbenaca L. | H scap | + | + | 1 | 1 | 1 | 1 | 3 | 3 |
| Caryophyllaceae | Euroasiat. | Silene vulgaris (Moench) Garcke | H scap | 1 | + | + | 2 | 2 | 1 | + | |
| Asteraceae | Euri-medit. | Urospermum dalechampii (L.) F.W. Schmidt | H scap | + | + | ||||||
| Geraniaceae | Subcosmop. | Erodium cicutarium (L.) L’Hér. | T caesp | + | |||||||
| Asteraceae | Steno-medit. | Anthemis arvensis L. | T scap | 1 | |||||||
| Poaceae | Euroasit. | Avena fatua L. | T scap | 2 | |||||||
| Asteraceae | Euri-medit. | Calendula arvensis (Vaill.) L. | T scap | 2 | 3 | 2 | 2 | ||||
| Asteraceae | C-Europ. | Crepis capillaris (L.) Wallr. | T scap | 1 | + | ||||||
| Poaceae | Euri-medit-Turan. | Dasypyrum villosum (L.) P. Candargy | T scap | + | + | + | 1 | 1 | 1 | 2 | |
| Asteraceae | Subcosmop. | Erigeron canadensis L. | T scap | 1 | |||||||
| Euphorbiaceae | Subcosmop. | Euphorbia helioscopia L. | T scap | + | 1 | ||||||
| Geraniaceae | Subcosmop. | Geranium molle L. | T scap | 1 | |||||||
| Asteraceae | Steno-medit. | Sonchus tenerrimus L. | T scap | 1 | |||||||
| Total number | 17 | 16 | 13 | 12 | 24 | 20 | 17 | 22 | |||
| FdL* | L* | C | CeP | |
|---|---|---|---|---|
| Summer | 123% | 132% | 19% | 101% |
| (100) | (100) | (20) | (65) | |
| Autumn | 165% | 183% | 112% | 182% |
| (100) | (100) | (70) | (100) | |
| Difference: D = A − S | 42% | 50.5% | 92.5% | 81.5% |
| 0.74 | 0.72 | 0.17 | 0.55 | |
| 0.25 | 0.28 | 0.83 | 0.44 |
| Tumuli | FdL* | L* | C | CeP | |
|---|---|---|---|---|---|
| Height (m) of sepulchral chambers | 2.15 | 2.1 | 2.61 | 2.5 (I room) 2.1 (II room) | |
| Volumes (m3) of sepulchral chambers | 40.86 | 23.94 | 38.35 | 38.4 (I room) 23.9 (IIroom) | |
| Months | January | 8.44 | 3.55 | 9.94 | 4.28 |
| February | 10.98 | 6.12 | 12.28 | 6.97 | |
| March | 15.42 | 10.54 | 16.52 | 11.42 | |
| April | 18.4 | 15.19 | 18.9 | 15.77 | |
| May | 21.72 | 20.02 | 21.69 | 20.35 | |
| June | 23.44 | 22.7 | 23.09 | 22.9 | |
| July | 23.26 | 22.06 | 23.04 | 22.34 | |
| August | 20.45 | 17.77 | 20.83 | 18.26 | |
| September | 16.39 | 12.37 | 17.39 | 13.1 | |
| October | 12.43 | 7.66 | 13.61 | 8.5 | |
| November | 8.79 | 4.27 | 10.1 | 5 | |
| December | 6.6 | 2.92 | 7.78 | 3.44 | |
| Annual | 186.32 | 145.17 | 195.17 | 152.33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caneva, G.; Langone, S.; Bartoli, F.; Cecchini, A.; Meneghini, C. Vegetation Cover and Tumuli’s Shape as Affecting Factors of Microclimate and Biodeterioration Risk for the Conservation of Etruscan Tombs (Tarquinia, Italy). Sustainability 2021, 13, 3393. https://doi.org/10.3390/su13063393
Caneva G, Langone S, Bartoli F, Cecchini A, Meneghini C. Vegetation Cover and Tumuli’s Shape as Affecting Factors of Microclimate and Biodeterioration Risk for the Conservation of Etruscan Tombs (Tarquinia, Italy). Sustainability. 2021; 13(6):3393. https://doi.org/10.3390/su13063393
Chicago/Turabian StyleCaneva, Giulia, Simone Langone, Flavia Bartoli, Adele Cecchini, and Carlo Meneghini. 2021. "Vegetation Cover and Tumuli’s Shape as Affecting Factors of Microclimate and Biodeterioration Risk for the Conservation of Etruscan Tombs (Tarquinia, Italy)" Sustainability 13, no. 6: 3393. https://doi.org/10.3390/su13063393
APA StyleCaneva, G., Langone, S., Bartoli, F., Cecchini, A., & Meneghini, C. (2021). Vegetation Cover and Tumuli’s Shape as Affecting Factors of Microclimate and Biodeterioration Risk for the Conservation of Etruscan Tombs (Tarquinia, Italy). Sustainability, 13(6), 3393. https://doi.org/10.3390/su13063393









