Plant-Microbe Synergism in Floating Treatment Wetlands for the Enhanced Removal of Sodium Dodecyl Sulphate from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Development of FTWs
2.3. Water Analysis
2.4. Determination of Persistence of Inoculated Bacteria
2.5. Data Analysis
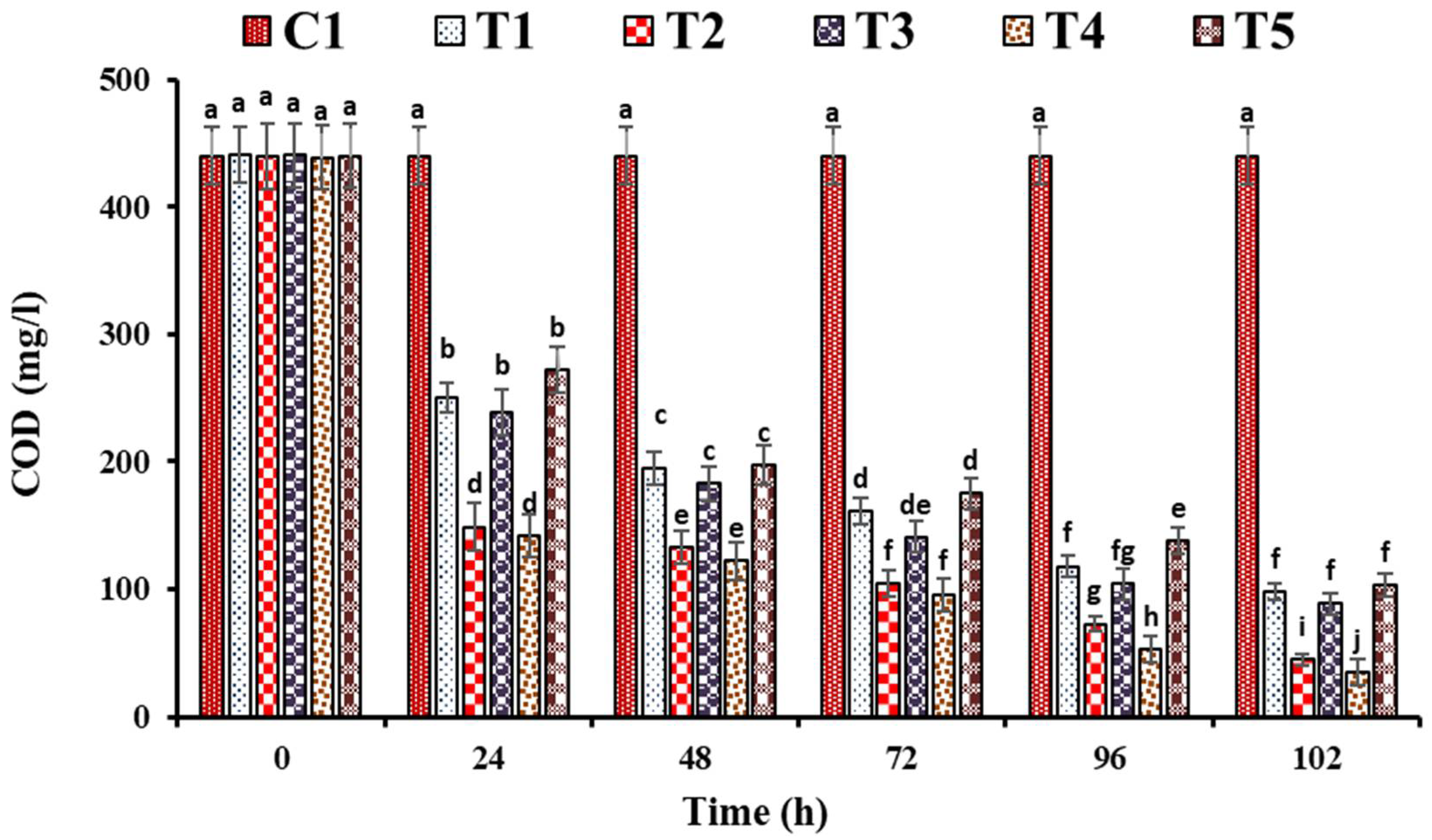
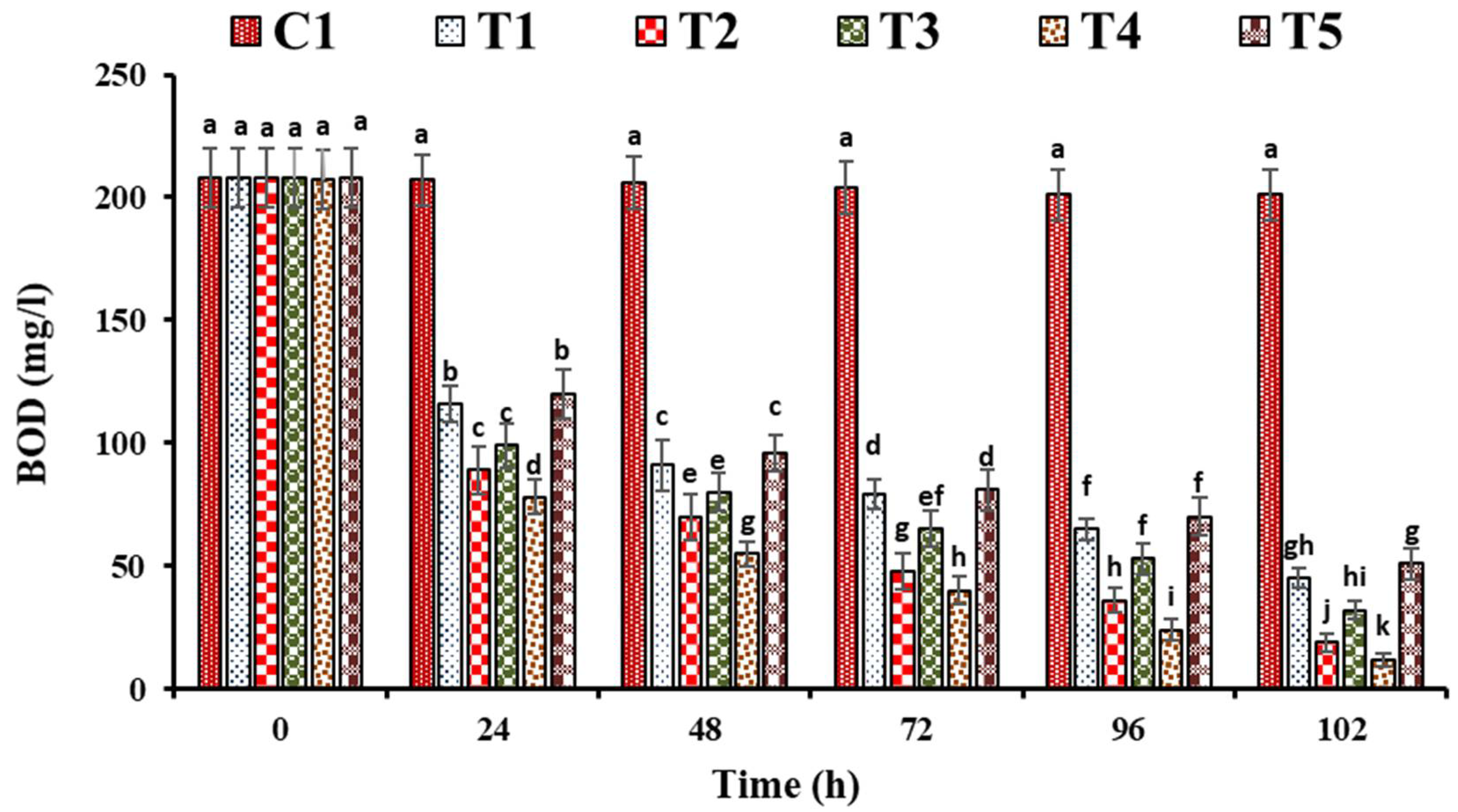
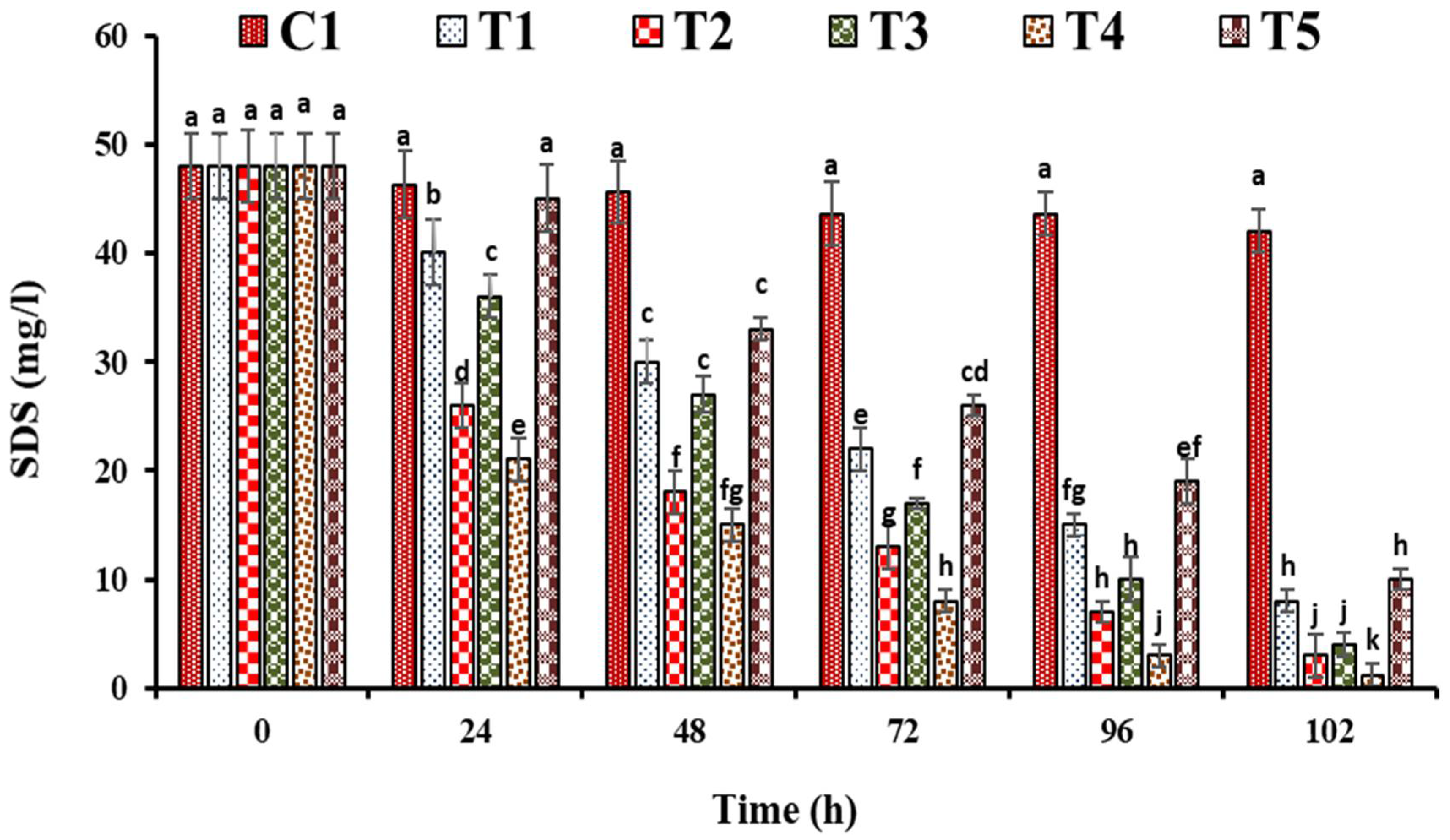
3. Results and Discussion
3.1. Performance Evaluation of FTWs
3.2. Persistence of Inoculated Bacteria in FTWs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahbazi, R.; Kasra-Kermanshahi, R.; Gharavi, S.; Moosavi-Nejad, Z.; Borzooee, F. Screening of SDS-degrading bacteria from car wash wastewater and study of the alkylsulfatase enzyme activity. Iran. J. Microbiol. 2013, 5, 153. [Google Scholar]
- Ambily, P.; Jisha, M. Metabolic profile of sodium dodecyl sulphate (SDS) biodegradation by Pseudomonas aeruginosa (MTCC 10311). J. Environ. Biol. 2014, 35, 827. [Google Scholar] [PubMed]
- Hosseini, F.; Malekzadeh, F.; Amirmozafari, N.; Ghaemi, N. Biodegradation of anionic surfactants by isolated bacteria from activated sludge. Int. J. Environ. Sci. Technol. 2007, 4, 127–132. [Google Scholar] [CrossRef]
- Dereszewska, A.; Cytawa, S.; Tomczak-Wandzel, R.; Medrzycka, K. The effect of anionic surfactant concentration on activated sludge condition and phosphate release in biological treatment plant. Pol. J. Environ. Stud. 2015, 24, 83–91. [Google Scholar] [CrossRef]
- Mazumder, D.; Mukherjee, S. Treatment of automobile service station wastewater by coagulation and activated sludge process. Int. J. Environ. Sci. Develop. 2011, 2, 64–69. [Google Scholar] [CrossRef]
- Jakovljević, V.D.; Vrvić, M.M. The Potential Application of Selected Fungi Strains in Removal of Commercial Detergents and Biotechnology. In Application and Characterization of Surfactants; IntechOpen: London, UK, 2017; p. 233. [Google Scholar] [CrossRef]
- Guerrini, A.; Romano, G.; Ferretti, S.; Fibbi, D.; Daddi, D. A performance measurement tool leading wastewater treatment plants toward economic efficiency and sustainability. Sustainability 2016, 8, 1250. [Google Scholar] [CrossRef]
- Muga, H.E.; Mihelcic, J.R. Sustainability of wastewater treatment technologies. J. Environ. Manag. 2008, 88, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Balkema, A.J.; Preisig, H.A.; Otterpohl, R.; Lambert, F.J. Indicators for the sustainability assessment of wastewater treatment systems. Urban Water 2002, 4, 153–161. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wang, H.-C.; Han, J.-L.; Liu, W.-Z.; Cheng, H.-Y.; Liang, B.; Wang, A.-J. The application of footprints for assessing the sustainability of wastewater treatment plants. J. Clean. Prod. 2020, 277, 124053. [Google Scholar] [CrossRef]
- Paulo, A.; Plugge, C.; García-Encina, P.; Stams, A. Anaerobic degradation of sodium dodecyl sulfate (SDS) by denitrifying bacteria. Int. Biodeterior. Biodegrad. 2013, 84, 14–20. [Google Scholar] [CrossRef]
- Headley, T.; Tanner, C. In Floating treatment wetlands: An innovative option for stormwater quality applications. In Proceedings of the 11th International Conference on Wetland Systems for Water Pollution Control, Indore, India, 1–7 November 2008; pp. 1101–1106. [Google Scholar]
- Weragoda, S.; Jinadasa, K.; Zhang, D.Q.; Gersberg, R.M.; Tan, S.K.; Tanaka, N.; Jern, N.W. Tropical application of floating treatment wetlands. Wetlands 2012, 32, 955–961. [Google Scholar] [CrossRef]
- Ladislas, S.; Gerente, C.; Chazarenc, F.; Brisson, J.; Andres, Y. Floating treatment wetlands for heavy metal removal in highway stormwater ponds. Ecol. Eng. 2015, 80, 85–91. [Google Scholar] [CrossRef]
- Yousaf, S.; Andria, V.; Reichenauer, T.G.; Smalla, K.; Sessitsch, A. Phylogenetic and functional diversity of alkane degrading bacteria associated with Italian ryegrass (Lolium multiflorum) and Birdsfoot trefoil (Lotus corniculatus) in a petroleum oil-contaminated environment. J. Hazard. Mater. 2010, 184, 523–532. [Google Scholar] [CrossRef]
- Olkowska, E.; Polkowska, Z.; Namiesnik, J. Analytics of surfactants in the environment: Problems and challenges. Chem. Rev. 2011, 111, 5667–5700. [Google Scholar] [CrossRef]
- Yen Doan, T.H.; Minh Chu, T.P.; Dinh, T.D.; Nguyen, T.H.; Tu Vo, T.C.; Nguyen, N.M.; Nguyen, B.H.; Pham, T.D. Adsorptive removal of Rhodamine B using novel adsorbent-based surfactant-nodified alpha alumina nanoparticles. J. Anal. Methods Chem. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.D.; Dang, T.H.M.; Nguyen, T.T.N.; Nguyen, T.A.H.; Pham, T.N.M.; Pham, T.D. Separation and determination of alkyl sulfate surfactants in wastewater by capillary electrophoresis coupled with contactless conductivity detection after preconcentration by simultaneous adsorption using alumina beads. Electrophoresis 2021, 42, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ishiguro, M. Adsorption of anionic surfactant (sodium dodecyl sulfate) on silica. Soil Sci. Plant Uutr. 2016, 62, 223–229. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater, Organic Pollutants—Monitoring, Risk and Treatment; IntechOpen: London, UK, 2013; pp. 167–194. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Yaseen, Z.M.; Zigale, T.T.; Kumar, R.; Salih, S.Q.; Awasthi, S.; Tung, T.M.; Al-Ansari, N.; Bhagat, S.K. Laundry wastewater treatment using a combination of sand filter, bio-char and teff straw media. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Ijaz, A.; Imran, A.; ul Haq, M.A.; Khan, Q.M.; Afzal, M. Phytoremediation: Recent advances in plant-endophytic synergistic interactions. Plant Soil 2016, 405, 179–195. [Google Scholar] [CrossRef]
- Ojo, O.A.; Oso, B.A. Biodegradation of synthetic detergents in wastewater. Afr. J. Biotechnol. 2009, 8, 229–249. [Google Scholar] [CrossRef]
- Sharman, S.H. Extensive biodegradation of synthetic detergents. Nature 1964, 201, 704–705. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ.Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochimica et Biophysica Acta (BBA)-Biomembranes 2000, 1508, 235–251. [Google Scholar] [CrossRef]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan, Q.M. Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 2013, 90, 1317–1332. [Google Scholar] [CrossRef]
- Abhilash, P.; Powell, J.R.; Singh, H.B.; Singh, B.K. Plant–microbe interactions: Novel applications for exploitation in multipurpose remediation technologies. Trends Biotechnol. 2012, 30, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.E. The need for sustainable soil remediation. Elements 2010, 6, 363–368. [Google Scholar] [CrossRef]
- Verhoeven, J.T.; Meuleman, A.F. Wetlands for wastewater treatment: Opportunities and limitations. Ecol. Eng. 1999, 12, 5–12. [Google Scholar] [CrossRef]
- Song, J.; Li, Q.; Dzakpasu, M.; Wang, X.C.; Chang, N. Integrating stereo-elastic packing into ecological floating bed for enhanced denitrification in landscape water. Bioresour. Technol. 2020, 299, 122601. [Google Scholar] [CrossRef]
- Park, J.B.; Sukias, J.P.; Tanner, C.C. Floating treatment wetlands supplemented with aeration and biofilm attachment surfaces for efficient domestic wastewater treatment. Ecol. Eng. 2019, 139, 105582. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Arslan, M.; Müller, J.A.; Shabir, G.; Islam, E.; Tahseen, R.; Anwar-ul-Haq, M.; Hashmat, A.J.; Iqbal, S.; Khan, Q.M. Floating treatment wetlands as a suitable option for large-scale wastewater treatment. Nat. Sustain. 2019, 2, 863–871. [Google Scholar] [CrossRef]
- Colares, G.S.; Dell’Osbel, N.; Wiesel, P.G.; Oliveira, G.A.; Lemos, P.H.Z.; da Silva, F.P.; Lutterbeck, C.A.; Kist, L.T.; Machado, Ê.L. Floating treatment wetlands: A review and bibliometric analysis. Sci. Total Environ. 2020, 714, 136776. [Google Scholar] [CrossRef] [PubMed]
- Yeh, N.; Yeh, P.; Chang, Y.-H. Artificial floating islands for environmental improvement. Renew. Sustain. Energy Rev. 2015, 47, 616–622. [Google Scholar] [CrossRef]
- Hu, Z.; Li, D.; Guan, D. Water quality retrieval and algae inhibition from eutrophic freshwaters with iron-rich substrate based ecological floating beds treatment. Sci. Total Environ. 2020, 712, 135584. [Google Scholar] [CrossRef]
- Afzal, M.; Rehman, K.; Shabir, G.; Tahseen, R.; Ijaz, A.; Hashmat, A.J.; Brix, H. Large-scale remediation of oil-contaminated water using floating treatment wetlands. NPJ Clean Water 2019, 2, 3. [Google Scholar] [CrossRef]
- Hussain, Z.; Arslan, M.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Treatment of the textile industry effluent in a pilot-scale vertical flow constructed wetland system augmented with bacterial endophytes. Sci. Total Environ. 2018, 645, 966–973. [Google Scholar] [CrossRef]
- Ijaz, A.; Shabir, G.; Khan, Q.M.; Afzal, M. Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol. Eng. 2015, 84, 58–66. [Google Scholar] [CrossRef]
- Karigar, C.; Rao, S. Role of microbial enzymes in bioremediation of pollutans. Enzyme Res. 2011, 2011805187. [Google Scholar] [CrossRef]
- Hussain, Z.; Arslan, M.; Shabir, G.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Remediation of textile bleaching effluent by bacterial augmented horizontal flow and vertical flow constructed wetlands: A comparison at pilot scale. Sci. Total Environ. 2019, 685, 370–379. [Google Scholar] [CrossRef]
- Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Abd-Allah, E.F. Bacterial augmented floating treatment wetlands for efficient treatment of synthetic textile dye wastewater. Sustainability 2020, 12, 3731. [Google Scholar] [CrossRef]
- Chen, Z.; Cuervo, D.P.; Müller, J.A.; Wiessner, A.; Köser, H.; Vymazal, J.; Kästner, M.; Kuschk, P. Hydroponic root mats for wastewater treatment. Environ. Sci. Pollut. Res. 2016, 23, 15911–15928. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Imran, A.; Khan, Q.M.; Afzal, M. Plant–bacteria partnerships for the remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2017, 24, 4322–4336. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating wetlands: A sustainable tool for wastewater treatment. Clean Soil Air Water. 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Lyu, J.; Lin, G.; Fan, Z.; Lin, W.; Dai, Z. Suitable plant combinations for ecological floating beds in eutrophic subtropical coastal wetlands under different salinities: Experimental evidences. Int. J. Environ. Sci. Technol. 2020, 17, 4505–4516. [Google Scholar] [CrossRef]
- Mustafa, H.M.; Hayder, G. Recent studies on applications of aquatic weed plants in phytoremediation of wastewater. Ain Shams Eng. J. 2020. [Google Scholar] [CrossRef]
- Sessitsch, A.; Coenye, T.; Sturz, A.; Vandamme, P.; Barka, E.A.; Salles, J.; Van Elsas, J.; Faure, D.; Reiter, B.; Glick, B. Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. Int. J. Syst. Evol. Microbiol. 2005, 55, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Afzal, M.; Imran, A.; Khan, Q.M. Bacterial rhizosphere and endosphere populations associated with grasses and trees to be used for phytoremediation of crude oil contaminated soil. Bull. Environ. Contam. Toxicol. 2015, 94, 314–320. [Google Scholar] [CrossRef]
- Federation, Water Environmental, and APH Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Enhancement of oil field-produced wastewater remediation by bacterially-augmented floating treatment wetlands. Chemosphere 2019, 217, 576–583. [Google Scholar] [CrossRef]
- Shehzadi, M.; Afzal, M.; Khan, M.U.; Islam, E.; Mobin, A.; Anwar, S.; Khan, Q.M. Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res. 2014, 58, 152–159. [Google Scholar] [CrossRef]
- Skrzypiecbcef, K.; Gajewskaad, M.H. The use of constructed wetlands for the treatment of industrial wastewater. J. Water Land Dev. 2017, 34, 233–240. [Google Scholar] [CrossRef]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.M.; Afzal, M. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Clean Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Kumar, A. Diversity of culturable sodium dodecyl sulfate (SDS) degrading bacteria isolated from detergent contaminated ponds situated in Varanasi city, India. Int. Biodeterior. Biodegrad. 2011, 65, 961–971. [Google Scholar] [CrossRef]
- Usharani, J.; Rajasekar, T.; Deivasigamani, B. Isolation and characterization of SDS (sodium dodecyl sulfate) degrading organisms from SDS contaminated areas. Open Access Sci. Rep. 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Effendi, I.; Nedi, S.; Pakpahan, R. Detergent disposal into our environmentand its impact on marine microbes. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 97, p. 012030. [Google Scholar] [CrossRef]

| Time (h) | Control (C) | L. fusca | B. mutica | T5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |||||||||
| pH | Turbidity | pH | Turbidity | pH | Turbidity | pH | Turbidity | pH | Turbidity | pH | Turbidity | |
| 0 | 8.62 (0.13) | 33.62 (2.07) | 8.60 (0.22) | 33.02 (1.52) | 8.57 (0.21) | 33.05 (2.78) | 8.62 (0.31) | 33.04 (1.65) | 8.52 (0.22) | 33.28 (1.72) | 8.62 (0.31) | 33.04 (1.15) |
| 24 | 8.57 (0.28) | 32.43 (2.19) | 8.43 (0.12) | 31.08 (1.23) | 8.3 (0.22) | 29.28 (1.55) | 8.25 (0.12) | 30.47 (1.53) | 8.15 (0.14) | 28.08 (1.56) | 8.52 (0.70) | 32.05 (1.23) |
| 48 | 8.4 (0.12) | 31.26 (2.54) | 8.32 (0.11) | 27.25 (1.05) | 8.2 (0.14) | 23.25 (1.05) | 8.14 (0.17) | 26.47 (2.05) | 8.05 (0.11) | 19.45 (1.03) | 8.47 (0.20) | 30.82 (1.52) |
| 72 | 8.3 (0.14) | 31.08 (3.04) | 8.27 (0.15) | 22.08 (3.35) | 8.1 (0.17) | 15.72 (2.08) | 8.02 (0.13) | 19.48 (1.08) | 7.92 (0.20) | 12.18 (1.64) | 8.32 (0.10) | 23.15 (1.58) |
| 96 | 8.2 (0.21) | 30.27 (2.45) | 8.13 (0.23) | 16.48 (2.95) | 8.0 (0.11) | 7.08 (1.52) | 8.03 (0.15) | 12.78 (2.05) | 7.83 (0.10) | 4.05 (1.17) | 8.25 (0.21) | 16.24 (1.39) |
| 102 | 8.2 (0.22) | 29.72 (2.06) | 8.05 (0.16) | 7.05 (1.85) | 7.9 (0.23) | 2.08 (1.62) | 7.92 (0.22) | 3.08 (1.04) | 7.84 (0.25) | 0.18 (0.05) | 8.23 (0.22) | 11.75 (1.05) |
| Treatment | Time | |||
|---|---|---|---|---|
| 0 h | 48 h | 96 h | 102 h | |
| Water (CFU ml−1) | ||||
| L. fusca and bacteria | 8.2 × 105 (5.2 × 103) | 5.0 × 103 (2 × 102) | 4.0 × 102 (2.2 × 102) | 1.5 × 102 (0.9×102) |
| B. mutica and bacteria | 8.2 × 105 (5.2 × 103) | 7.3 × 103 (2.5 × 102) | 9.1 × 102 (1.5 × 102) | 2.1 × 102 (1.0×102) |
| Bacteria | 8.2 × 105 (5.2 × 103) | 4.5 × 102 (1.2 × 102) | 2.6 × 102 (1.0 × 102) | 1.2 × 102 (0.8×102) |
| Root interior (CFU g−1) | ||||
| L. fusca and bacteria | -- | 6.6 × 103 (2.3 × 102) | 4.8 × 104 (2.2 × 102) | 6.4 × 104 (2.7×102) |
| B. mutica and bacteria | -- | 3.0 × 104 (1.8 × 102) | 6.0 × 105 (4.3 × 102) | 8.7 × 105 (6.2×102) |
| Shoot interior (CFU g−1) | ||||
| L. fusca and bacteria | -- | 1.7 × 102 (0.9 × 102) | 5.1 × 103 (2.6 × 102) | 6.2 × 103 (3.5×102) |
| B. mutica and bacteria | -- | 2.7 × 102 (1.1 × 102) | 5.8 × 103 (3.0 × 102) | 7.0 × 103 (1.1×102) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasin, M.; Tauseef, M.; Zafar, Z.; Rahman, M.; Islam, E.; Iqbal, S.; Afzal, M. Plant-Microbe Synergism in Floating Treatment Wetlands for the Enhanced Removal of Sodium Dodecyl Sulphate from Water. Sustainability 2021, 13, 2883. https://doi.org/10.3390/su13052883
Yasin M, Tauseef M, Zafar Z, Rahman M, Islam E, Iqbal S, Afzal M. Plant-Microbe Synergism in Floating Treatment Wetlands for the Enhanced Removal of Sodium Dodecyl Sulphate from Water. Sustainability. 2021; 13(5):2883. https://doi.org/10.3390/su13052883
Chicago/Turabian StyleYasin, Momina, Muhammad Tauseef, Zaniab Zafar, Moazur Rahman, Ejazul Islam, Samina Iqbal, and Muhammad Afzal. 2021. "Plant-Microbe Synergism in Floating Treatment Wetlands for the Enhanced Removal of Sodium Dodecyl Sulphate from Water" Sustainability 13, no. 5: 2883. https://doi.org/10.3390/su13052883
APA StyleYasin, M., Tauseef, M., Zafar, Z., Rahman, M., Islam, E., Iqbal, S., & Afzal, M. (2021). Plant-Microbe Synergism in Floating Treatment Wetlands for the Enhanced Removal of Sodium Dodecyl Sulphate from Water. Sustainability, 13(5), 2883. https://doi.org/10.3390/su13052883









