Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Materials and Growth Conditions
2.3. Measurements
2.4. Statistical Analysis
3. Results
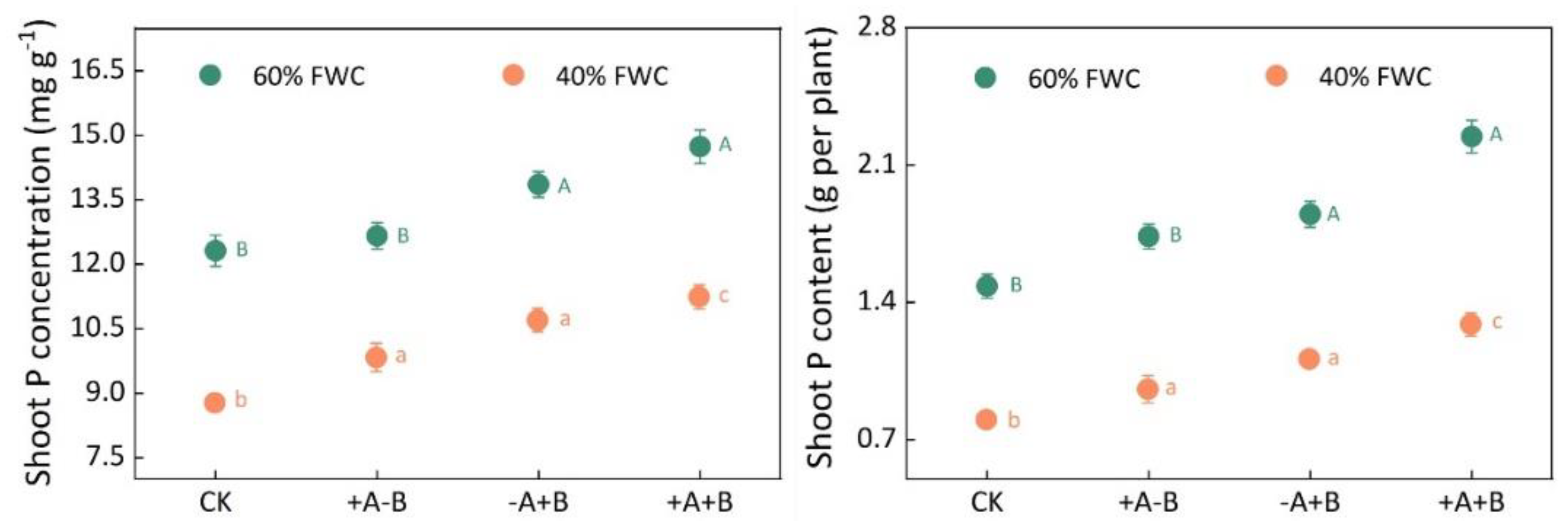
3.1. Biomass and Phosphorus Content of Maize
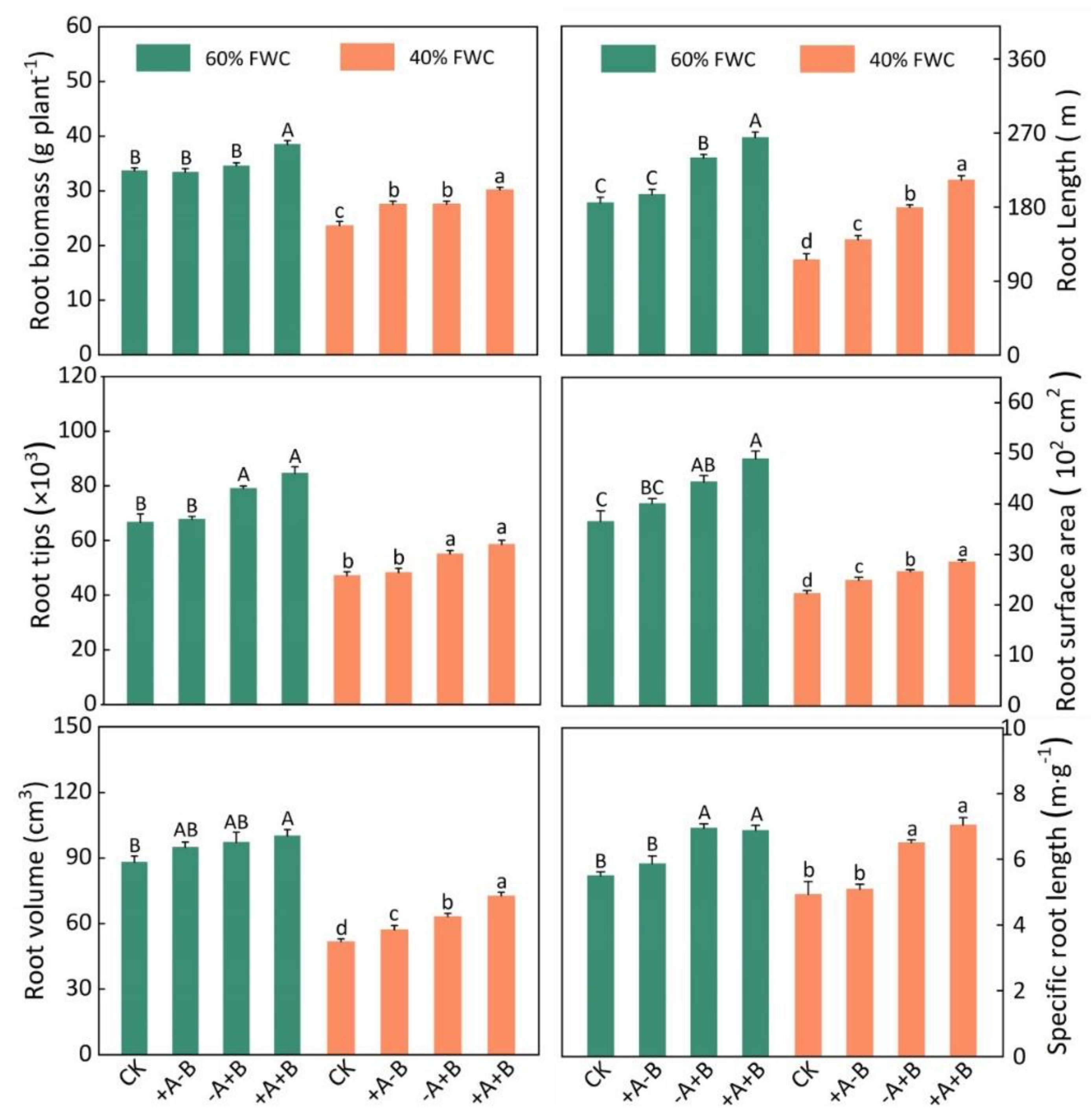
3.2. Root Morphological Parameters
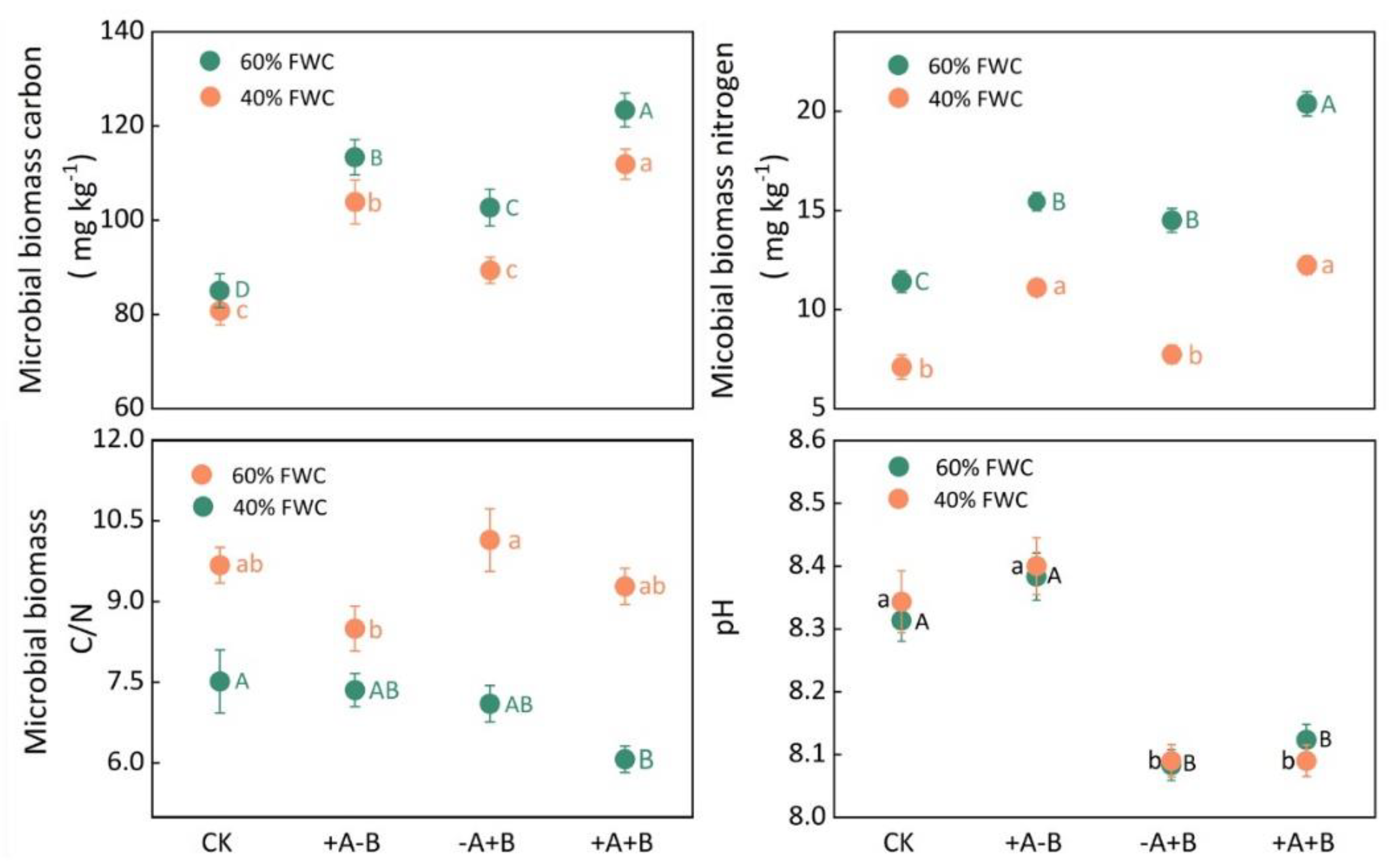
3.3. Rhizosphere Microbial Activity and Available Phosphorus Content
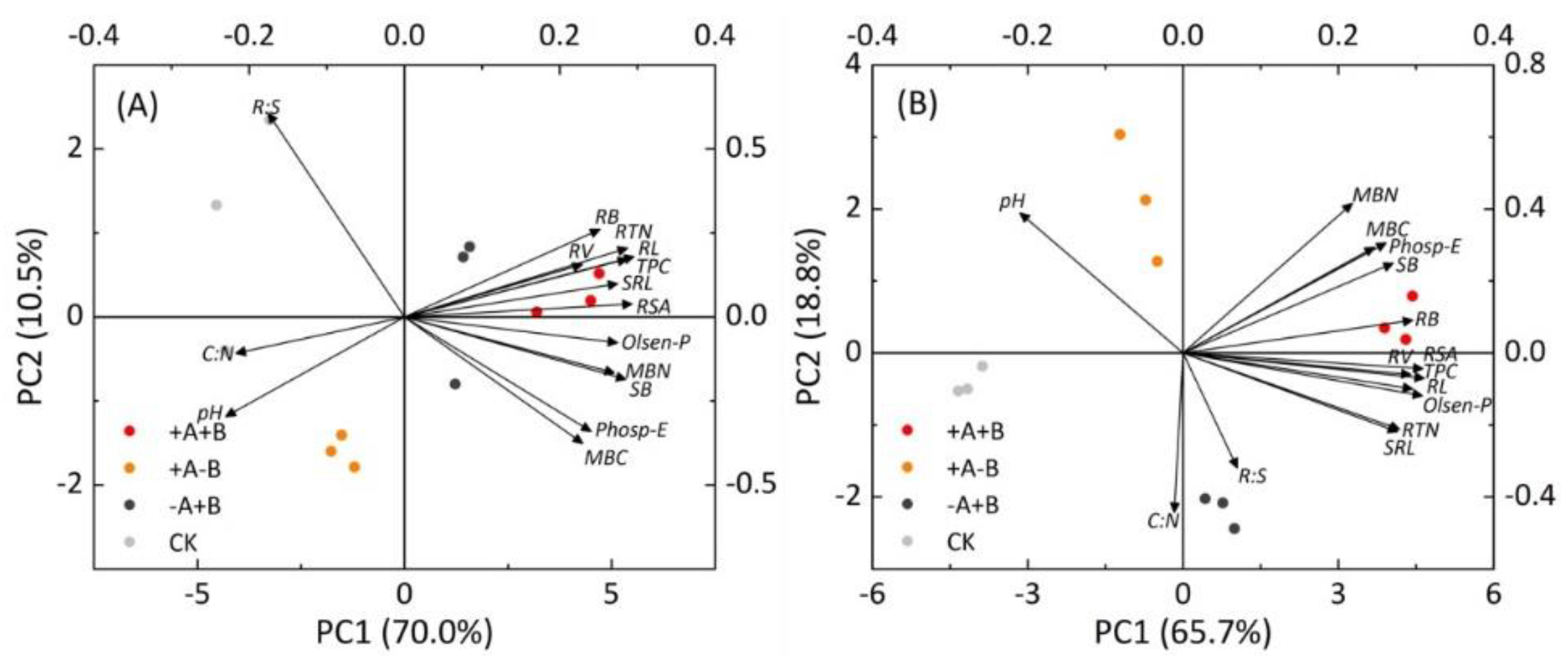
3.4. Principal Component Analysis
4. Discussion
4.1. The Effect of AMF and Biochar on Maize Growth and Phosphorus Acquisition
4.2. Effects of AMF and Biochar on the Morphology of the Maize Root System
4.3. The Effect of AMF and Biochar on Maize Growth and Phosphorus Uptake
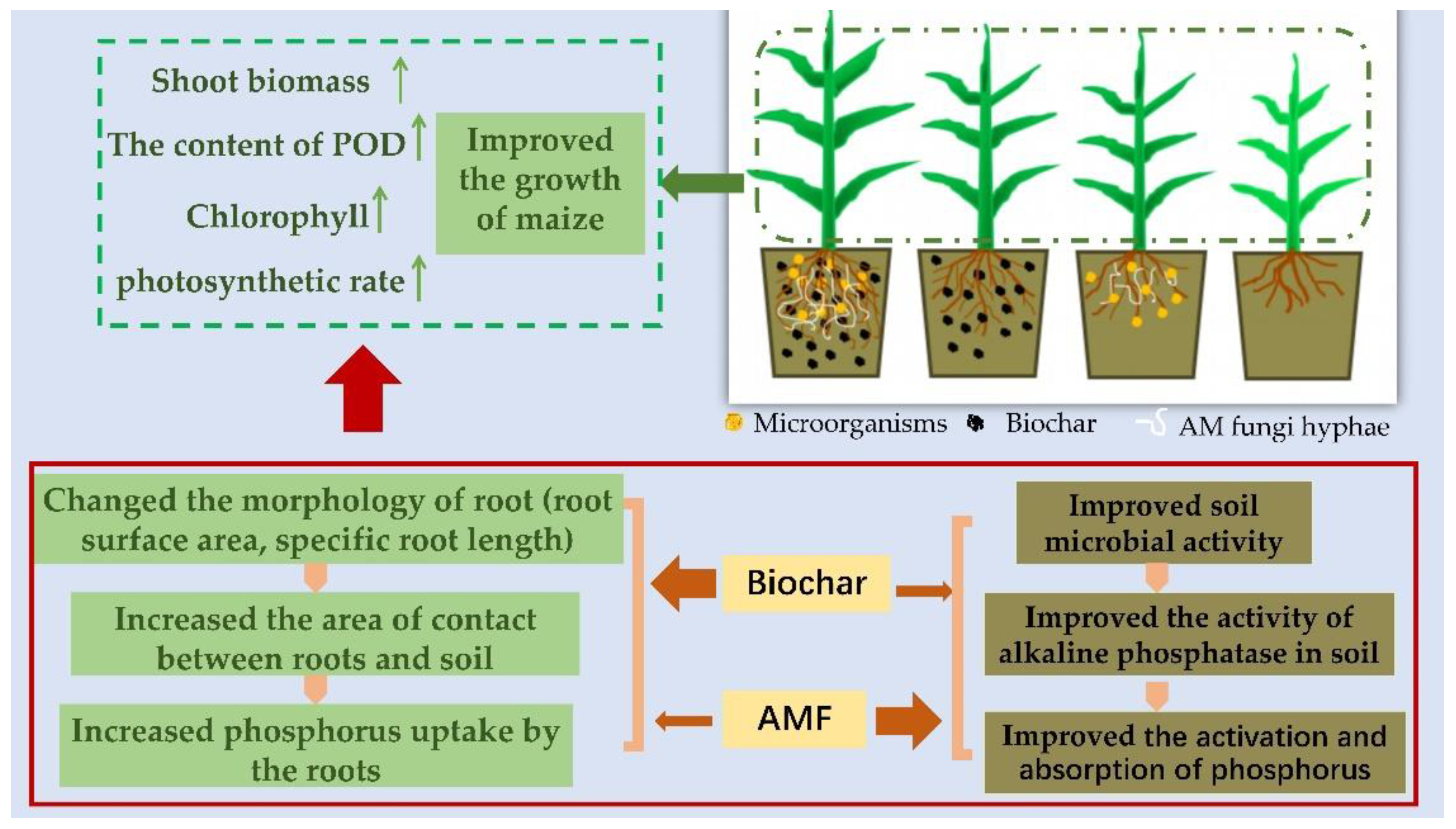
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Efthymiou, A.; Grønlund, M.; Müller-Stöver, D.S.; Jakobsen, I. Augmentation of the phosphorus fertilizer value of biochar by inoculation of wheat with selected Penicillium strains. Soil Biol. Biochem. 2018, 116, 139–147. [Google Scholar] [CrossRef]
- Shen, S.M. On our phosphorus fertilizer production and countermeasures. Chin. J. Soil Sci. 1985, 3, 97–103. [Google Scholar]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Temporal and spatial distribution of roots and competition for nitrogen in pea-barley intercrops-A field study employing 32p technique. Plant Soil. 2001. [Google Scholar] [CrossRef]
- Latati, M.; Bargaz, A.; Belarbi, B.; Lazali, M.; Benlahrech, S.; Tellah, S.; Kaci, G.; Drevon, J.J.; Ounane, S.M. The intercropping common bean with maize improves the rhizobial efficiency, resource use and grain yield under low phosphorus availability. Eur. J. Agron. 2016, 72, 80–90. [Google Scholar] [CrossRef]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust. J. Plant Physiol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frei Dit Frey, N.; Gianinazzi-Pearson, V.; et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Cosme, M.; Wurst, S. Interactions between arbuscular mycorrhizal fungi, rhizobacteria, soil phosphorus and plant cytokinin deficiency change the root morphology, yield and quality of tobacco. Soil Biol. Biochem. 2013, 57, 436–443. [Google Scholar] [CrossRef]
- Efthymiou, A.; Jensen, B.; Jakobsen, I. The roles of mycorrhiza and Penicillium inoculants in phosphorus uptake by biochar-amended wheat. Soil Biol. Biochem. 2018, 127, 168–177. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Bauerle, T.; Vanek, S.; Hestrin, R.; Nigussie, A. Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae-biochar interactions. Plant Soil 2016, 408, 95–105. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H. Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal. For. Ecol. Manag. 2006. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Vassilev, N.; Martos, E.; Mendes, G.; Martos, V.; Vassileva, M. Biochar of animal origin: A sustainable solution to the global problem of high-grade rock phosphate scarcity? J. Sci. Food Agric. 2013, 93, 1799–1804. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; delle Vedove, G.; De Neve, S. C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol. Biochem. 2014, 78, 195–203. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.S.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Blackwell, P.; Abbott, L.K.; Storer, P. Direct and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. J. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Vanek, S.J.; Lehmann, J. Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil. 2015. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Stefanova, K.; Solaiman, Z.M. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza 2016. [Google Scholar] [CrossRef] [PubMed]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil-Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Turner, N.C. Imposing and maintaining soil water deficits in drought studies in pots. Plant Soil 2019. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar]
- Kwak, S.S.; Kim, S.K.; Lee, M.S.; Jung, K.H.; Park, I.H.; Liu, J.R. Acidic peroxidases from suspension-cultures of sweet potato. Phytochemistry 1995. [Google Scholar] [CrossRef]
- Lüttge, U. Plant physiology. Encycl. Ecol. 1949, 24, 549–557. [Google Scholar] [CrossRef]
- Wu, S.J.; Ren, Y.T.; Wu, C.X. Comparative study on measurement methods of pH value of grassland soil. Chin. J. Soil Sci. 2018, 49, 343–348. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987. [Google Scholar] [CrossRef]
- Gao, Y.L.; Chao, X.X.; Fu, G.N.; Jia, T.; Zhang, X.M. Available phosphorus in different soil types. 2011, 162–164. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Tang, X.; Li, H.; Zhang, F.; Rengel, Z.; Whalley, W.R.; Davies, W.J.; Shen, J. Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol. 2016, 209, 823–831. [Google Scholar] [CrossRef]
- Kra, S.; Green, D.M. Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar]
- Ren, A.T.; Zhu, Y.; Chen, Y.L.; Ren, H.X.; Li, J.Y.; Kay Abbott, L.; Xiong, Y.C. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, G.C.; Chen, Y.L.; Gong, X.F.; Peng, Y.N.; Wang, Z.Y.; Ren, A.T.; Xiong, Y.C. Inoculation of arbuscular mycorrhizal fungi with plastic mulching in rainfed wheat: A promising farming strategy. Field Crops Res. 2017, 204, 229–241. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil. 2010. [Google Scholar] [CrossRef]
- Doubková, P.; Vlasáková, E.; Sudová, R. Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil. 2013. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, G.; Chen, X.; Zou, C. Arbuscular mycorrhizal fungal colonization is considerable at optimal Olsen-P levels for maximized yields in an intensive wheat-maize cropping system. Field Crops Res. 2017, 209, 1–9. [Google Scholar] [CrossRef]
- Mackay, J.E.; Cavagnaro, T.R.; Müller Stöver, D.S.; Macdonald, L.M.; Grønlund, M.; Jakobsen, I. A key role for arbuscular mycorrhiza in plant acquisition of P from sewage sludge recycled to soil. Soil Biol. Biochem. 2017, 115, 11–20. [Google Scholar] [CrossRef]
- de Figueiredo, C.C.; Farias, W.M.; Coser, T.R.; Monteiro de Paula, A.; Sartori da Silva, M.R.; Paz-Ferreiro, J. Sewage sludge biochar alters root colonization of mycorrhizal fungi in a soil cultivated with corn. Eur. J. Soil Biol. 2019, 93, 103092. [Google Scholar] [CrossRef]
- Koide, R.T.; Kabir, Z. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol. 2000. [Google Scholar] [CrossRef]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of pyrolysis parameters on phosphorus fractions of biosolids derived biochar. Sci. Total Environ. 2019, 695, 133846. [Google Scholar] [CrossRef]
- de Figueiredo, C.C.; Pinheiro, T.D.; de Oliveira, L.E.Z.; de Araujo, A.S.; Coser, T.R.; Paz-Ferreiro, J. Direct and residual effect of biochar derived from biosolids on soil phosphorus pools: A four-year field assessment. Sci. Total Environ. 2020, 739, 140013. [Google Scholar] [CrossRef]
- Berta, G.; Sampo, S.; Gamalero, E.; Massa, N.; Lemanceau, P. Suppression of Rhizoctonia root-rot of tomato by Glomus mossae BEG12 and Pseudomonas fluorescens A6RI is associated with their effect on the pathogen growth and on the root morphogenesis. Eur. J. Plant Pathol. 2005, 111, 279–288. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, J.; Song, Z.; Wang, Z.; Qiu, L.; Hu, J.; Gong, Y. Arbuscular mycorrhizal fungi alleviate root damage stress induced by simulated coal mining subsidence ground fissures. Sci. Total Environ. 2019, 652, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H. Is there an association between root architecture and mycorrhizal growth response? New Phytol. 2014, 204, 192–200. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Shen, G.; Wang, R.; Gao, L.; Kong, F.; Zhang, J. Growth performance, nutrient absorption of tobacco and soil fertility after straw biochar application. Int. J. Agric. Biol. 2016. [Google Scholar] [CrossRef]
- McKay Fletcher, D.M.; Ruiz, S.; Dias, T.; Petroselli, C.; Roose, T. Linking root structure to functionality: The impact of root system architecture on citrate-enhanced phosphate uptake. New Phytol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Honvault, N.; Houben, D.; Nobile, C.; Firmin, S.; Lambers, H.; Faucon, M.P. Tradeoffs among phosphorus-acquisition root traits of crop species for agroecological intensification. Plant Soil 2020. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Fan, M.; Wu, Y.; Shangguan, Z. Interactions between biochar and nitrogen impact soil carbon mineralization and the microbial community. Soil Tillage Res. 2020, 196, 104437. [Google Scholar] [CrossRef]

| Treatments | Addition or Inoculation | Biochar (%) | AMF (g) | |||
|---|---|---|---|---|---|---|
| Control | AMF | Bio | Bio + AMF | |||
| 40% FWC | −A − B | +A − B | −A + B | +A + B | 5% | 40 g |
| 60% FWC | −A − B | +A − B | −A + B | +A + B | 5% | 40 g |
| Treatment | Colonization Rate (%) | POD Activity (ΔA470/min·gFW) | Chlorophyll Content (mg g−1 FW) | Photosynthetic Rate (μmol CO2 m−2 s−1) | Shoot Biomass (g·plot-1) | Plant Height (cm) | Leaf Area (cm2·Plant) | |
|---|---|---|---|---|---|---|---|---|
| CK | - | 316.83 ± 14.37C | 1.43 ± 0.04C | 18.27 ± 0.38C | 101.17 ± 0.96C | 162.24 ± 2.31C | 6580.33 ± 46.91C | |
| 60% FWC | +A − B | 18.0 ± 0.81 | 250.50 ± 20.10B | 1.59 ± 0.02AB | 21.3 ± 0.50B | 113.57 ± 1.68B | 168.33 ± 2.03BC | 6701.27 ± 32.96BC |
| −A + B | - | 256.66 ± 18.56B | 1.56 ± 0.03B | 21.3 ± 0.46B | 117.13 ± 2.50B | 172.34 ± 1.76B | 6828.87 ± 34.67B | |
| +A + B | 22.7 ± 0.68 | 221.67 ± 23.9A | 1.66 ± 0.02A | 24.17 ± 0.35A | 129.02 ± 2.41A | 180.10 ± 2.08A | 7141.51 ± 47.76A | |
| 40% FWC | CK | - | 395.23 ± 9.33a | 1.26 ± 0.02b | 12.60 ± 0.39c | 73.57 ± 1.98c | 137.33 ± 3.52c | 4869.37 ± 53.76c |
| +A − B | 27.7 ± 0.52 | 319.35 ± 12.07b | 1.48 ± 0.03a | 16.8 ± 0.32a | 84.76 ± 2.07b | 149.05 ± 1.53ab | 5472.45 ± 31.06b | |
| −A + B | - | 359.74 ± 6.58a | 1.34 ± 0.03b | 14.77 ± 0.68b | 84.01 ± 1.20b | 144.67 ± 2.01bc | 5326.47 ± 44.08b | |
| +A + B | 31.3 ± 0.77 | 329.33 ± 4.62b | 1.50 ± 0.03a | 17.53 ± 0.55a | 90.97 ± 1.84a | 153.56 ± 2.19a | 5834.40 ± 48.32a | |
| Sig. | Water | - | *** | *** | *** | *** | *** | *** |
| AMF | - | *** | *** | *** | *** | *** | ** | |
| Biochar | - | * | ** | *** | *** | * | ** | |
| W × AMF | - | ns | ns | ns | ns | ns | *** | |
| W × Bio | - | ns | ns | * | * | ns | ns | |
| AMF × Bio | - | ns | ns | * | * | ns | ns | |
| W × AMF × Bio | - | ns | ns | ns | ns | ns | * | |
| Treatment | RL | RSA | RTN | RB | RV | SRL | Phosp-E | Olsen-P | pH | MBC | MBN | C:N | PC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | *** | *** | *** | *** | *** | * | *** | *** | ns | *** | *** | ns | *** |
| AMF | * | * | ns | * | * | ns | *** | *** | ns | *** | *** | * | *** |
| Biochar | *** | *** | *** | ** | *** | *** | ns | *** | *** | ** | * | ns | *** |
| W × AMF | ns | ns | ns | * | ns | ns | * | * | ns | ns | ns | ns | ns |
| W × Bio | * | * | * | ns | ns | ns | ns | ns | ns | * | ** | * | ns |
| AMF × Bio | ns | ns | ns | * | ns | ns | ns | * | ns | ns | ns | ns | ns |
| W × AMF × Bio | ns | ns | ns | ** | ns | ns | ns | ns | ns | ns | ns | ns | * |
| RL | RSA | RTN | RB | RV | SRL | Phosp-E | Olsen-P | pH | MBC | MBN | C:N | SB | PC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RL | 1 | 0.939 ** | 0.929 ** | 0.839 ** | 0.831 ** | 0.935 ** | 0.665 * | 0.816 ** | −0.808 ** | 0.583 * | 0.765 ** | −0.681 * | 0.829 ** | 0.927 ** |
| RSA | 1 | 0.919 ** | 0.843 ** | 0.840 ** | 0.845 ** | 0.667 * | 0.751 ** | −0.647 * | 0.681 * | 0.811 ** | −0.686 * | 0.912 ** | 0.829 ** | |
| RTN | 1 | 0.848 ** | 0.823 ** | 0.832 ** | 0.600 * | 0.809 ** | −0.786 ** | 0.542 | 0.733 ** | −0.707 * | 0.825 ** | 0.907 ** | ||
| RB | 1 | 0.660 * | 0.593 * | 0.362 | 0.578 * | −0.582 * | 0.600 * | 0.761 ** | −0.614 * | 0.792 ** | 0.817 ** | |||
| RV | 1 | 0.806 ** | 0.776 ** | 0.822 ** | −0.564 | 0.800 ** | 0.905 ** | −0.733 ** | 0.863 ** | 0.853 ** | ||||
| SRL | 1 | 0.746 ** | 0.830 ** | −0.819 ** | 0.476 | 0.636 * | −0.608 * | 0.713 ** | 0.841 ** | |||||
| Phosp−E | 1 | 0.882 ** | −0.642 * | 0.719 ** | 0.634 * | −0.3 | 0.715 ** | 0.685 * | ||||||
| Olsen−P | 1 | −0.816 ** | 0.592 * | 0.710 ** | −0.557 | 0.779 ** | 0.873 ** | |||||||
| pH | 1 | 0.293 | 0.413 | 0.351 | −0.511 | −0.810 ** | ||||||||
| MBC | 1 | 0.861 ** | −0.368 | 0.819 ** | 0.619 * | |||||||||
| MBN | 1 | −0.776 ** | 0.892 ** | 0.774 ** | ||||||||||
| C:N | 1 | −0.649 * | −0.633 * | |||||||||||
| SB | 1 | 0.773 ** | ||||||||||||
| TPC | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Cai, L. Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus. Sustainability 2021, 13, 3244. https://doi.org/10.3390/su13063244
Li M, Cai L. Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus. Sustainability. 2021; 13(6):3244. https://doi.org/10.3390/su13063244
Chicago/Turabian StyleLi, Mengying, and Liqun Cai. 2021. "Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus" Sustainability 13, no. 6: 3244. https://doi.org/10.3390/su13063244
APA StyleLi, M., & Cai, L. (2021). Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus. Sustainability, 13(6), 3244. https://doi.org/10.3390/su13063244






