Abstract
Introduction of quinoa (Chenopodium quinoa willd.), a gluten-free nutritious pseudo-cereal, outside its traditional growing areas exposed it to seedling damping-off. Here, we isolated eleven phosphate-solubilizing bacteria from the quinoa rhizosphere and assessed their effect on germination and seedlings growth. All isolates solubilized phosphate, produced indole3-acetic acid, hydrocyanic acid, siderophores, and ammonia. Genotypic analysis revealed that our strains are related to the genus of Bacillus, Pseudomonas, and Enterobacter. Strains Enterobacter asburiae (QD14, QE4, QE6, and QE16), Enterobacter sp. QE3, and Enterobacter hormaechei QE7 withstood 1.5 mg·L−1 of cadmium sulfate, 0.5 mg·mL−1 of nickel nitrate, and 1 mg·mL−1 of copper sulfate. Moreover, all strains solubilized zinc from ZnO; P. Stutzeri QD1 and E. asburiae QD14 did not solubilize Zn3(PO4)2 and CO3Zn, whereas CO3Zn was not solubilized by E. asburiae QE16. Bacillus atrophaeus S8 tolerated 11% NaCl. P. frederiksbergensis S6 and Pseudomonas sp. S7 induced biofilm formation. Anti-fusarium activity was demonstrated for E. asburiae QE16, P. stutzeri QD1, P. frederiksbergensis S6, Pseudomonas sp. S7, and B. atrophaeus S8. Lastly, inoculation of quinoa seeds with B. atrophaeus S8 and E. asburiae QB1 induced the best germination rate and seedling growth, suggesting their potential use as inoculants for salty and heavy metal or zinc contaminated soils.
1. Introduction
In recent years, pseudo-cereal quinoa (Chenopodium quinoa willd.) has received much attention as a versatile crop that can thrive in extreme soil and climatic conditions [1]. Quinoa is also an important gluten-free nutritious food crop, and its proteins content is higher than maize, barley, or wheat and can be used as an alternative to milk proteins [2,3]. Quinoa is found natively in the Andean region, where it grows at >4000 m above-sea-level. It is also the traditional pseudo-cereal in South America and considered a source of starch and a high-quality protein content; hence, why it was introduced in Morocco in early 2000. Quinoa has been studied as potential source of food security for people in mountainous areas with a post-drought period leading to increased soil salinity [4]. Quinoa can tolerate high levels of salinity and drought stress, and some varieties can even be irrigated using sea water [5]. The high nutritional value of the grains supports recent interest in cultivation in the USA and European countries [6]. Under both Andean and European conditions, issues associated with germination of quinoa have been observed [7]. The introduction of the novel crop in areas outside its traditional growing regions could expose it to fungal diseases. Drimalkova M. et al. (2003) [8] have isolated different fungal species from infected parts of quinoa seedlings responsible for damping-off the quinoa seedlings growth. Now, it is well established that free-living and root-colonizing microorganisms, when applied to seeds or roots, improve plant growth, reduce damage caused by phytopathogens, and alleviate abiotic stresses [9]. These microorganisms play a key role in a large number of processes in distinct ecosystems, including the acquisition of plant nutrients [10].
A significant number of phosphate-solubilizing bacteria (PSB) isolated from the plant rhizosphere, such as Pseudomonas, Bacillus, Rhodococcus, Arthrobacter, Serratia, Chryseobacterium, Gordonia, Phyllobacterium, Delftia [11,12], Azotobacter [13], Enterobacter, Pantoea, and Klebsiella [14], play a key role in enhancing plant growth [14]. PSB have the ability to solubilize phosphate by converting insoluble phosphate compounds present in the soil to soluble forms that are assimilable by plants [15]. In this context, PSB have been considered as suitable ecological candidates for plant phosphorus (P) nutrition [16]. P plays an important role in all metabolic processes, including photosynthesis, energy transfer, signal transduction, macromolecule biosynthesis, and nucleic acid synthesis in plants [17]. Besides carbon and nitrogen, which can be taken directly from the atmosphere, P uptake can only be facilitated via the plant root [18]. P exists in different forms that interact through a variety of physicochemical, biological, and biochemical mechanisms involving adsorption/desorption, precipitation/dissolution, mineralization, and immobilization reactions [19]. The P acquisition, in sufficient amounts, is critical for plant growth and development [20]. Despite its undeniable importance in the life cycle, P supply remains a limiting factor for plant growth [21] as only 0.5% of total P in soil is accessible to plants [22]. The main mechanisms of phosphate solubilization employed by soil microorganisms include the release of mineral-dissolving complexing agents and compounds including organic acid, protons, siderophores, hydroxyl ions, and CO2. Such a process is facilitated by extracellular enzyme release (biochemical phosphate mineralization), and the liberation of P during various substrate degradation (biological phosphate mineralization) [19,23]. Also, these PSB exhibit multifunctional properties that benefit plants growth/germination by producing indole-3 acetic acid (IAA), ammonia, hydrocyanic acid (HCN), biofilm, etc. [19]. Moreover, they play an important role in alleviating stresses related to drought, salinity, and heavy metal contamination [24,25].
To preserve the sustainability of the quinoa crop in Morocco and prevent potential plant disease issues, we sought here to isolate potent PSB from the quinoa rhizosphere and to evaluate their effect(s) on quinoa seed germination, seedling growth, and biocontrol. We also investigated our strains for their: (i) conventional plant growth promoting traits, (ii) capacity to resist selected antibiotics, (iii) ability to withstand heavy metals, and finally (vi) ability to mitigate salt stress.
2. Materials and Methods
2.1. Soil Sampling and PSB Screening
Twelve representative soil samples from three-month-old quinoa plants’ rhizosphere were collected in July 2018 from the experimental farm (32.219731 E, −7.892268 N) in Ben Guerir, Morocco. These samples were randomly taken from different locations so that the entire quinoa field was covered. Soil and root samples of each plant were collected under aseptic conditions (washing and disinfecting each piece of equipment with NaClO at 2% and ethanol at 70% before reuse) at a depth ranging from 10 to 20 cm. Each sample was put in a sterile plastic bag and immediately transported to the laboratory. PSB isolation was performed according to the suspension–dilution method. One gram of soil from each sample was mixed with 9 mL of sterilized distilled water and then stirred for 30 min. One hundred microliters of the stock solution were introduced into 1.5 mL Eppendorf tube containing 900 µL of sterile distilled water to obtain 10−1 dilution. A dilution series was prepared until 10−5 dilution. Afterwards, 100 µL of each suspension were spread on Petri dishes containing National Botanical Research Institute’s phosphate growth medium (NBRIP) [26] containing 5 g of tricalcium phosphate (Ca3PO4)2 as a sole source of P. The pH of the medium was adjusted to 7.0. The transparent halo formation around the bacterial colonies, post-incubation for 7 days at 28 ± 2 °C, indicated positive P solubilization. Colonies surrounded by clear zones were cryo-preserved using 10% dimethyl sulfoxide (DMSO) at −80 °C for further use.
2.2. P Solubilization Measurement
Based on the results obtained on solid medium, the phosphate solubilization capacity of each bacterial isolate was determined in NBRIP broth. Briefly, 50 mL of NBRIP broth were inoculated with 100 µL of a bacterial suspension (OD600nm = 0.8, n = 3) (UV/visible spectrophotometer, UV-6300PC) and then incubated for 5 days at 28 ± 2 °C under shaking at 150 rpm (New Brunswick™ Innova® 43 Incubator Shaker Series). Next, the contents of each Erlenmeyer flask were centrifuged at 5000 rpm for 30 min, and the supernatant was filtered through 0.22 μm sterile syringe filter to get rid of insoluble materials [27]. The P content of the supernatant was estimated by colorimetric assay using SKALAR SAN++ system. The amount of total soluble P was calculated from the regression equation of a calibration curve and expressed in µg·mL−1. The pH of bacterial culture supernatants was also monitored [13].
2.3. In Vitro Assessment of PGP Properties
2.3.1. Indole3-Acetic Acid (IAA) Production Assay
The release of IAA was assessed by colorimetry. Tryptic soy broth (TSB) medium supplemented with 0.1% L-tryptophan [28] was inoculated with 100 μL of a bacterial culture (0.8 OD600nm) (n = 3) then incubated at 30 °C with shaking at 150 rpm [29]. Seven days post-incubation, the bacterial cultures were centrifuged for 10 min at 5000 rpm. One milliliter of each supernatant was vigorously mixed with 2 mL of Salkowski reagent (1 mL of 0.5 M FeCl3 and 50 mL of 35% HClO4) and then placed in the dark for 30 min. The absorbance of the resulting pink color, indicating the production of IAA, was measured at OD530nm. The concentrations of released IAA were calculated from the regression equation of a previously prepared standard curve and expressed in µg·mL−1.
2.3.2. Siderophores Production Assay
The production of siderophores by selected isolates was monitored according to the method described by Schwyn and Neilands (1987) [30] using chromium azurol S medium (CAS), which contains the ternary complex CAS-Fe+3-hexadecyltrimethyl-ammonium bromide as an indicator. Each isolate was spot inoculated on the solid CAS medium and the plates were incubated at 28 ± 2 °C for 5 days. The efficiency of PSB to produce siderophores was measured by monitoring the size of the halo zone and the intensity of the color change. The halo diameter was used as an indicator of siderophore production intensity.
2.3.3. Production of Hydrocyanic Acid
The bacterial capacity to produce hydrocyanic acid was also measured. Briefly, overnight culture of each bacterium (OD600nm = 0.8) was inoculated using a sterile loop on nutrient agar supplemented with 0.44% glycine [31]. Whatman papers soaked with a yellow solution (5% picric acid and 2% sodium carbonate) were placed into the lid of each Petri dish. Next, plates were sealed using parafilm and incubated for 4 days at 28 ± 2 °C. The bacterial HCN-production was indicated by Whatman paper color changing from yellow to orange.
2.3.4. Ammonia Production Assay
All the bacterial isolates were tested for ammonia production by using Cappuccino and Sherman’s (1992) method [32]. Fresh bacterial cultures (OD600nm = 0.8) were inoculated into 10 mL of peptone water, incubated (at 28 ± 2 °C), and shaken at 150 rpm. After 48 h of incubation, 0.5 mL of the Nessler reagent was added to the bacterial culture. Color changing from yellow to dark brown indicated ammonia production. Next, the absorbance was measured at OD450nm and the concentrations of ammonia were estimated based on a standard curve using various concentrations of ammonium sulphate ranging from 0 to 0.3 µmol·mL−1 [33].
2.3.5. Cellulase and Protease Production Assay
To evaluate the production of extracellular cellulase, bacteria were plated on the mineral–salt agar medium containing 0.4% (NH4)2SO4, 0.6% NaCl, 0.1% K2HPO4, 0.01% MgSO4, 0.01% CaCl2 with 0.5% carboxymethyl cellulose (CMC), and 2% agar: pH = 6.75 ± 0.25. The plates were then spot inoculated and incubated at 30 °C. After 2 days of incubation, a Congo red solution (1%) was added to the surface of each plate. Twenty minutes post incubation, the plates were flooded with a 1 M NaCl solution for 30 min. The appearance of a clear halo around the colonies was indicative of CMC degradation, reflecting the production of extracellular cellulase [34].
Protease activity assay was performed according to the method of Kavitha et al. (2013) [35] using a media composed of the following ingredients (g·L−1 of distilled water): pancreatic casein (5); yeast extract (2.5); glucose (1), and agar (15). Medium pH was adjusted to 7 and autoclaved. After cooling, 100 mL of a 10% skimmed milk solution was added to the medium and then seeded by spot inoculation method. After 48 h of incubation at 30 °C, the apparition of transparent halos around colonies indicated the protease activity [36].
2.3.6. Bacterial Biofilm Formation
To assess the biofilm formation, we used a colorimetric assay [37]. Briefly, bacterial cultures were first diluted to 1/100 with fresh TSB medium, and 200 μL of each bacterial suspension was inoculated, in triplicate, in a 48-well microtiter microplate, and further incubated at 30 °C for 24 h. A sterile non-inoculated broth was used as a control. Next, the cultures suspensions were discarded, and the bacterial pellets were washed three times by a phosphate buffered saline (PBS) solution to remove planktonic bacteria. Biofilm formation was revealed by addition of 2% crystal violet for 15 min at room temperature. Dye excess was removed by washing with distilled water. The bacterial biofilm was solubilized using 95% ethanol, and the OD600nm was measured using the VICTOR NivoTM multimode plate reader (Perkin Elmer, Casablanca, Morocco). The OD600nm values were considered as an index of bacterial adherence to the surface, forming biofilms.
2.3.7. Zinc Solubilization Assay
Zinc (Zn) solubilization by selected PSB was evaluated in Tris-mineral agar medium [38] containing in 1 L of distilled water: D-glucose 10 g, (NH4)2SO4 1 g, KCl 0.2 g, K2HPO4 0.1 g, MgSO4 2 g, pH = 6.75 ± 0.25. Prepared media were separately amended with three sources of insoluble Zn, namely, zinc oxide (ZnO, 15.23 mM), zinc phosphate (Zn3(PO4)2, 5.0 mM), and zinc carbonate (CO3Zn, 5.2 mM), at a 0.1% Zn final concentration [39]. Bacteria were spot inoculated on plates and further incubated at 30 °C for 10 days. Zn solubilization was associated with the observation of a clear zone around colonies. Efficiency was noted as absent (−), low (+), moderate (++), and strong (+++).
2.4. PSB Tolerance Assessment
2.4.1. Salt and Heat Tolerance
To investigate our isolated PSB salinity tolerance, a single colony of each strain was streaked on TSA plates supplemented with various concentrations of NaCl (from 0 to 14%) (w/v) and incubated at 30 °C for 48 h in the dark [25]. In addition, all isolates were examined for heat stress tolerance. Bacteria were streaked on TSA agar medium and the plates were then placed at different temperatures of 30, 37, 42, 45, and 50 °C for 24 h in the dark [40]. The salt and heat tolerance of each bacterium were qualitatively confirmed by observing its growth on salt-supplemented and nutrient agar media.
2.4.2. Antibiotic Resistance Profiling
Selected PSB were tested for resistance to antibiotics on TSA agar medium [41]. Plates were prepared by adding various concentrations of antibiotics, namely, ampicillin (100 μg·mL−1), kanamycin (50 μg·mL−1), tetracycline (10 μg·mL−1), streptomycin (100 μg·mL−1), chloramphenicol (20 μg·mL−1), and spectinomycin (60 μg·mL−1). A single colony of each bacterial strain was streaked on these plates and further evaluated for resistance after 24 h of incubation at 30 °C. E. coli DH5α, known for its sensitivity to tested antibiotics, was used as negative control [42].
2.4.3. Heavy Metal Tolerance
Due to the irrigation practices, some Moroccan soils, water, and crops were reported to be polluted by heavy metals, especially Zn, Cu, and Cd [43]. PSB strains were tested for their tolerance to three heavy metal salts, mainly cadmium sulfate (CdSO4), copper sulfate (CuO4S·5H2O), and nickel nitrate (N2NiO8). Stock solutions were prepared in distilled water, sterilized through 0.45 μm sterile syringe filters, and stored at 4 °C. Agar dilution method was followed [44]. Freshly prepared agar plates were supplemented with increasing soluble heavy metal salt concentrations ranging from 0 to 2.000 μg·mL−1 [24]. A single colony from each strain was streaked in the metal amended and control plates (without metals). Heavy metal tolerance was determined post 48 h incubation period at 30 °C.
2.5. Genotypic Identification
The polymerase chain reaction (PCR) was performed on bacterial 16S rRNA gene using forward pA (5′-AGAGTTTGATCCTGG CTCAG-3′) and reverse 926R primers (5′-CCGYCAATTYMTTTRAGTTT-3′) [45], generating an amplicon of 1000-bp. The PCR reaction mixture contained 23 μL of DNAase free water, 1 μL of both primers at 20 µM final concentration), 25 μL of PCR SuperMix (ThermoFisher 10572014, Casablanca, Morocco), and 1 μL of overnight bacterial cultures as DNA matrix. The amplification process was launched using the following program: initial denaturation step for 5 min at 94 °C, denaturation for 1 min at 94 °C, hybridization for 1 min at 52 °C, elongation step for 1 min 30 s at 72 °C with 35 cycles, and finally, an incubation for 10 min at 72 °C. Then, 5 μL of each PCR sample was loaded on 1% agarose gel, and the amplicon length was checked. The amplicons were sequenced and compared to the homologous sequences using the Blast program (Basic Local Alignment Search Tools) (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 27 December 2020) and SILVA database (https://www.arb-silva.de/, accessed on 27 December 2020). The phylogenetic dendrogram was constructed by the neighbor-joining method using Interactive Tree Of Life (iTOL) [46]. The sequences were deposited in the NCBI GenBank and the sequence accession numbers were provided.
2.6. Seed Germination Assay
Selected PSB were tested for their effects on quinoa seed germination and seedling growth. First, the surface of seeds was sterilized with 2% sodium hypochlorite solution, shaken in 70% ethanol, washed several times with sterilized distilled water, before being air-dried under laminar flow hood. Seed inoculation was carried out with bacterial suspensions, and the optical density (OD600nm) was adjusted to 0.8 (UV/visible spectrophotometer, UV-6300PC) [47]. An overnight culture of each bacteria was centrifuged at 5000 rpm for 10 min, and bacterial cell pellets were resuspended in sterile distilled water, vortexed for 10 s, and used for seed treatment. Bacterial suspensions were applied as a seed drench with a ratio of 10 mL per 90 seeds for 1 h [48]. Seeds treated with sterilized distilled water only were used as negative control [25]. Afterwards, seeds were air-dried and placed on sterilized Petri dishes containing 0.7% agar [49]. Thirty seeds (n = 3) were placed on each plate. Triplicates of each treatment were maintained in a dark space at 25 °C for 48 h [48]. The germination rate was monitored after 24 h of incubation. Seed germination was annotated when the radicle pierced the seed coat. At the third day, the plates were maintained at room temperature in a day/night cycle (~10/14 h) for another 3 days. The length of root and shoot, as well as the fresh and dry weights of seedlings, were measured after day 5. Germination rate and seedling vigor index were recorded using the following equations [50]:
where: “n” is the number of germinated seeds, and “N” is the total number of seeds.
Germination rate (%) = (n ÷ N) × 100
Vigor index = % germination × total plant length
2.7. Antifungal Assay
The bacterial isolates were tested in an in vitro preliminary assay (n = 3) to check their potential antagonism activities against Fusarium oxysporum growth. A loop of bacterial cells from 24-h-old cultures were streaked on potato dextrose agar (PDA) plates. Then, a fungal spot (5 mm diameter) of Fusarium oxysporum was located at the same distance from the bacterial line [51]. Plate containing only F.oxysporum was used as control. Plates were sealed with Parafilm, incubated at room temperature for 4 days, and daily observed to investigate the growth of Fusarium oxysporum in each plate. Strains QF11 and E. coli DH5α were used as positive (C+) and negative (C−) controls, respectively.
2.8. Statistical Analysis
All experiments were conducted in triplicate. The results of different parameters were subjected to analysis of variance (ANOVA) using single factor ANOVA in Microsoft Excel 2016, followed by comparison of means two by two according to the Tukey test at p ≤ 0.05.
3. Results
3.1. Isolation of Eleven P Solubilization Rhizobacteria
Following the qualitative assay on NBRIP plates, up to 76 single colonies were screened for P solubilization. Based on the solubilization efficiency index, bacteria were grown in NBRIP broth. The quantification of solubilized P in liquid medium allowed us to select 11 isolates named S6, S7, S8, QB1, QD1, QD14, QE3, QE4, QE6, QE7, and QE16. These isolates showed dispersed capacities to solubilize P, varying between 137.33 ± 3.78 and 320 ± 22.62 mg·L−1. The highest concentrations of soluble phosphate were expressed by QB1 (320 ± 22.62 mg·L−1), followed by S6 (315 ± 8.88 mg·L−1), while the lowest was recorded for QD1 (137.33 ± 3.78 mg·L−1) (Figure 1). P solubilization is often accompanied by the acidification of the medium. Thus, we measured the final pH of the culture supernatants. The pH values were negatively correlated to soluble phosphate concentrations (r = −0.84). Maximum pH decline was seen for S8 (4.06 ± 0.05), while the minimal value (4.9 ± 0.05) was recorded for the QD14 isolate.
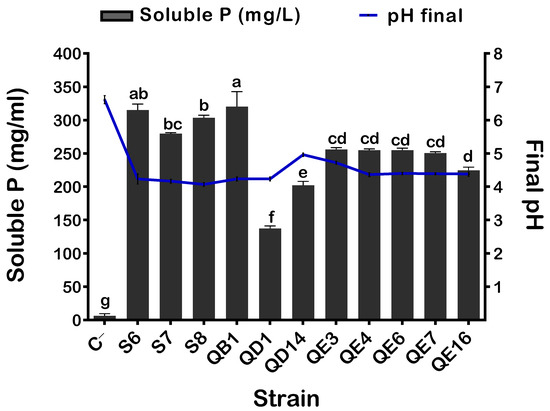
Figure 1.
Free phosphorus level and final pH of bacterial supernatants. The values represent means of replicates (n = 3) ± standard deviations. The letters in superscript indicate the statistically significant difference at 95% between treatments. Uninoculated media were used as a negative control (C−).
3.2. The QD1 and QE6 Isolates Are the Best IAA Producers
Bacterial IAA production is one of the most desirable functions of plant growth-promoting rhizobacteria (PGPR). Thus, our isolates were evaluated for their in vitro capacity to synthesize indole-3-acetic acid in TSB supplemented with 0.1% L-tryptophan as a precursor (See M&M). Following 7 days of incubation, all tested bacteria produced IAA with various concentrations ranging from 88.26 ± 4.8 to 438.9 ± 6.9 µg·mL−1 for S6 and QE6 isolates, respectively. The highest IAA values were recorded for QD1 and QE6 isolates (438.9 ± 6.9 mg·L−1), whereas the lowest value was recorded for the S6 isolate (88.28 ± 4.8 µg·mL−1) (Figure 2).
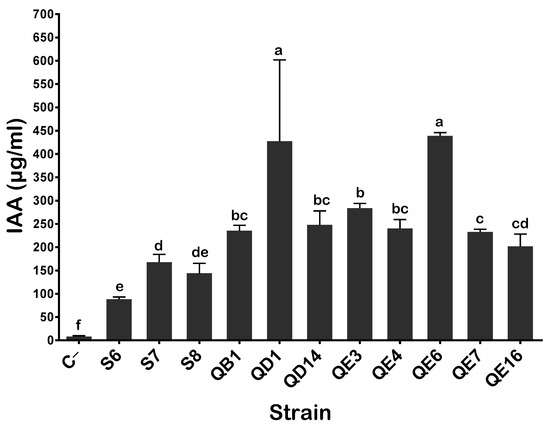
Figure 2.
IAA production by selected PSB strains in the presence of 0.1% of L-tryptophan. The values represent means of replicates (n = 3) ± standard deviations. The letters in superscript indicate the statistically significant difference at 95% between treatments. Uninoculated media were used as a negative control (C−).
3.3. The S8 Isolate Tolerated High Salt Concentrations and Is Thermoresistant
To assess PSB capacity to grow under salty conditions, bacteria were grown on plates containing increasing NaCl concentrations ranging from 0 to 14% (w/v) on TSA (See M&M). We found that bacterial growth was inhibited by 5% NaCl in all isolates except for S8 isolate, which tolerated up to 11% NaCl final concentration (Table 1).

Table 1.
Summary of other phenotypic traits of selected PSB isolates.
As temperature is a critical parameter, we next checked the isolates for optimal growth on plates incubated at various temperature from 30 to 50 °C. With an exception made for S6, S7, and QD1, the remaining isolates grew up to 42 °C (Table 1).
In both salt and heat test, no visible change in colony morphology was noted when colonies were compared to those growing in stress-free media.
3.4. The QB1 Isolate Exhibited a Strong Zn Solubilization
PSB isolates were next tested for their abilities to solubilize zinc on solid media containing ZnO, Zn3(PO4)2, or CO3Zn as a sole source of zinc. All tested isolates solubilized zinc from ZnO, while QB1, QE3, QE4, QE6, and QE7 mobilized zinc from the three tested substrates, with the high solubilization performed by the QB1 isolate (Table 1).
3.5. The QD1, S6, S7, S8 and QB1 Isolates Are The Best Siderophores Producers
Isolated PSB were tested for their siderophores production on CAS-agar medium (See M&M). QD1, S6, S7, S8, and QB1 produced a notable level of siderophores, which contrasted with low production in QD14, QE6, QE7, and QE16 isolates. Remarkably, siderophore production was not detected at all in the QE3 and QE4 isolates (Table 1).
3.6. The S6 and S7 Isolates Induced a Strong Biofilm Formation
The ability of our PSB isolates to form biofilm was assessed based on the colorimetric assay (see M&M). The values of the OD600nm of S6 (0.36) and S7 (0.34) contrasted with 0.03 measured in the control and in the QD14 isolate. These results indicated strong biofilm formation by S6 and S7. The remaining isolates could be considered as moderate biofilm producers (Figure 3).
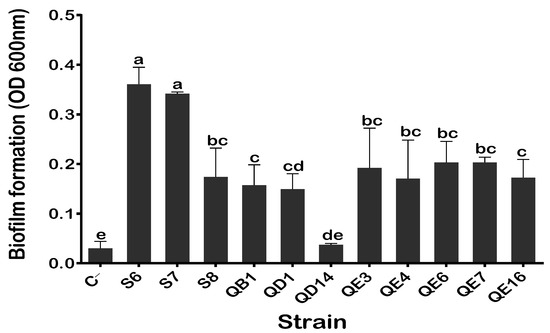
Figure 3.
Quantification of biofilm formation by the PSB isolates. The values represent means of replicates (n = 3) ± standard deviations. The letters in superscript indicate the statistically significant difference at 95% between treatments. Uninoculated media were used as a negative control (C−).
3.7. Ammonia and HCN Production and the Lack of Cellulase and Protease Activities
Ammonia and HCN exert various benefits for plants, mainly by acting as metabolic inhibitors against plant pathogens. Our PSB isolates produced ammonia. The highest values were measured for isolates S6 (0.68 μmol·mL−1) and S7 (0.58 μmol·mL−1) (Figure 4). The lowest value was detected in the QB1 isolate (0.15 μmol·mL−1). When tested for the production of HCN, all isolates were positive; however, the most remarkable performance was seen in the S6, QD1, and QE16 isolates (Table 1). Lastly, none of the tested isolates were able to produce either cellulase or proteases.
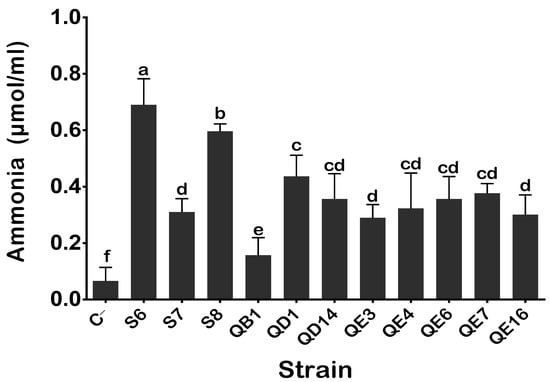
Figure 4.
Ammonia production by isolated PSB in peptone water. The values represent means of replicates (n = 3) ± standard deviations. The letters in superscript indicate the statistically significant difference at 95% between treatments. Uninoculated media were used as a negative (C−).
3.8. Antibiotic Resistance Profile of Selected PSB Isolates
PSB isolates were checked for their potential antibiotic resistance to six different antibiotics (See M&M). The isolates S8, QB1, QD1, QD14, QE6, and QE7 were sensitive to all tested antibiotics. The isolates S6, S7, QE3, QE4, and QE16 were resistant to ampicillin, S6 and S7 to chloramphenicol, and QE3, QE4, and QE16 to tetracycline. Lastly, none of the tested strains conferred resistance to either streptomycin, spectinomycin, or kanamycin (Table 2).

Table 2.
Antibiotic resistance patterns of selected PSB strains.
3.9. Selected PSB Isolates Withstood Heavy Metal Stress
We investigated the ability of our isolates to grow under increasing concentrations of CdSO4, CuO4S.5H2O, and N2NiO8 (see M&M). The QE3, QE4, QE4, QE6, QE7, and QE16 isolates tolerated up to 1.5 mg·mL−1 of CdSO4 and 1 mg·mL−1 of CuO4S.5H2O (Table 3). The growth of the S8 isolate was inhibited at 100 μg·mL−1 of CdSO4, 100 μg·mL−1 of CuO4S.5H2O, and at 500 μg·mL−1 of N2NiO8, whereas S6, S7, and QD1 were the less tolerant isolates (Table 3).

Table 3.
Heavy metal tolerance patterns of selected PSB strains.
3.10. PSB Isolates Are Related to The Genus of Pseudomonas, Bacillus, and Enterobacter
Phylogenetic analysis of the 16S rRNA gene revealed that 11 isolates belong to the genus of Pseudomonas, Bacillus, and Enterobacter (Table 4, Figure 5). The sequence of the 16S rRNA genes of S6, S7, and QD1 isolates were 100% identical to Pseudomonas frederiksbergensis, Pseudomonas sp., and Pseudomonas stutzeri, respectively. The S8 isolate corresponded to Bacillus atrophaeus GQJK17, QE7 to Enterobacter hormaechei subsp. Xiangfangensis, while the five isolates QB1, QD14, QE4, QE6, and QE16 were closely related (99.6 to 99.9% identity) to Enterobacter asburiae. Lastly, the QE3 isolate shared 99% sequence identity with Enterobacter sp. The provided GenBank accession number of each 16S rRNA sequence is represented in Table 4.

Table 4.
Genetic characterization of the selected PSB isolates based on 16S rRNA gene sequencing
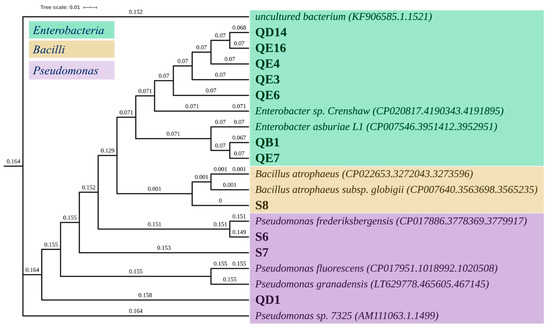
Figure 5.
Phylogenetic tree of the selected PSB strains generated using Interactive Tree of Life (iTOL) (https://itol.embl.de/, accessed on 29 December 2020). 16S rDNA 1000-bp gene sequences multiple alignment was done using the NCBI GenBank and SILVA databases.
3.11. Bacillus atrophaeus GQJK17 S8 Strain Induced the Best Seeds Germination and Quinoa Seedling Growth
Bacterization of quinoa seeds showed that B. atrophaeus GQJK17 S8 and E. asburiae (QB1 and QE16) strains increased the germination rate post 24 h of incubation (p < 0.05) (Figure 6). Synoptically coated seeds with bacterial suspensions of these three strains showed a significant increase in germination rate ranging from 16.35 to 20.41% as compared to non-inoculated seeds (treated with sterile distilled water). The remaining eight strains showed no significant difference in terms of germination rate promotion (p < 0.05). However, except for P. frederiksbergensis S6, the remaining strains, although at various extend levels, significantly enhanced seedling growth and biomass (Figure 6 and Figure 7). Indeed, the total length of seedlings was increased significantly by 18.64% up to 84.14% compared to control. Similarly, germinated quinoa seeds ranged between 42.7% and 112.9%, while the increase in fresh and dry weights varied from 31.03% to 102.06%, respectively. The germination vigor index values varied between 450, recorded in the negative control, and 781, recorded in B. atrophaeus S8. Seed germination and seedling growth differences between strains are highlighed in Figure 7. Data analysis indicates that seeds treated with strains B. atrophaeus GQJK17 S8, Pseudomonas sp. S7, P. Stutzeri QD1, and E. asburiae (QB1, QE16, QE7, QE4, and QE3) provide better results in terms of early plant growth compared to untreated seeds. Performances of studied strains can be classified in the following order: S8 > S7 > QB1 > QD1 > QE16 > QE7 > QE4 > QE3 > QE6 > QD14 > S6 (Figure 7).
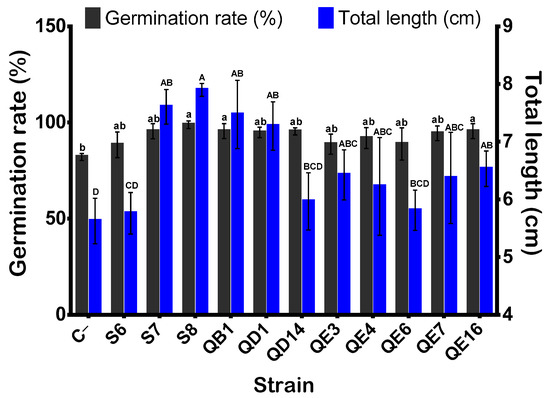
Figure 6.
Effect of bacterial inoculation on quinoa seed germination rate and total lengths of seedlings. Germinated seeds and total lengths of seedlings were monitored after 24 h and 5 days of incubation, respectively. The values represent means of replicates (n = 3) ± standard deviations. The letters in superscript indicate the statistically significant difference at 95% between treatments.
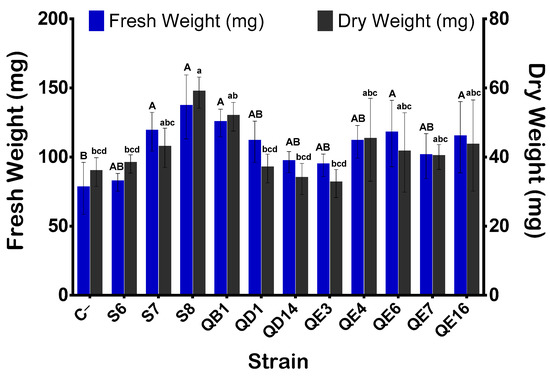
Figure 7.
Effect of bacterial inoculation on seedlings biomass. Fresh weights of seedlings were recorded after 5 days of incubation and weights of 48-oven dried seedlings were measured. The values represent means of replicates (n = 3) ± standard deviations. The letters in superscript indicate the statistically significant difference at 95% between treatments.
3.12. Five PSB Strains Exhibit Anti-Fusarium Oxysporum Activity
Here, we investigated the anti-fungic activity of the 11 strains using QA2 isolate as a positive control. Three different phenotypes emerged in the presence of Fusarium oxysporum (Figure 8). The first class included strains E. hormaechei subsp. xiangfangensis QE7, E. asburiae QE4, and Enterobacter sp. QE3, with no antagonist effect (no halo formation). The second class included the E. asburiae strains QD14, QB1, and QE6, which exhibited a moderate antagonism effect (Figure 9). The third class included five strains; E. asburiae QE16, P. stutzeri QD1, P. frederiksbergensis S6, Pseudomonas sp. S7, B. atrophaeus GQJK17 S8; all of them induced the formation of halos limiting fungi dissemination. Finally, the aspect of Pseudomonas sp. S7 on the plate was likely associated to its ability to secrete exopolysaccharide (EPS).
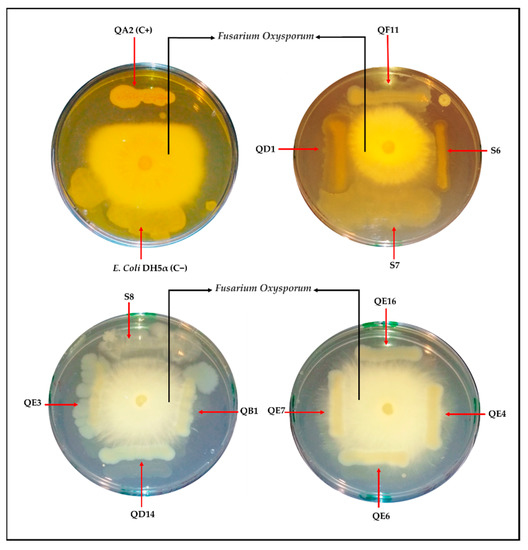
Figure 8.
In vitro antagonism assay of selected PSB strains with Fusarium oxysporum using potato dextrose a(PDA) plates. Bacterial strains were streaked on divided plates in different compartments (the four sides) with F. oxysporum (in the center) and incubated for 4 days at room temperature. Strains QA2 and E. coli DH5α were used as positive (C+) and negative (C−) controls, respectively.
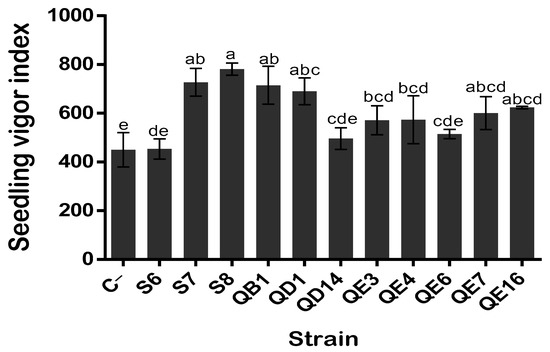
Figure 9.
Effect of bacterial inoculation on quinoa seedling vigor index. The values represent means of replicates (n = 3) ± standard deviations. The letters in superscript indicate the statistically significant difference at 95% between treatments.
4. Discussion
4.1. Isolation and Identification of Eleven PSB Strains from Rhizosphere of the Quinoa Plant
The present study describes the capacity of our quinoa phosphate-solubilizing rhizobacteria to stimulate quinoa seed germination and seedling growth. Based on phosphate solubilization criteria, out of 76 isolates, 11 PSB were isolated on NBRIP plates. Genotyping analysis revealed they belonged to three different genera: one Bacillus, three Pseudomonas, and seven Enterobacter. Comparatively, Ortuno et al. (2013) [52] reported that the native microorganisms associated with quinoa plants (isolated from leaves, stems, and roots) belong to Bacillus (B. amyloliquefaciens, B. tequilensis, B. subtilis, B. pumilus, B. licheniformis, B. horikoshii, B. atrophaeus, and B. thuringiensis) and to Paenibacillus sp. In this study, we isolated seven Enterobacter strains, including E. asburiae (QB1, QD14, QE4, QE6, and QE16), Enterobacter sp. QE3, and E. hormaechei subsp. Xiangfangensis QE7. We also isolated three Pseudomonas species (P. frederiksbergensis S6, Pseudomonas sp. S7, and P. stutzeri QD1), and one Bacillus atrophaeus GQJK17 S8.
Considering the abundance of PSB in different soils and their functional diversity, it becomes highly desirable to search for PSB with multitasking properties. It is well known now that plant immune signaling and root microbiome assembly are functionally linked. To evaluate the capacity of the 11 PSB strains to stimulate quinoa plant growth and to tolerate abiotic stress under in vitro conditions, we performed a set of conventional tests reflecting plant improvement growth.
4.2. What Are the PGPR Features of Quinoa Isolated PSB Strains?
When used as inoculants for quinoa seed bacterization, eight strains positively affected seedling growth, although to different degrees (Figure 9 and Figure 10). The highest vigor index of quinoa plant growth was obtained upon inoculation with Pseudomonas sp. S7, B. atrophaeus GQJK17 S8, and E. asburiae QB1 strains. B. atrophaeus GQJK17 S8 was the best seedling inducer (Figure 9). This result is likely attributed to the strains’ capacity to produce phytohormones. Indeed, the three strains are among the P solubilizers and, in addition, they produced intermediate levels of IAA. Conversely, P. frederiksbergensis S6 produced a low amount of IAA, which may explain its reduced capacity to stimulate quinoa plant growth (vigor index = 453.5). Hence, despite its higher P solubilization and the best HCN and ammonia production, P. frederiksbergensis S6 did not stimulate efficient growth of seedling.
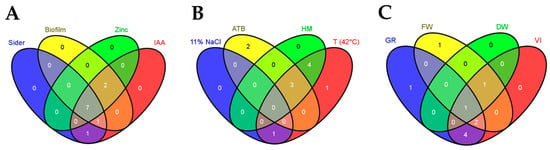
Figure 10.
Venn diagrams illustrating the overlapping relationship between the relevant (A) PGP attributes, (B) extreme growth properties, and (C) quinoa seed germination parameters using selected PSB. Sider (siderophore), ATB (antibiotic resistance), HM (heavy metal tolerance), T (temperature), GR (germination rate), FW (fresh weight), DW (fry weight), VI (vigor index). The Venn diagrams were drawn using VENNY 2.0 (https://bioinfogp.cnb.csic.es/tools/venny/, accessed on 20 November 2020).
Production of siderophores is a desired trait of dual interest as they represent an important source of assimilable iron for plants and act as a stimulatory signal for the production of metabolites involved in the control of phytopathogens [53]. We demonstrated that strains Pseudomonas sp. S7, B. atrophaeus GQJK17 S8, and E. asburiae QB1 produced siderophores and exhibited antifungal activity on Fusarium oxysporum (Figure 8). Comparatively, due to its capacity to produce lipopeptides and to secrete proteins and volatile compounds, B. atrophaeus CAB-1 was shown to display a high antifungal activity against various pathogens [54]. Furthermore, when applied to seedlings and harvested fruits, another B. atrophaeus UCMB-5137 was shown to colonize plant roots and to improve plants’ resistance to bacterial and fungal phytopathogens [54]. The anti-Fusarium solani activity was also reported in the B. atrophaeus GQJK17 strain, isolated from Lycium barbarum [55].
The antagonist assay showed that Pseudomonas sp. S7 produced exopolysaccharides (EPS), likely known to inhibit the dissemination of Fusarium oxysporum. There is increasing evidence that rhizobacteria improve plant growth via the production of plant growth substances rather than by their action of solubilizing phosphate. Plants can induce defense responses (elicitation of induced systemic resistance, ISR) against biotic or abiotic stresses by producing agents against pathogens [55,56]. When a quinoa crop is introduced in areas outside its traditional growing regions, it becomes very sensitive to fungal diseases. For example, in 1999, quinoa stands were severely damaged in the Czech Republic by Fusarium avenaceum and Fusarium spp fungi, which were responsible for damping-off of quinoa seedlings [8].
Besides the role of siderophores in fighting harmful effects caused by phytopathogenic microorganisms, HCN and ammonia also play an important role in the biocontrol of a wide range of fungi-caused diseases in plants [57,58,59]. Ammonia affects pathogen growth and fungal activity by acting as a metabolic inhibitor [58,60], and at the same time, it provides nitrogen directly to the plant [61]. Under our hands, both HCN and ammonia were significantly produced by E. asburiae QB1 and B. atrophaeus GQJK17 S8, which may explain their remarkable anti-Fusarium activities.
The remaining four E. asburiae strains (QD14, QE4, QE6, and QE16) showed a significant growth effect on quinoa compared to E. asburiae QB1. Strain E. asburiae QE6 was the stronger IAA producer but not the best in terms of growth stimulation (vigor index 514.67). In literature, Enterobacter sp. Fs-11, an isolated strain from the sunflower rhizosphere, exhibited a remarkable IAA production [62]. Very recently, we reported that the E. asburiae QF11 strain produced up to 795.31 μg·mL−1 of IAA and promoted quinoa root development [63]. Although strain E. asburiae QE6 produced HCN, ammonia, and a reduced amount of siderophores, it lacked the anti-Fusarium oxysporum activity (Figure 9). We showed that E. asburiae QE16 stimulated seedling growth (growth vigor index = 623.36) and produced a high level of HCN. Collectively, it appears clearly that the identified seven E. asburiae strains induce various effects on seedling growth. Assuming that these strains originated from the same rhizospheric microbiome, one may underline their potential synergic action to promote plant growth. In-line with this, other Enterobacter strains were associated with the secretion of homoserine lactones quorum sensing (QS) molecules (AHL, C4-HSL, and C6-HSL), responsible for bacterial communication [64] through the regulation of genes expression in response to bacterial population density [65].
P. stutzeri QD1 was also among the strains that remarkably improved quinoa seed germination. P. stutzeri’s simultaneous capacity for nitrogen fixation and denitrification may be of relevance to overall nitrogen cycling in several ecosystems [66]. In China, rice paddy rhizospheric P.stutzeri A15 is extensively used as an inoculant for rice culture [67,68]. P. stutzeri QD1 is not a strong ammonia producer but its remarkable capacity to fix N2 could explain its biostimulator effect. Some strains of P. stutzeri synthesize siderophores [69,70]; for example, P. stutzeri RC7 produces a catechol-like siderophore [71]. However, no siderophores were detected in P. stutzeri YPL-1 [72]. Interestingly, our P. stutzeri QD1 was the best producer of both siderophores and HCN, supporting its anti-Fusarium oxysporum activity.
Plant growth by PGPR in most cases involves rhizospheric biofilm formation [73,74]. Both Pseudomonas strains S6 and S7 form biofilms, which may explain their resistance to acidity, heavy metal, and antibiotics, as previously reported [75]. We report here that P. frederiksbergensis S6, Enterobacter asburiae QD14, and Enterobacter asburiae QE6 improved quinoa seedling vigor index but Pseudomonas sp. S7, Enterobacter asburiae QB1, and B. atrophaeus GQJK17 S8 remain the best performants.
4.3. Antibiotic, Salt, Heavy Metal, and Rare Elements Resistance Effects on PSB Strain Behavior
Zinc (Zn) is a rare element required for several biological processes within plants, such as enzyme activation and protein synthesis. Zn deficiency occurs in calcareous, sodic, sandy, and in intensively cultivated soils, which consequently affects the yield of crops, such as maize, rice, potato, wheat, and tomato [39]. The use of zinc fertilizers overcomes this problem [76,77,78]. Zn predominantly occurs in soil as zincite (zinc oxide, ZnO) and sphalerite (zinc sulfide, ZnS), which may alter the plant−soil ecosystem [79,80]. Here, we demonstrated a heterogenous Zn solubilization efficiency by the studied strains. For example, E. asburiae QB1 showed the highest vigor index of quinoa seed growth, a finding likely attributed to its capacity to solubilize complex forms of Zn. This solubilization could be mediated by numerous mechanisms, one of which is acidification. The culture supernatant of E. asburiae QB1 was found to be the more acidic one, which was likely due to the production of organic acids that decreased the pH via the Zn cations sequestration. The second reported Zn solubilization mechanism is linked to siderophore production [81]. Again, E. asburiae QB1 was the best siderophore producer. However, no direct correlation has been established between Zn solubilization and siderophore production yet. For example, strains E. asburiae QE3 and QE4 did not produce siderophores, although they solubilized Zn. Comparatively, it is well known that Pseudomonas and Bacillus strains are good Zn solubilizers [38,82]. Under our hands, we showed that Pseudomonas strains (S6 and S7) solubilized the three tested complex forms of Zn, which contrasts with P. stutzeri QD1, which solubilized only ZnO.
Beside Zn solubilization, E. asburiae (QB1, QD14, QE4, QE6, and QE16) strains tolerated up to 0.5 mg·L−1 of copper and showed adaptive response to both 1.5 mg·L−1 of cadmium and 1.0 mg·L−1 of copper. As expected, nickel was highly toxic to all strains, which corroborates previous findings [83]. Kang et al. (2015) [84] reported that the association of E. asburiae KE17 with soybean not only promoted growth but also mitigated the toxic stress effects of Cu and Zn. The third mechanism used by some bacteria to solubilize divalent heavy metals, such as Zn+, Cu+, and Ni2+, involves an active efflux transporter present at the bacterial membrane. Tamilnadu J. et al. (2016) described CZC (Cobalt-zinc-cadmium) genes in the genome of E. asburiae [85], encoding resistance to Cd2+, Zn2+, and Co2+ by metal-dependent efflux driven by the proton motive force. We analyzed the published sequence of the complete genome of a clinical isolate of E. asburiae [86] and found that several genes were conserved (yebI, znuB, ZntB, ZnuC, zntA, etc.) encoding for zinc ABC (ATP-binding cassette) transporter permease; zinc-binding protein; zinc transporter ZntB; the zinc import ATP-binding protein ZnuC; zinc ABC transporter substrate-binding protein; and, cadmium-, zinc-, and mercury-transporting ATPase. Bacteria also adapt themselves to heavy metal stress by developing various resistance mechanisms via intracellular metal-binding proteins [87]. The ability of our PSB strains, especially Enterobacteria, to tolerate higher concentrations of heavy metal provides a scope of their potential use as potential bioremediator agents, as previously reported [88].
Zn is known to be a common toxic metal that co-selects for antibiotic resistance, especially when genes associated with resistance are clustered on the same genetic element. Antibiotic resistance is a major concern as emergence and spread become increasingly worse. Contact with heavy metals may co-select antibiotic resistant bacteria (ARB) [89], which is one of the major neglected issues in deploying bacterial-based biofertilizers. Many PGPR biofertilizers harbor more than one plasmid and chromosome-borne antibiotic resistance genes (ARGs) [90,91]. Hence, the potential risks of transferring ARGs to neighboring bacteria and even to crops [90] is a major challenge. We proceeded here to the evaluation of resistance profiles against six available antibiotics. Two Pseudomonas strains, i.e., S6 and S7 showed resistance to both ampicillin and chloramphenicol, and three Enterobacter strains (QE3, QE4, and QE16) exhibited resistance to ampicillin and tetracycline (Figure 10).
To fight biotic stress such as salinity, plants induce several physiological responses, including phytohormone synthesis, antioxidant production, and nutrient uptake regulation. Furthermore, plant-associated bacteria are good boosters for plant growth under harsh conditions [92,93]. We demonstrated that B. atrophaeus GQJK17 S8 remarkably mitigated up to 11% NaCl (Figure 10), whereas the growth of the remaining strains was halted at 5% NaCl.
5. Conclusions
In this study, Bacillus atrophaeus GQJK17 S8 and Enterobacter asburiae QB1 emerged as potential bio-safe strains that induced early quinoa plant growth by improving the germination rate, seedling biomass, and growth vigor index. Their use may enhance greatly the assimilation of nutrients brought by chemical fertilizers and participate to generate healthy plants. Further agronomic tests are required to underpin the mechanisms by which our elite strains promote plant growth. To summarize, we propose here the use of either E. asburiae QB1 or B. atrophaeus GQJK17 S8 strain as a seed’s inoculant to prevent damping-off of quinoa seedlings by reducing heavy metal toxicity and mediating protection against fungal phytopathogens.
Author Contributions
Conceptualization, L.B., A.A., and I.M.; Funding acquisition, L.B. and A.A.; Investigation I.M. and L.B.; Methodology, I.M., N.F., and M.H. Project administration: L.B. and A.A.; Supervision, L.B. and A.A.; Validation, I.M., L.B., and A.A.; statistical analysis S.B. and I.M.; Writing—original draft, I.M.; Writing—review and editing, L.B. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a welcome grant from the Mohammed VI Polytechnic University (UM6P) of Ben Guerir, Morocco.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jacobsen, S.-E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.-E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Stikic, R.; Glamoclija, D.; Demin, M.; Vucelic-Radovic, B.; Jovanovic, Z.; Milojkovic-Opsenica, D.; Jacobsen, S.-E.; Milovanovic, M. Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J. Cereal Sci. 2012, 55, 132–138. [Google Scholar] [CrossRef]
- Benlhabib, O.; Yazar, A.; Qadir, M.; Lourenço, E.; Jacobsen, S.-E. How Can We Improve M editerranean Cropping Systems? J. Agron. Crop Sci. 2014, 200, 325–332. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.-E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Jacobsen, S.-E. Adaptation of quinoa (Chenopodium quinoa) to Northern European agriculture: Studies on developmental pattern. Euphytica 1997, 96, 41–48. [Google Scholar] [CrossRef]
- Jacobsen, S.; Bach, A. The influence of temperature on seed germination rate in quinoa (Chenopodium quinoa Willd. Seed Sci. Technol. 1998, 26, 515–523. [Google Scholar]
- Dřímalková, M. Mycoflora of Chenopodium quinoa Willd. seeds. Plant Prot. Sci. 2003, 39, 146–150. [Google Scholar]
- Zablotowicz, R.M.; Tipping, E.M.; Lifshitz, R.; Kloepper, J.W. Plant growth promotion mediated by bacterial rhizosphere colonizers. In The Rhizosphere and Plant Growth; Springer: Berlin/Heidelberg, Germany, 1991; pp. 315–326. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Wani, P.A.; Zaidi, A.; Khan, A.A.; Khan, M.S. Effect of phorate on phosphate solubilization and indole acetic acid releasing potentials of rhizospheric microorganisms. Ann. Plant Prot. Sci. 2005, 13, 139–144. [Google Scholar]
- Kumar, V.; Kumar Behl, R.; Narula, N. Establishment of phosphate-solubilizing strains of Azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under green house conditions. Microbiol. Res. 2001, 156, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Park, M.; Madhaiyan, M.; Seshadri, S.; Song, J.; Cho, H.; Sa, T. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol. Biochem. 2005, 37, 1970–1974. [Google Scholar] [CrossRef]
- Pradhan, N.; Sukla, L. Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr. J. Biotechnol. 2006, 5, 850–854. [Google Scholar]
- Sharma, K.; Dak, G.; Agrawal, A.; Bhatnagar, M.; Sharma, R. Effect of phosphate solubilizing bacteria on the germination of Cicer arietinum seeds and seedling growth. J. Herb. Med. Toxicol. 2007, 1, 61–63. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Plassard, C.; Robin, A.; Le Cadre, E.; Marsden, C.; Trap, J.; Herrmann, L.; Waithaisong, K.; Lesueur, D.; Blanchart, E.; LARDY, L.; et al. Améliorer la biodisponibilité du phosphore: Comment valoriser les compétences des plantes et les mécanismes biologiques du sol. Innov. Agron. 2015, 43, 115–138. [Google Scholar]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- McGill, W.; Cole, C. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 1981, 26, 267–286. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Adnan Shahid, M.; Yang, J. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front. Microbiol. 2018, 9, 2507. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Chandra Dash, M.; Sarkar, S.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Shahid, I.; Mehnaz, S.; Malik, K. Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on growth of wheat (Triticum aestivum L.). Microbiol. Res. 2017, 205. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.; Lindow, S.E. Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl. Environ. Microbiol. 2005, 71, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Latif Khan, A.; Ahmed Halo, B.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I.-J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solarium lycopersicum. Electron. J. Biotechnol. 2016, 19, 58–64. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Lorck, H. Production of hydrocyanic acid by bacteria. Physiol. Plant. 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Cappuccino, J.C.N.S. Microbiology: A Laboratory Manual, 3rd ed.; Co, B.C.P., Ed.; Pearson: London, UK, 1992. [Google Scholar]
- Chrouqi, L.; Lahcen, O.; Jadrane, I.; Koussa, T.; Alfeddy, M.N. Screening of soil rhizobacteria isolated from wheat plants grown in the Marrakech region (Morocco, North Africa) for plant growth promoting activities. JMES 2017, 8, 3382–3390. [Google Scholar]
- Kasana, R.C.; Salwan, R.; Dhar, H.; Dutt, S.; Gulati, A. A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr. Microbiol. 2008, 57, 503–507. [Google Scholar] [CrossRef]
- Kavitha, T.; Nelson, R.; Jesi, S.J. Screening of rhizobacteria for plant growth promoting traits and antifungal activity against charcoal rot pathogen Macrophomina phaseolina. Int. J. Pharma Bio Sci. 2013, 4, 177–186. [Google Scholar]
- Smibert, R. Phenotypic characterization. In Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994; pp. 607–654. [Google Scholar]
- Christensen, G.D.; Simpson, W.A.; Younger, J.; Baddour, L.; Barrett, F.; Melton, D.; Beachey, E. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Muralidharan, G. Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. Eur. J. Soil Biol. 2016, 76, 1–8. [Google Scholar] [CrossRef]
- Khanghahi, M.Y.; Ricciuti, P.; Allegretta, I.; Terzano, R.; Crecchio, C. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ. Sci. Pollut. Res. 2018, 25, 25862–25868. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.S.; Naik, J.H.; Chaudhari, S.; Amaresan, N. Characterization of culturable bacteria isolated from hot springs for plant growth promoting traits and effect on tomato (Lycopersicon esculentum) seedling. C. R. Biol. 2017, 340, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Armalytė, J.; Skerniškytė, J.; Bakienė, E.; Krasauskas, R.; Šiugždinienė, R.; Kareivienė, V.; Kerzienė, S.; Klimienė, I.; Sužiedėlienė, E.; Ružauskas, M. Microbial Diversity and Antimicrobial Resistance Profile in Microbiota From Soils of Conventional and Organic Farming Systems. Front. Microbiol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Halda-Alija, L. Incidence of antibiotic-resistant Klebsiella pneumoniae and Enterobacter species in freshwater wetlands. Lett. Appl. Microbiol. 2004, 39, 445–450. [Google Scholar] [CrossRef]
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Cervantes-Vega, C.; Chavez, J.; Córdova, N.; Amador, J.V. Resistance to metals by Pseudomonas aeruginosa clinical isolates. Microbios 1986, 48, 159–163. [Google Scholar] [PubMed]
- Hill, J.E.; Hemmingsen, S.M.; Town, J.R. Strong PCR Primers and Primer Cocktails. 2009. Available online: https://patents.google.com/patent/US7507535B2/en (accessed on 4 August 2020).
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Asghar, H.; Arshad, M.; Shahbaz, M. Screening of multi-traits rhizobacteria to improve maize growth under axenic conditions. J. Anim. Plant Sci. 2013, 23, 514–520. [Google Scholar]
- Ji, S.H.; Gururani, M.A.; Chun, S.C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef]
- Islam, S.; Mannan Akanda, A.; Prova, A.; Islam, T.; Hossain, M. Isolation and Identification of Plant Growth Promoting Rhizobacteria from Cucumber Rhizosphere and Their Effect on Plant Growth Promotion and Disease Suppression. Front. Microbiol. 2016, 6, 1360. [Google Scholar] [CrossRef]
- De Boer, W.; Wagenaar, A.-M.; Klein Gunnewiek, P.J.; Van Veen, J.A. In vitro suppression of fungi caused by combinations of apparently non-antagonistic soil bacteria. FEMS Microbiol. Ecol. 2007, 59, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, N.; Castillo, J.A.; Claros, M.; Navia, O.; Angulo, M.; Barja, D.; Gutiérrez, C.; Angulo, V. Enhancing the sustainability of quinoa production and soil resilience by using bioproducts made with native microorganisms. Agronomy 2013, 3, 732–746. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Sella, S.R.; Vandenberghe, L.P.; Soccol, C.R. Bacillus atrophaeus: Main characteristics and biotechnological applications—A review. Crit. Rev. Biotechnol. 2015, 35, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Walsh, U.F.; Morrissey, J.P.; O’Gara, F. Pseudomonas for biocontrol of phytopathogens: From functional genomics to commercial exploitation. Curr. Opin. Biotechnol. 2001, 12, 289–295. [Google Scholar] [CrossRef]
- Howell, C.; Beier, R.; Stipanovic, R. Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Phytopathology 1988, 78, 1075–1078. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Noumavo, P.A.; Baba-Moussa, F.; Salami, H.A.; Sina, H.; Sèzan, A.; Bankolé, H.; Adjanohoun, A.; Baba-Moussa, L. Characterization of potential plant growth promoting rhizobacteria isolated from Maize (Zea mays L.) in central and Northern Benin (West Africa). Appl. Environ. Soil Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Bensidhoum, L.; Nabti, E.; Tabli, N.; Kupferschmied, P.; Weiss, A.; Rothballer, M.; Schmid, M.; Keel, C.; Hartmann, A. Heavy metal tolerant Pseudomonas protegens isolates from agricultural well water in northeastern Algeria with plant growth promoting, insecticidal and antifungal activities. Eur. J. Soil Biol. 2016, 75, 38–46. [Google Scholar] [CrossRef]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Hameed, S.; Zafar, M.; Tahir, M.; Ijaz, M.; Tariq, M.; Hussain, K.; Ali, A. Enterobacter sp. strain Fs-11 adapted to diverse ecological conditions and promoted sunflower achene yield, nutrient uptake, and oil contents. Braz. J. Microbiol. 2019, 50, 459–469. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Sulaiman, J.; Chen, J.W.; Yin, W.-F.; Chan, K.-G. Quorum sensing activity of Enterobacter asburiae isolated from lettuce leaves. Sensors 2013, 13, 14189–14199. [Google Scholar] [CrossRef]
- Smith, R.S.; Iglewski, B.H.P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003, 6, 56–60. [Google Scholar] [CrossRef]
- Lalucat, J.; Bennasar, A.; Bosch, R.; García-Valdés, E.; Palleroni, N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 2006, 70, 510–547. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.; Kloepper, J. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Barraquio, W.L.; Segubre, E.M.; Gonzalez, M.A.S.; Verma, S.C.; James, E.K.; Ladha, J.K.; Tripathi, A.K. Diazotrophic enterobacteria: What is their role in the rhizosphere of rice. In Quest Nitrogen Fixat Rice; International Rice Research Institute: Los Baños, Philippines, 2000; pp. 93–118. [Google Scholar]
- MEYER, J.-M.; Abdallah, M.A. The siderochromes of non-fluorescent pseudomonads: Production of nocardamine by Pseudomonas stutzeri. Microbiology 1980, 118, 125–129. [Google Scholar] [CrossRef]
- Meyer, J.-M.; Geoffroy, V.A.; Baida, N.; Gardan, L.; Izard, D.; Lemanceau, P.; Achouak, W.; Palleroni, N.J. Siderophore typing, a powerful tool for the identification of fluorescent and nonfluorescent pseudomonads. Appl. Environ. Microbiol. 2002, 68, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.N.; Patel, H.N.; Desai, S.B. Isolation and partial characterization of catechol-type siderophore fromPseudomonas stutzeri RC 7. Curr. Microbiol. 1990, 20, 283–286. [Google Scholar] [CrossRef]
- Lim, H.-S.; Kim, Y.-S.; Kim, S.-D. Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl. Environ. Microbiol. 1991, 57, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, G.; Weerasekara, M.; Seneviratne, K.; Zavahir, J.; Kecskés, M.; Kennedy, I. Importance of biofilm formation in plant growth promoting rhizobacterial action. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 81–95. [Google Scholar]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Snehi, S.K.; Singh, V. Role of PGPR in biofilm formations and its importance in plant health. Biofilms Plant Soil Health 2017, 27. [Google Scholar] [CrossRef]
- Ali, S.; Shah, A.; Arif, M.; Miraj, G.; Ali, I.; Sajjad, M.; Farhatullah, M. Khan and NM Khan. 2009. Enhanc. Wheat Grain Yield Yield Compon. Foliar Appl. Zinc Boron. Sarhad J. Agric. 2009, 25, 15–19. [Google Scholar]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.M.; Broadley, M.R. Approaches to reduce zinc and iron deficits in food systems. Glob. Food Secur. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Ahmad, H.R.; Aziz, T.; Hussain, S.; Akraam, M.; Sabir, M.; Hanafi, M.M. Zinc-enriched farm yard manure improves grain yield and grain zinc concentration in rice grown on a saline-sodic soil. Int. J. Agric. Biol. 2012, 14, 787–792. [Google Scholar]
- Isaure, M.-P.; Laboudigue, A.; Manceau, A.; Sarret, G.; Tiffreau, C.; Trocellier, P.; Lamble, G.; Hazemann, J.-L.; Chateigner, D. Quantitative Zn speciation in a contaminated dredged sediment by μ-PIXE, μ-SXRF, EXAFS spectroscopy and principal component analysis. Geochim. Cosmochim. Acta 2002, 66, 1549–1567. [Google Scholar] [CrossRef]
- Adele, N.C.; Ngwenya, B.T.; Heal, K.V.; Mosselmans, J.F.W. Soil bacteria override speciation effects on zinc phytotoxicity in zinc-contaminated soils. Environ. Sci. Technol. 2018, 52, 3412–3421. [Google Scholar] [CrossRef]
- Saravanan, V.; Kumar, M.R.; Sa, T. Microbial zinc solubilization and their role on plants. In Bacteria in Agrobiology: Plant Nutrient Management; Springer: Berlin/Heidelberg, Germany, 2011; pp. 47–63. [Google Scholar]
- Saravanan, V.S.; Subramoniam, S.R.; Raj, S.A. Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Braz. J. Microbiol. 2004, 35, 121–125. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Shahid, M.A.; Yang, J. Corrigendum: Impact of Salicylic acid and PGPR on the Drought Tolerance and Phytoremediation potential of Helianthus annus. Front. Microbiol. 2019, 10, 2222. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Khan, A.L.; Lee, K.E.; Lee, J.D.; Lee, I.J. Enterobacter asburiae KE 17 association regulates physiological changes and mitigates the toxic effects of heavy metals in soybean. Plant Biol. 2015, 17, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Joonu, J.; Averal, H.I. Heavy metal resistant CZC genes identification in Bacillus cereus, Enterobacter asburiae and Pseudomonas aeruginosa isolated from BHEL industry, Tamilnadu. J. Microbiol. Biotechnol. 2016, 5, 27–31. [Google Scholar]
- Liu, F.; Yang, J.; Xiao, Y.; Li, L.; Yang, F.; Jin, Q. Complete genome sequence of a clinical isolate of Enterobacter asburiae. Genome Announc. 2016, 4. [Google Scholar] [CrossRef]
- Hashem, A.; Abed, K. Arsenic, lead and microorganisms in hair and nails of some women from Saudi Arabia. J. Med. Sci. 2002, 2, 82–84. [Google Scholar] [CrossRef][Green Version]
- Calomiris, J.J.; Armstrong, J.L.; Seidler, R.J. Association of metal tolerance with multiple antibiotic resistance of bacteria isolated from drinking water. Appl. Environ. Microbiol. 1984, 47, 1238–1242. [Google Scholar] [CrossRef]
- Abelenda-Alonso, G.; Padullés, A.; Rombauts, A.; Gudiol, C.; Pujol, M.; Alvarez-Pouso, C.; Jodar, R.; Carratalà, J. Antibiotic prescription during the COVID-19 pandemic: A biphasic pattern. Infect. Control Hosp. Epidemiol. 2020, 41, 1371–1372. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Kang, Y.; Shen, M.; Xia, D.; Ye, K.; Zhao, Q.; Hu, J. Caution of intensified spread of antibiotic resistance genes by inadvertent introduction of beneficial bacteria into soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 576–582. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, E.; Rahimian, H.; Pirdashti, H.; Nematzadeh, G.A. Phosphate solubilization potential and modeling of stress tolerance of rhizobacteria from rice paddy soil in northern Iran. World J. Microbiol. Biotechnol. 2014, 30, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).