Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Materials
2.3. Experimental Design
2.4. Analyses
2.4.1. Chemical and Physical Analyses
2.4.2. Soil DNA Extraction and PCR Amplification
2.4.3. Next Generation Sequencing
2.5. Statistical Analysis
3. Results
3.1. Composting Properties
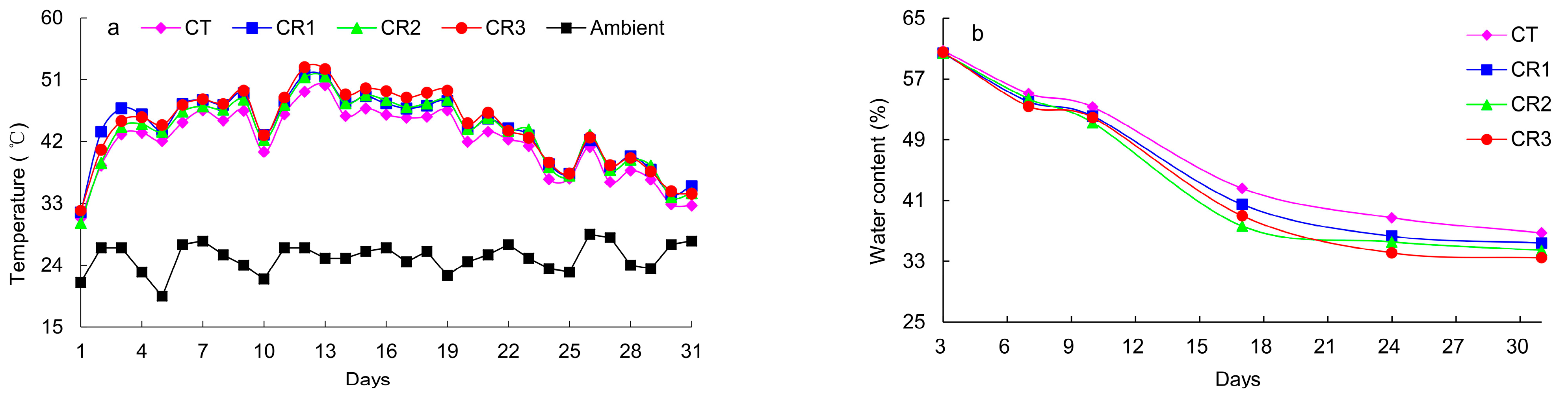
3.1.1. Changes in Soil Temperature and Water Content
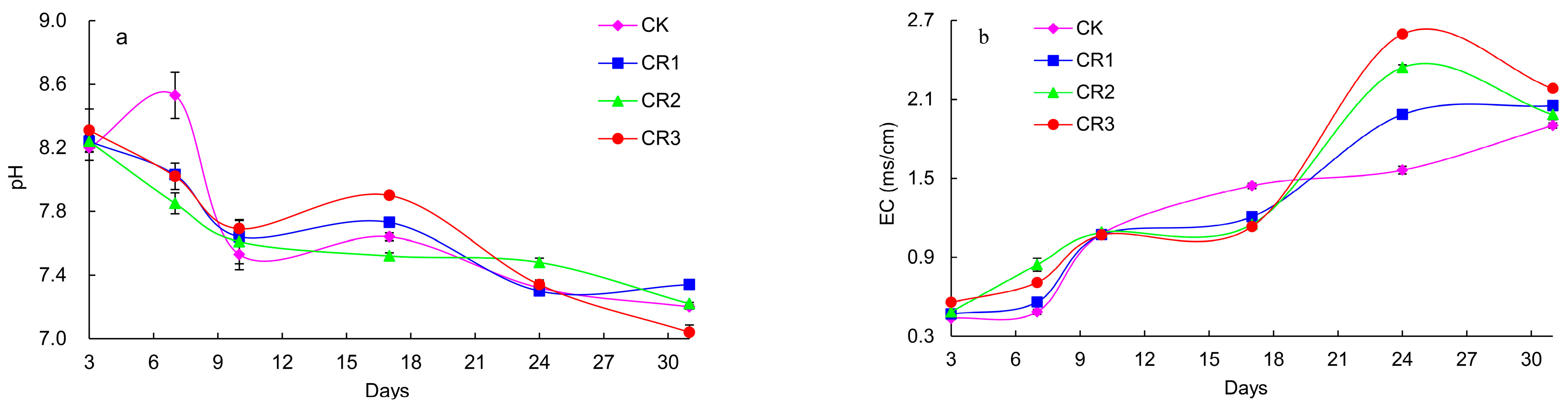
3.1.2. Changes in pH and EC
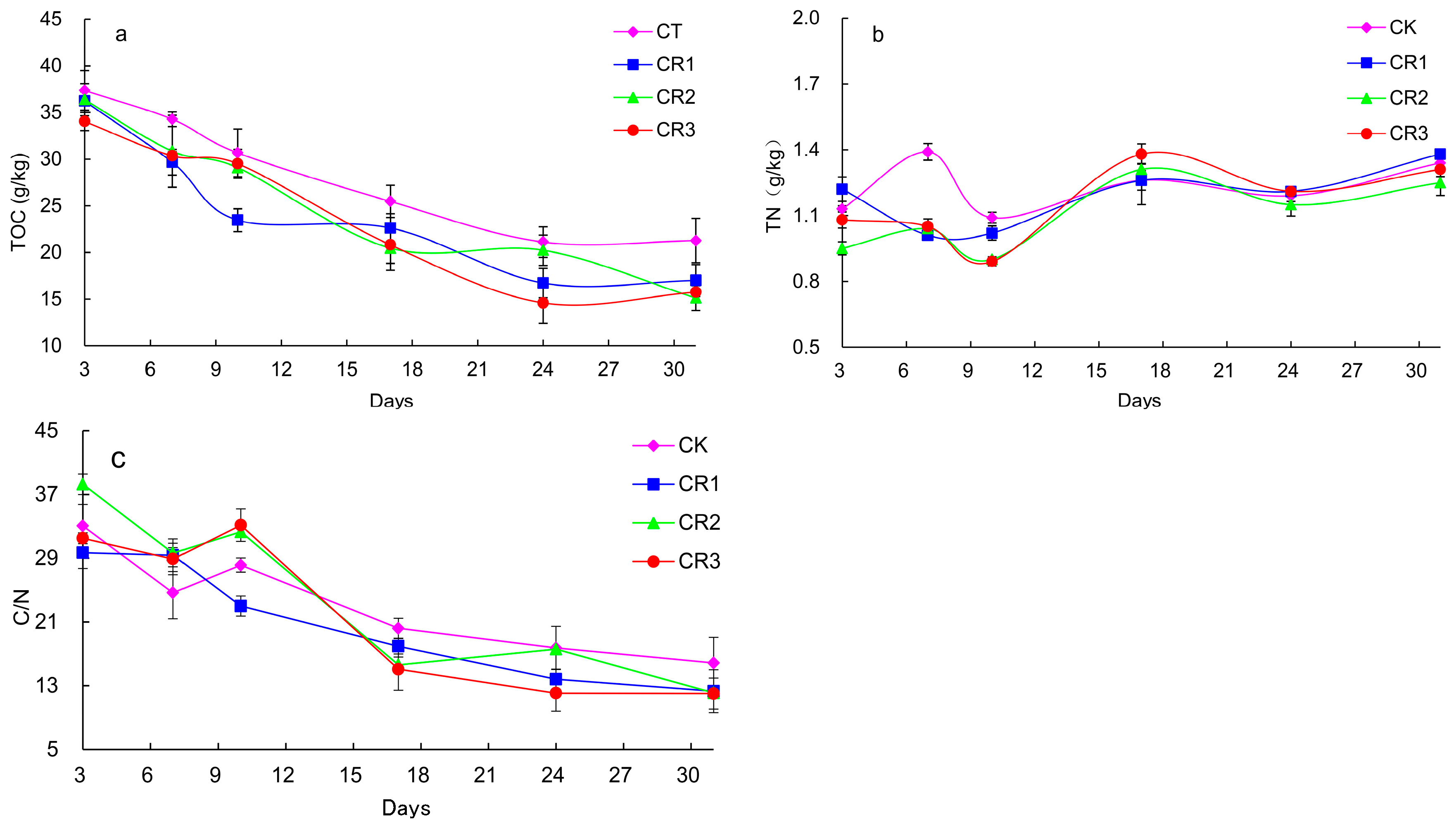
3.1.3. Changes in TOC and TN Content
3.2. Distribution of the Soil Microbiome
3.2.1. Predominant Bacterial and Fungal Taxa at the Phylum Level
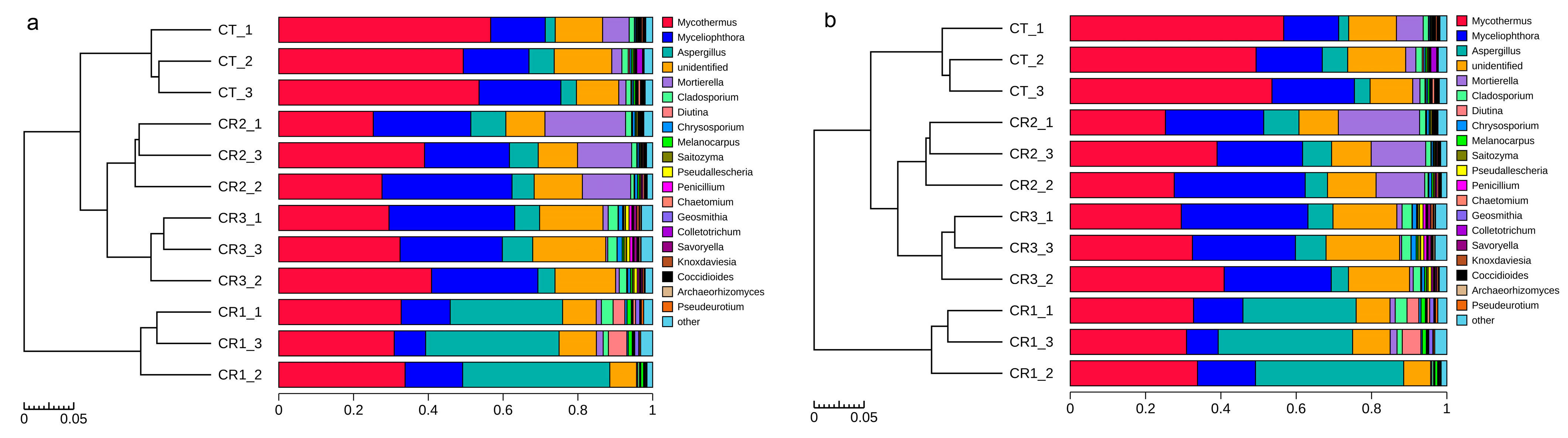
3.2.2. Taxonomic Composition of Bacterial and Fungal Communities at the Genus Level
3.3. Richness and Diversity of Bacterial and Fungal Communities in Soil Samples Following Composting
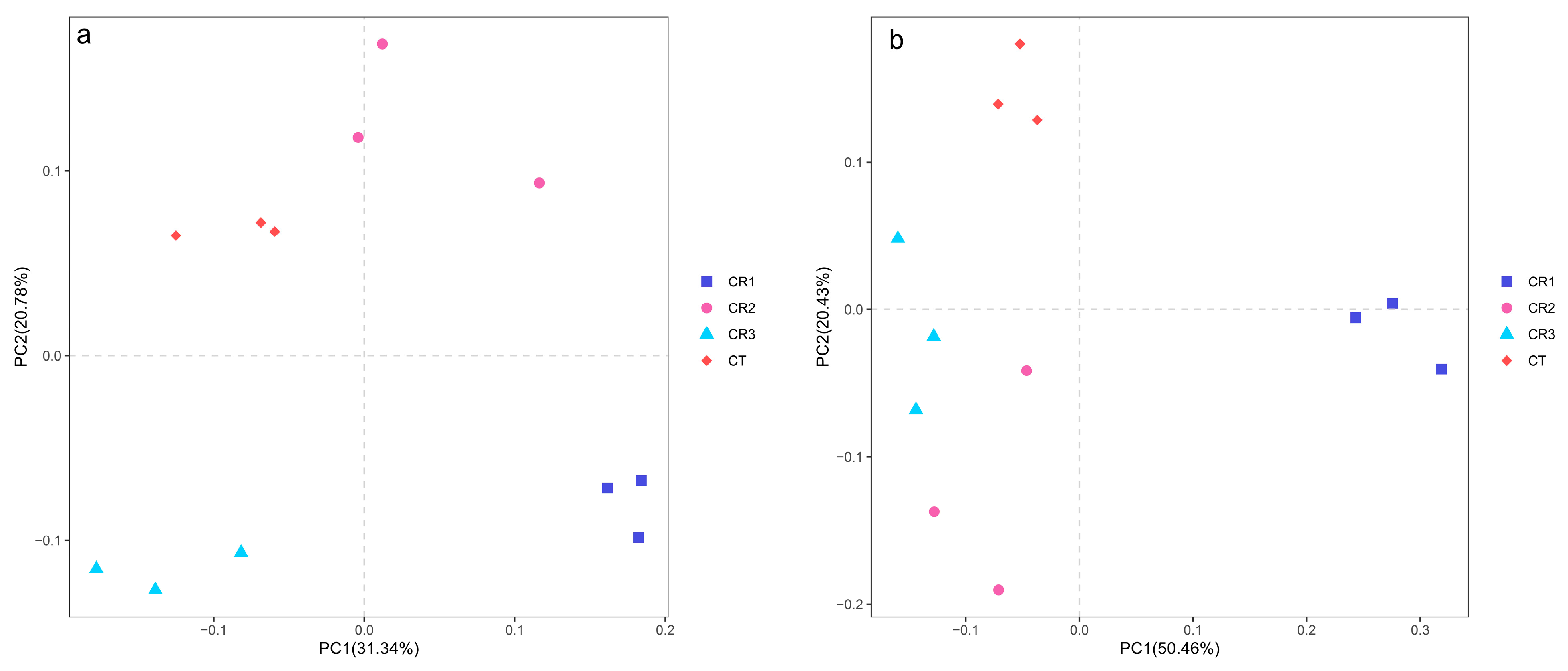
3.4. Treatment Effect on Bacterial and Fungal Beta Diversity
3.5. Relationship between Predominant Soil Microflora and Environmental Factors
4. Discussion
4.1. Effect of Exogenous Microbial Agents on the Physicochemical Properties of Topsoil Composting
4.2. Effects of Exogenous Microbial Agents on the Bacterial and Fungal Communities
4.3. Correlation between Bacterial and Fungal Communities and the Physicochemical Properties of the Soil
5. Conclusions
- The addition of exogenous microbial agents to cucumber straw during the in situ processing of these samples aided the aerobic fermentation process necessary to initiate the composting reaction in the plow layer. The results demonstrated that the addition of exogenous microbial agents prolong the high-temperature duration, reduce the total organic carbon (TOC) content, and accelerated the decline in the C/N ratio, ensuring compost maturity and effectively shortening the composting time.
- Proteobacteria, Chloroflexi, Actinobacteria, Firmicutes, and Acidobacteria were the dominant phyla in the bacterial community structure. Compared with the CT, the CR1, CR2, and CR3 treatments significantly increased the relative abundance of Firmicutes. For the fungal community, the CR2 treatment significantly increased the relative abundance of Mortierellomycota but decreased the relative abundance of Ascomycota. Moreover, the addition of exogenous microbial agents can significantly increase the richness and diversity of the fungal community.
- Redundancy analysis (RDA) revealed that the total nitrogen (TN) content was the most predominant main factor affecting the composition of the bacterial community, while the TN content, pH, and temperature were the most important factors that determined the fungal community composition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turmel, M.-S.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop residue management and soil health: A systems analysis. Agric. Syst. 2015, 134, 6–16. [Google Scholar] [CrossRef]
- He, J.-Z.; Zheng, Y.; Chen, C.-R.; He, Y.-Q.; Zhang, L.-M. Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches. J. Soils Sediments 2008, 8, 349–358. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, W.; He, X.; Yu, H.; Fan, J.; Liu, D. Influence of 20–year organic and inorganic fertilization on organic carbon accumulation and microbial community structure of aggregates in an intensively cultivated sandy loam soil. PLoS ONE 2014, 9, e92733. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Lidén, G. Semi-continuous co-digestion of solid slaughterhouse waste, manure, and fruit and vegetable waste. Renew. Energy 2008, 33, 726–734. [Google Scholar] [CrossRef]
- Mahmoud, M.; Elreedy, A.; Pascal, P.; Sophie, L.R.; Tawfik, A. Hythane (H2 and CH4) production from unsaturated polyester resin wastewater contaminated by 1,4-dioxane and heavy metals via up-flow anaerobic self-separation gases reactor. Energy Convers. Manag. 2017, 152, 342–353. [Google Scholar] [CrossRef]
- Das, A.; Mondal, C. Studies on the utilization of fruit and vegetable waste for generation of biogas. Int. J. Eng. Sci. 2013, 3, 24–32. [Google Scholar]
- Gale, W.J.; Cambardella, C.A. Carbon dynamics of surface residue- and root-derived organic matter under simulated no-till. Soil Sci. Soc. Am. J. 2000, 64, 190–195. [Google Scholar] [CrossRef]
- Arcand, M.M.; Knight, J.D.; Farrell, R.E. Differentiating between the supply of N to wheat from above and belowground residues of preceding crops of pea and canola. Biol. Fertil. Soils 2014, 50, 563–570. [Google Scholar] [CrossRef]
- Ibrahim, M.; Cao, C.G.; Zhan, M.; Li, C.-F.; Iqbal, J. Changes of CO2 emission and labile organic carbon as influenced by rice straw and different water regimes. Int. J. Environ. Sci. Technol. 2013, 12, 263–274. [Google Scholar] [CrossRef]
- Briassoulis, D.; Dejean, C. Critical review of norms and standards for biodegradable agricultural plastics Part Ι. Biodegradation in soil. J. Polym. Environ. 2010, 18, 384–400. [Google Scholar] [CrossRef]
- Andrea, C.; Muniyasami, S.; Emo, C. Assessment of the whole environmental degradation of oxo-biodegradable linear low density polyethylene (LLDPE) films designed for mulching applications. J. Polym. Environ. 2012, 20, 1007–1018. [Google Scholar]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.; Teng, Y.; Ma, W.; Christie, P.; Li, Z. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 024010. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.-B.; Hu, W.-L.; Qin, X.-H.; Ma, X.-W.; Yan, C.-R.; Wang, H.-Y. The status and distribution characteristics of residual mulching film in Xinjiang, China. J. Integr. Agric. 2016, 15, 2639–2646. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Yang, Y.; Wang, C. Physical and degradable properties of mulching films prepared from natural fibers and biodegradable polymers. Appl. Sci. 2016, 6, 147. [Google Scholar] [CrossRef]
- Dahman, Y.; Ugwu, C.U. Production of green biodegradable plastics of poly(3-hydroxybutyrate) from renewable resources of agricultural residues. Bioprocess Biosyst. Eng. 2014, 37, 1561–1568. [Google Scholar] [CrossRef]
- Cozzolino, E.; Giordano, M.; Fiorentino, N.; El-Nakhel, C.; Pannico, A.; Di Mola, I.; Mori, M.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Appraisal of biodegradable mulching films and vegetal-derived biostimulant application as eco-sustainable practices for enhancing lettuce crop performance and nutritive value. Agronomy 2020, 10, 427. [Google Scholar] [CrossRef]
- Zang, X.Y.; Liu, M.; Wang, H.; Fan, Y.; Zhang, H.; Liu, J.; Xing, E.; Xu, X.; Li, H. The distribution of active beta-glucosidase producing microbial communities in composting. Can. J. Microbiol. 2017, 63, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Yogev, A.; Raviv, M.; Hadar, Y.; Cohen, R.; Wolf, S.; Gil, L.; Katan, J. Induced resistance as a putative component of compost suppressiveness. Biol. Control. 2010, 54, 46–51. [Google Scholar] [CrossRef]
- Liu, N.; Hou, T.; Yin, H.; Han, L.; Huang, G. Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J. Hazard. Mater. 2019, 362, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Yao, T.; Su, M.; Li, J.; Liu, Z.; Xin, Y.; Wang, L.; Chen, J.; Gun, S. Effects of microbial inoculation on enzyme activity, available nitrogen content, and bacterial succession during pig manure composting. Bioresour. Technol. 2020, 306, 123167. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, J.; Wang, X.; Tuo, X.; Zhang, K.; Zhang, L.; Guo, A.; Zhang, X. Effects of copper on the composition and diversity of microbial communities in laboratory-scale swine manure composting. Can. J. Microbiol. 2018, 64, 409–419. [Google Scholar] [CrossRef]
- Ma, S.; Fang, C.; Sun, X.; Han, L.; He, X.; Huang, G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 259, 221–227. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, J.; Han, L.; Ma, S.; Sun, X.; Huang, G. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour. Technol. 2017, 241, 190–199. [Google Scholar] [CrossRef]
- Mao, H.; Lv, Z.; Sun, H.; Li, R.; Zhai, B.; Wang, Z.; Awasthi, M.K.; Wang, Q.; Zhou, L. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour. Technol. 2018, 258, 195–202. [Google Scholar] [CrossRef]
- Wu, J.; He, S.; Liang, Y.; Li, G.; Li, S.; Chen, S.; Nadeem, F.; Hu, J. Effect of phosphate additive on the nitrogen transformation during pig manure composting. Environ. Sci. Pollut. Res. 2017, 24, 17760–17768. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Ren, G.; Xu, X.; Qu, J.; Zhu, L.; Wang, T. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J. Microbiol. Biotechnol. 2016, 32, 1–11. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Chen, Z.; Wan, J.; Hu, L.; Zhang, Y. Effect of Phanerochaete chrysosporium inoculation on bacterial community and metal stabilization in lead-contaminated agricultural waste composting. Bioresour. Technol. 2017, 243, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Effects of brown sugar and calcium superphosphate on the secondary fermentation of green waste. Bioresour. Technol. 2013, 131, 68–75. [Google Scholar] [CrossRef]
- Hoshino, Y.T.; Morimoto, S. Comparison of 18S rDNA primers for estimating fungal diversity in agricultural soils using polymerase chain reaction-denaturing gradient gel electrophoresis. Soil Sci. Plant Nutr. 2008, 54, 701–710. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, Q.; Chen, H.; Wang, M.; Ren, X.; Zhao, J.; Li, J.; Guo, D.; Li, D.-S.; Awasthi, S.K.; et al. Evaluation of biochar amended biosolids co-composting to improve the nutrient transformation and its correlation as a function for the production of nutrient-rich compost. Bioresour. Technol. 2017, 237, 156–166. [Google Scholar] [CrossRef]
- Sun, D.; Lan, Y.; Xu, E.G.; Meng, J.; Chen, W. Biochar as a novel niche for culturing microbial communities in composting. Waste Manag. 2016, 54, 93–100. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Effects of earthworm casts and zeolite on the two-stage composting of green waste. Waste Manag. 2015, 39, 119–129. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; El-Din, M.N.; Refaat, B.M.; Abdel-Shakour, E.H.; Ewais, E.E.-D.; Alrefaey, H.M. Biotechnological application of thermotolerant cellulose-decomposing bacteria in composting of rice straw. Ann. Agric. Sci. 2016, 61, 135–143. [Google Scholar] [CrossRef]
- Sharifi, Z.; Sinegani, A.A.S. Arsenic and other irrigation water quality indicators of ground water in an agricultural area of Qorveh Plain, Kurdistan, Iran. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 548–555. [Google Scholar]
- Liu, L.; Wang, S.; Guo, X.; Zhao, T.; Zhang, B. Succession and diversity of microorganisms and their association with physicochemical properties during green waste thermophilic composting. Waste Manag. 2018, 73, 101–112. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Pandey, A.K.; Khan, J.; Bundela, P.S.; Wong, J.W.; Selvam, A. Evaluation of thermophilic fungal consortium for organic municipal solid waste composting. Bioresour. Technol. 2014, 168, 214–221. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Q.; Wei, Y.; Cui, H.; Zhang, X.; Wang, X.; Shan, S.; Wei, Z. Effect of actinobacteria agent inoculation methods on cellulose degradation during composting based on redundancy analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Ran, F.; Han, B.; Li, J.; Lan, X.; Zhang, Y.; Yang, X.; et al. Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef]
- Harshitha, J.; Krupanidhi, S.; Kumar, S.; Wong, J. Design and development of indoor device for recycling of domestic vegetable scrap. Environ. Technol. 2015, 37, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Wen, L.; Cao, H.; Liu, K.; An, X.; Li, D.; Wang, H.; Du, X.; Li, C. Role of psychrotrophic bacteria in organic domestic waste composting in cold regions of China. Bioresour. Technol. 2017, 236, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Men, M.; Xu, B.; Xu, X.; Cheng, L.; Meng, Q.; Deng, L.; Jiang, X.; Wu, X.; Sheng, S. Dynamic characteristics of the composition of the fungal community in a novel static composting system of dairy manure and rice straw. J. Agro-Environ. Sci. 2018, 37, 2029–2036. [Google Scholar]
- Natvig, D.O.; Taylor, J.W.; Tsang, A.; Hutchinson, M.I.; Powell, A.J. Mycothermus thermophilus gen. et comb. nov., a new home for the itinerant thermophile Scytalidium thermophilum (Torula thermophila). Mycologia 2015, 107, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ranjard, L.; Dequiedt, S.; Jolivet, C.; Saby, N.P.A.; Thioulouse, J.; Harmand, J.; Loisel, P.; Rapaport, A.; Fall, S.; Simonet, P.; et al. Biogeography of soil microbial communities: A review and a description of the ongoing french national initiative. Agron. Sustain. Dev. 2010, 30, 359–365. [Google Scholar] [CrossRef]
- Roy, K.; Ghosh, D.; Debruyn, J.M.; Dasgupta, T.; Wommack, K.E.; Liang, X.; Wagner, R.E.; Radosevich, M. Temporal dynamics of soil virus and bacterial populations in agricultural and early plant successional soils. Front. Microbiol. 2020, 11, 1494. [Google Scholar] [CrossRef]
- Liang, X.; Wagner, R.E.; Li, B.; Zhang, N.; Radosevich, M. Quorum sensing signals alter in vitro soil virus abundance and bacterial community composition. Front. Microbiol. 2020, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Change Biol. 2011, 18, 336–348. [Google Scholar] [CrossRef]
- Frey, S.; Drijber, R.; Smith, H.; Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 2008, 40, 2904–2907. [Google Scholar] [CrossRef]


| Material | WC (%) | TOC (g/kg) | TN (g/kg) | C/N |
|---|---|---|---|---|
| Chicken manure | - | 31.53 | 5.36 | 5.88 |
| Cucumber straw | 76.02 | 39.58 | 2.11 | 18.76 |
| Processing | Clean Tags | OTUs | Chao1 | PD Whole Tree | Shannon | |
|---|---|---|---|---|---|---|
| Bacteria | CT | 70841 b | 2201 b | 2856 b | 184.45 b | 8.84 a |
| CR1 | 75231 b | 2299 ab | 2725 c | 183.08 b | 8.87 a | |
| CR2 | 104396 a | 2249 ab | 2968 a | 197.54 a | 8.92 a | |
| CR3 | 95333 a | 2404 a | 2743 c | 177.95 b | 8.68 a | |
| Fungi | CT | 110437 c | 327 b | 342 d | 59.6 d | 3.49 b |
| CR1 | 141453 b | 260 c | 418 b | 68.38 b | 3.52 b | |
| CR2 | 142419 b | 316 b | 589 a | 77.32 a | 3.79 a | |
| CR3 | 158449 a | 392 a | 395 c | 63.76 c | 3.41 b |
| Number | Species |
|---|---|
| Proteobacteria | |
| A1 | Alphaproteobacteria |
| A2 | Gammaproteobacteria |
| A3 | Betaproteobacteria |
| A4 | Deltaproteobacteria |
| Firmicutes | |
| B1 | Bacilli |
| B2 | Clostridia |
| Actinobacteria | |
| C1 | Actinobacteria |
| C2 | Acidimicrobiia |
| C3 | Thermomicrobia |
| Chloroflexi | |
| D1 | Anaerolineae |
| D2 | Chloroflexia |
| D3 | Gitt-GS-136 |
| Number | Species |
|---|---|
| Ascomycota | |
| E1 | Sordariomycetes |
| E2 | Eurotiomycetes |
| E3 | Dothideomycetes |
| E4 | Saccharomycetes |
| E5 | Leotiomycetes |
| Mortierellomycota | |
| F1 | Mortierellomycetes |
| Basidiomycota | |
| G1 | Tremellomycetes |
| G2 | Agaricomycetes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, C.; Yan, C.; Xue, Y.; Xu, Z.; Jin, T.; Liu, Q. Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts. Sustainability 2021, 13, 2925. https://doi.org/10.3390/su13052925
Yun C, Yan C, Xue Y, Xu Z, Jin T, Liu Q. Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts. Sustainability. 2021; 13(5):2925. https://doi.org/10.3390/su13052925
Chicago/Turabian StyleYun, Cuixia, Changrong Yan, Yinghao Xue, Zhiyu Xu, Tuo Jin, and Qin Liu. 2021. "Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts" Sustainability 13, no. 5: 2925. https://doi.org/10.3390/su13052925
APA StyleYun, C., Yan, C., Xue, Y., Xu, Z., Jin, T., & Liu, Q. (2021). Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts. Sustainability, 13(5), 2925. https://doi.org/10.3390/su13052925






