Effects of a Short-Term Trampling Experiment on Alpine Vegetation in the Tatras, Slovakia
Abstract
1. Introduction
2. Materials and Methods
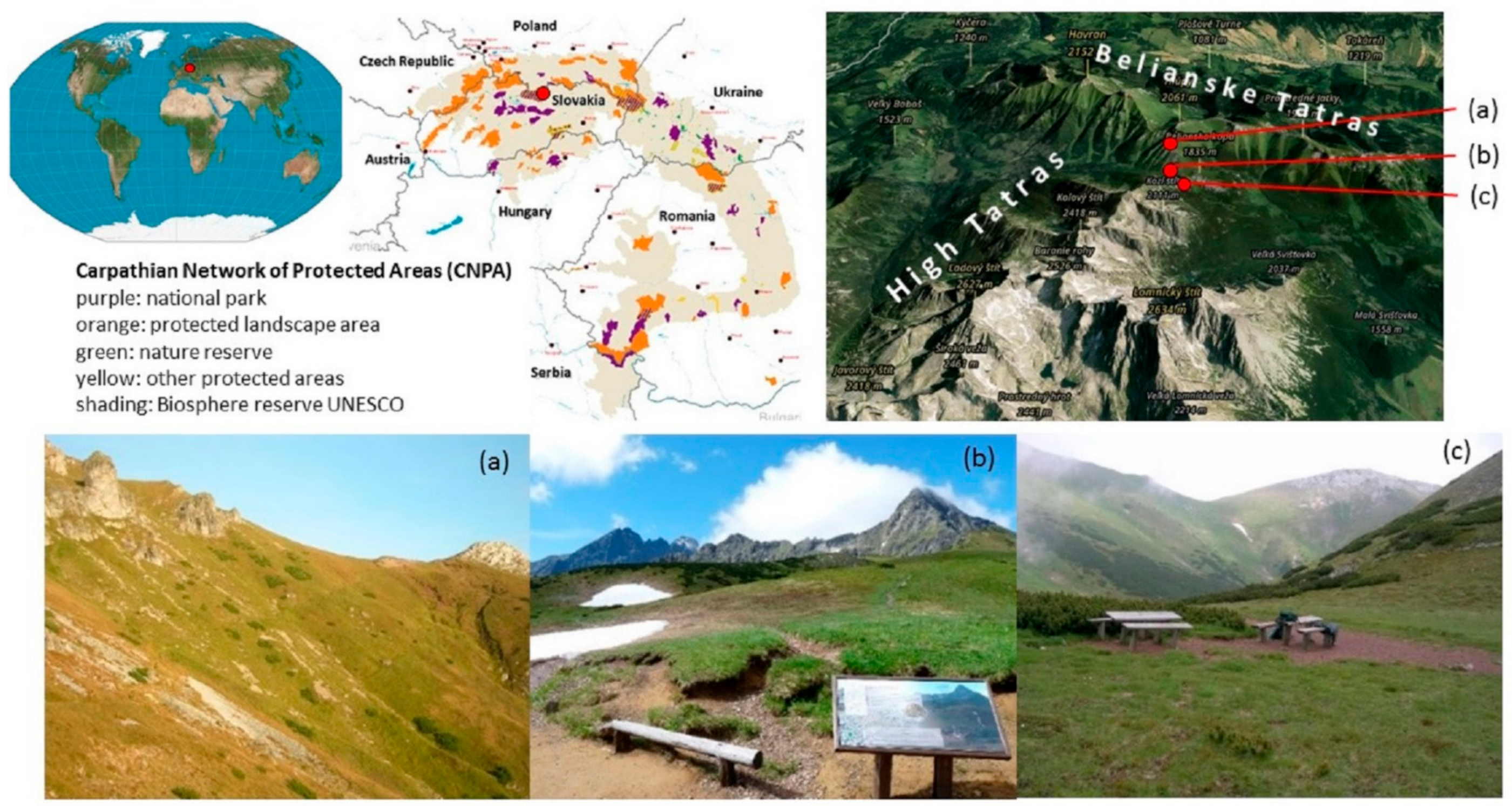
2.1. Study Area
- (1)
- The community Juncetum trifidi (Krajina 1933) is not one of the endangered phytocenoses, although it contains endemic taxa (Campanula tatrae, Leucanthemopsis tatrae, Soldanella carpatica). As a pioneering community, it has an important soil protection function. The community is formed by tufted hemicryptophytes (Juncus trifidus, Oreochloa disticha, Festuca supina) and rosette hemicryptophytes (Hieracium alpinum, Campanula alpina) with non-dominant shrub chamaephytes (Vaccinium vitis-idaea, Vaccinium myrthillus). The undergrowth consists of scaly and bushy lichens (Cetraria is-landica, Cladonia rangiferina, Cladonia gracilis, Cladonia arbuscula, Cladonia uncialis) and mosses (Polytrichum alpinum, Polytrichum piliferum). The bedrock consists of limestone, dolomites, and shales. The community spreads over rankers on the border of the High and Belianske Tatras. An experimental block was established on the NW site with a slope of 22°, at an altitude of 1754 m. In 2008, the average number of people visiting the path in this community was 298 per day.
- (2)
- The community Junco trifidi-Callunetum vulgaris (Krajina 1933) Hadač ex Šibík et al. 2007 with a small-scale and rare occurrence in the Western Carpathians is rare, not yet endangered. The community is formed by chamaephytes (Calluna vulgaris, Vaccinium myrthillus, Vaccinium vitis-idaea) and hemicryptophytes (Juncus trifidus, Avenella flexuosa, Hieracium alpinum, Campanula alpina). The undergrowth consists of lichens (Cetraria islandica, Cladonia gracilis). The bedrock consists of lime-stone, dolomites and shales. The community spreads over rankers on the border of the High and Be-lianske Tatras. An experimental block was established on the NE site with a slope of 4°, at an alti-tude of 1778 m. In 2008, the average number of people visiting the path in this community was 249 per day.
- (3)
- The community Seslerietum tatrae occurs in a narrow altitude range 1900–2000 m a.s.l. with long-lasting high snow cover. The community is formed by hemicryptophytes (Sesleria tatrae, Carex tatrorum, Anthoxanthum alpinum, Bastrsia alpina, Bistorta vivipara, Campanula tatrae, Helianthemum grandiflorum, Homogyne alpina, Pedicularis verticillata, Potentilla aura, Soldanella carpatica, Thymus pulcherrimus). The mosses reach a cover of 30% (Pleurozium schreberi). The bedrock consists of limestone, dolomites, and shales. The community spreads over lithosols in the National Nature Reserve Belianske Tatry. An experimental block was established on the SW site with a slope of 39°, at an altitude of 1924 m. In 2008, the average number of people visiting the path in this community was 86 per day.
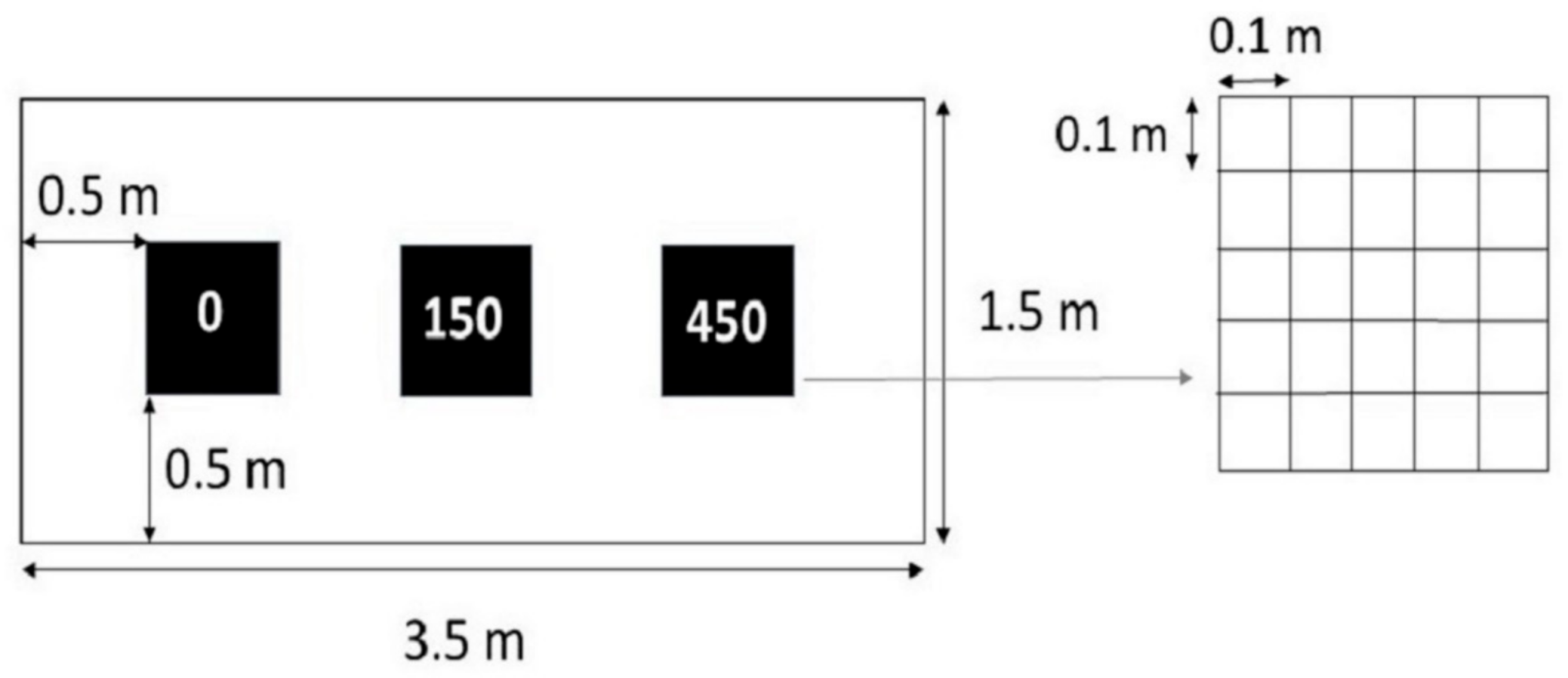
2.2. Experimental Design
2.3. Trampling Treatment and Timing
- visual estimates of the top cover perpendicular to the slope angle of each vascular plant species and of mosses and lichens;
- visual estimates of the top cover perpendicular to the slope angle of bare ground;
- visual estimates of the top cover perpendicular to the slope angle of litter.
- (1)
- Visual estimates of the coverage (%) of each vascular plant species and of mosses and lichens. Only green photosynthetic material should be included in cover estimates. It is inappropriate to include the cover of surviving stems that had been defoliated by trampling. Cover values are round integral numbers, and if the cover is less than 1% the value 0.5% or 0% can be used, indicating a complete lack of cover.
- (2)
- Visual estimates of the cover (%) of bare ground (ground not covered by live vegetation). Bare ground can be either mineral or soil.
- (3)
- Visual estimates of the cover (%) of litter (including the litter of recently trampled plants).
2.4. Data Analysis
Relative Cover
2.5. Loss of Species
2.6. Statistical Processing
3. Results
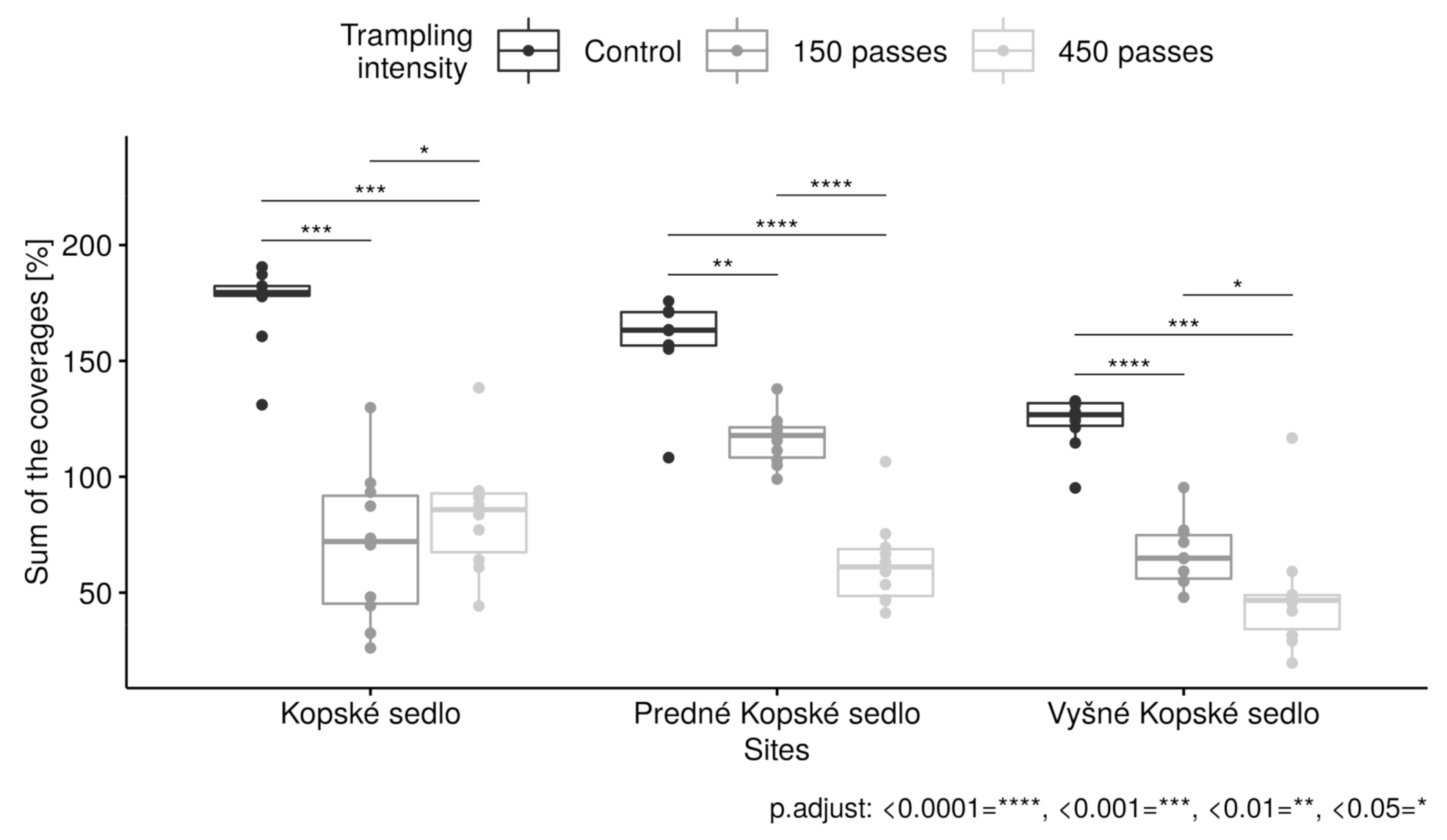
3.1. Interaction between Trampling Intensities and Sites
3.2. Impacts of Trampling Disturbance On Individual Species
- (1)
- The Juncetum trifidi community is dominated by hemicryptophytes (88%) and woody chamaephytes (12%). The most damaged were hemicryptophytes Bistorta major, Hieracium alpinum, Campanula alpina, Campanula tatrae, and Juncus trifidus. Particularly, woody chamaephyt Vaccinium myrtillus was more resistant to the process of trampling than broadleaf hemicryptophytes.
- (2)
- The Junco trifidi-Callunetum vulgaris community is dominated by hemicryptophytes (86%) and woody chamaephytes (14%). The most damaged species were hemikryptophytes Pulsatilla scherfelii, Bistorta major, Campanula alpina, Campanula tatrae, and Juncus trifidus. Woody chamaephytes Calluna vul-garis, Vaccinium myrtillus and Vaccinium vitis-idaea were more resistant to the process of trampling than broadleaf hemicryptophytes.
- (3)
- The Seslerietum tatrae community is dominated by hemicryptophytes (67%), woody and herbaceous chamaephytes (26%), annual terophytes (4%), and geophytes (3%). The most damaged species were hemikrypthytes Sesleria tatrae, Carex sempervirens, Luzula alpinopilosa subsp, obscura, Rhodiola rosea, and Phyteuma orbiculare. In this community, the woody chamephytes of Salix reticulata and Salix kitaibeliana were severely damaged during the trampling process.
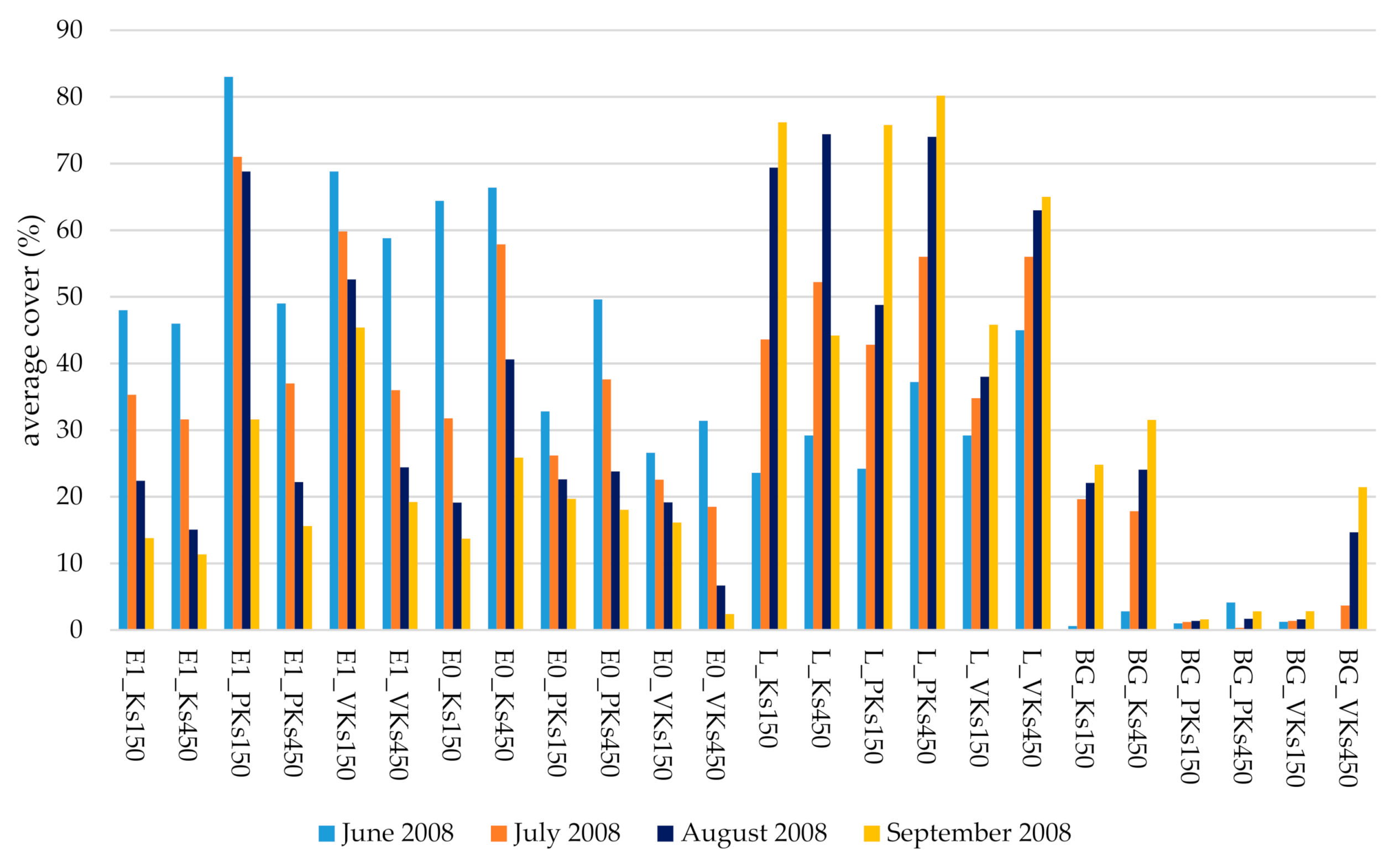
3.3. Impacts of Trampling Disturbance on Vegetation Layers, Litter and Bare Ground
3.4. Species Loss
3.5. Relative Cover of Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 1st ed.; Springer: Berlin, Germany, 2003; p. 343. [Google Scholar]
- Körner, C.; Spehn, E.M. Mountain Biodiversity: A Global Assessment, 1st ed.; Routledge: London, UK, 2002; p. 350. [Google Scholar]
- Willard, B.E.; Cooper, D.J.; Forbes, B.C. Natural Regeneration of Alpine Tundra Vegetation after Human Trampling: A 42-year Data Set from Rocky Mountain National Park, Colorado, U.S.A. Arct. Antarct. Alp. Res. 2007, 39, 177–183. [Google Scholar] [CrossRef][Green Version]
- Ram, J.; Singh, J.S.; Singh, S.P. Plant Biomass, Species Diversity and Net Primary Production in a Central Himalayan High Altitude Grassland. J. Ecol. 1989, 77, 456. [Google Scholar] [CrossRef]
- Dhar, U. Conservation implications of plant endemism in high altitude Himalaya. Curr. Sci. 2002, 82, 141–148. [Google Scholar]
- Price, M.F. Conservation and Sustainable Development in Mountain Areas; IUCN: Gland, Switzerland, 2004. [Google Scholar]
- Wang, X.X.; Dong, S.K.; Yang, B.; Li, Y.Y.; Su, X.K. A comparison of biodiversityeecosystem function relationships in alpine grasslands across a degradation gradient on the Qinghai-Tibetan Plateau. Rangel. Ecol. Manag. 2014, 37, 45–55. [Google Scholar]
- Buckley, R. Tourism in the Most Fragile Environments. Tour. Recreat. Res. 2000, 25, 31–40. [Google Scholar] [CrossRef]
- Roovers, P.; Verheyen, K.; Hermy, M.; Gulinck, H. Experimental trampling and vegetation recovery in some forest and heathland communities. Appl. Veg. Sci. 2004, 7, 111–118. [Google Scholar] [CrossRef]
- Scott, J.J.; Kirkpatrick, J.B. Effects of human trampling on the sub-Antarctic vegetation of Macquarie Island. Polar Rec. 1994, 30, 207–220. [Google Scholar] [CrossRef]
- Cole, D.N. Impacts of hiking and camping on soils and vegetation: A review. In Environmental Impact of Ecotourism, 1st ed.; Buckley, R., Ed.; CABI Publishing: Oxfordshire, UK, 2004; pp. 41–60. [Google Scholar]
- Pickering, C.M.; Growcock, A.J. Impacts of experimental trampling on tall alpine herbfields and subalpine grasslands in the Australian Alps. J. Environ. Manag. 2009, 91, 532–540. [Google Scholar] [CrossRef]
- Crisfield, V.E.; Macdonald, S.E.; Gould, A.J. Effects of Recreational Traffic on Alpine Plant Communities in the Northern Canadian Rockies. Arct. Antarct. Alp. Res. 2012, 44, 277–287. [Google Scholar] [CrossRef]
- Barros, A.; Gonnet, J.; Pickering, C. Impacts of informal trails on vegetation and soils in the highest protected area in the Southern Hemisphere. J. Environ. Manag. 2013, 127, 50–60. [Google Scholar] [CrossRef]
- Cole, D.N. Experimental Trampling of Vegetation. I. Relationship Between Trampling Intensity and Vegetation Response. J. Appl. Ecol. 1995, 32, 203. [Google Scholar] [CrossRef]
- Xu, L.; Freitas, S.M.A.; Yu, F.-H.; Dong, M.; Anten, N.P.R.; Werger, M.J.A. Effects of Trampling on Morphological and Mechanical Traits of Dryland Shrub Species Do Not Depend on Water Availability. PLoS ONE 2013, 8, e53021. [Google Scholar] [CrossRef] [PubMed]
- Liddle, M. A selective review of the ecological effects of human trampling on natural ecosystems. Biol. Conserv. 1975, 7, 17–36. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Alatalo, J.M. Effects of human trampling on abundance and diversity of vascular plants, bryophytes and lichens in alpine heath vegetation, Northern Sweden. SpringerPlus 2015, 4, 95. [Google Scholar] [CrossRef]
- Forbes, B.C.; Monz, C.; Tolvanen, A. Tourism ecological impacts in terrestrial polar ecosystems. In Environmental Impact of Ecotourism, 1st ed.; Buckley, R., Ed.; CABI Publishing: Oxfordshire, UK, 2004; pp. 155–170. [Google Scholar]
- Pertierra, L.R.; Lara, F.; Tejedo, P.; Quesada, A.; Benayas, J. Rapid denudation processes in cryptogamic communities from Maritime Antarctica subjected to human trampling. Antarct. Sci. 2013, 25, 318–328. [Google Scholar] [CrossRef]
- Li, W.; He, S.; Cheng, X.; Zhang, G. Short-term effects of experimental trampling on alpine grasslands in Shangri-la, China. Glob. Ecol. Conserv. 2020, 23, e01161. [Google Scholar] [CrossRef]
- Cole, D.N. Experimental Trampling of Vegetation. II. Predictors of Resistance and Resilience. J. Appl. Ecol. 1995, 32, 215. [Google Scholar] [CrossRef]
- Whinam, J.; Chilcott, N. Impacts of trampling on alpine environments in central Tasmania. J. Environ. Manag. 1999, 57, 205–220. [Google Scholar] [CrossRef]
- Cole, D.N.; Monz, C.A. Trampling Disturbance of High-Elevation Vegetation, Wind River Mountains, Wyoming, U.S.A. Arct. Antarct. Alp. Res. 2002, 34, 365. [Google Scholar] [CrossRef]
- Whinam, J.; Chilcott, N.M. Impacts after four years of experimental trampling on alpine/sub-alpine environments in western Tasmania. J. Environ. Manag. 2003, 67, 339–351. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Gray, A.; Vanbergen, A.J.; Bergès, L.; Bohner, A.; Brooker, R.W.; De Bruyn, L.; De Cinti, B.; Dirnböck, T.; Grandin, U.; et al. Functional traits and local environment predict vegetation responses to disturbance: A pan-European multi-site experiment. J. Ecol. 2011, 99, 777–787. [Google Scholar] [CrossRef]
- Pescott, O.L.; Stewart, G.B. Assessing the impact of human trampling on vegetation: A systematic review and meta-analysis of experimental evidence. PeerJ 2014, 2, e360. [Google Scholar] [CrossRef]
- Gremmen, N.J.M.; Smith, V.R.; Van Tongeren, O.F.R. Impact of Trampling on the Vegetation of Subantarctic Marion Island. Arct. Antarct. Alp. Res. 2003, 35, 442–446. [Google Scholar] [CrossRef]
- Longton, R.E. Bryophyte vegetation in polar regions. In Bryophyte Ecology, 1st ed.; Smith, A.J.E., Ed.; Springer: Amsterdam, The Netherlands, 1982; pp. 123–165. [Google Scholar]
- Matveyeva, N.; Chernov, Y. Biodiversity of terrestrial ecosystems. In The Arctic: Environment, People, Policy; Nuttall, M., Callaghan, T.V., Eds.; Harwood Academic Publishers: Groningen, The Netherlands, 2000; pp. 233–273. [Google Scholar]
- Vitt, D.H.; Pakarinen, P. The bryophyte vegetation production and organic components of Truelove Lowland. In Truelove Lowland, Canada: A High Arctic Ecosystem; Bliss LC: Edmonton, AB, Canada, 1977; pp. 225–244. [Google Scholar]
- Wielgolaski, F.E.; Bliss, L.C.; Svoboda, J.; Doyle, G. Primary production of tundra. In Tundra Ecosystems: A Comparative Analysis, 1st ed.; Heal, O.W., Moore, J.J., Eds.; Bliss LC: Cambridge, UK, 1981; pp. 187–226. [Google Scholar]
- Andersen, U.V. Resistance of Danish coastal vegetation types to human trampling. Biol. Conserv. 1995, 71, 223–230. [Google Scholar] [CrossRef]
- Arnesen, T. Vegetation dynamics following trampling in grassland and heathland in Sølendet Nature Reserve, a boreal upland area in Central Norway. Nord. J. Bot. 1999, 19, 47–69. [Google Scholar] [CrossRef]
- Liddle, M.J.; Greig-Smith, P. A survey of tracks and paths in a sand dune ecosystem II. Vegetation. J. Appl. Ecol. 1975, 12, 909–930. [Google Scholar] [CrossRef]
- Bayfield, N.G. Recovery of four montane heath communities on Cairngorm, Scotland, from disturbance by trampling. Biol. Conserv. 1979, 15, 165–179. [Google Scholar] [CrossRef]
- Gallet, S.; Roze, F. Resistance of Atlantic Heathlands to trampling in Brittany (France): Influence of vegetation type, season and weather conditions. Biol. Conserv. 2001, 97, 189–198. [Google Scholar] [CrossRef]
- Gallet, S.; Rozé, F. Long-term effects of trampling on Atlantic Heathland in Brittany (France): Resilience and tolerance in relation to season and meteorological conditions. Biol. Conserv. 2002, 103, 267–275. [Google Scholar] [CrossRef]
- Kostrakiewicz-Gierałt, K.; Pliszko, A.; Gmyrek-Gołąb, K. The Effect of Visitors on the Properties of Vegetation of Calcareous Grasslands in the Context of Width and Distances from Tourist Trails. Sustainability 2020, 12, 454. [Google Scholar] [CrossRef]
- Cole, D.N. Effects of three seasons of experimental trampling on five montane forest communities and a grassland in Western Montana, USA. Biol. Conserv. 1987, 40, 219–244. [Google Scholar] [CrossRef]
- Cole, D.N.; Bayfield, N.G. Recreational trampling of vegetation: Standard experimental procedures. Biol. Conserv. 1993, 63, 209–215. [Google Scholar] [CrossRef]
- Kelly, J.R.; Harwell, M.A. Indicators of ecosystem recovery. Environ. Manag. 1990, 14, 527–545. [Google Scholar] [CrossRef]
- Kuss, F.R.; Hall, C.N. Ground flora trampling studies: Five years after closure. Environ. Manag. 1991, 15, 715–727. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 21 January 2021).
- Apollo, M.; Andreychouk, V. Trampling Intensity and Vegetation Response and Recovery according to Altitude: An Experimental Study from the Himalayan Miyar Valley. Resources 2020, 9, 98. [Google Scholar] [CrossRef]
- Bates, G.H. The Vegetation of Footpaths, Sidewalks, Cart-Tracks and Gateways. J. Ecol. 1935, 23, 470. [Google Scholar] [CrossRef]
- Speight, M.C. Outdoor Recreation and Its Ecological Effects: A Bibliography and Review; University College: London, UK, 1973; Volume 4. [Google Scholar]
- Dale, D.; Weaver, T. Trampling Effects on Vegetation of the Trail Corridors of North Rocky Mountain Forests. J. Appl. Ecol. 1974, 11, 767. [Google Scholar] [CrossRef]
- Nepal, S.K. Tourism in protected areas: The Nepalese Himalaya. Ann. Tour. Res. 2000, 27, 661–681. [Google Scholar] [CrossRef]
- Marek, A.; Wieczorek, M. Tourist Traffic in the Aconcagua Massif Area. Quaest. Geogr. 2015, 34, 65–76. [Google Scholar] [CrossRef][Green Version]
- Barros, A.; Monz, C.; Pickering, C. Is tourism damaging ecosystems in the Andes? Current knowledge and an agenda for future research. Ambio 2014, 44, 82–98. [Google Scholar] [CrossRef]
- Hertlová, B.; Popelka, O.; Zeidler, M.; Banaš, M. Alpine plant communities responses to simulated mechanical disturbances of tourism, case study from the High Sudetes Mts. J. Landsc. Manag. 2016, 7, 16–21. [Google Scholar]
- Lemauviel, S.; Gallet, S.; Roze, F. Responses of three heathland shrubs to single or repeated experimental trampling. Environ. Manag. 2004, 33, 821–829. [Google Scholar] [CrossRef]
- Grabherr, G. The impact of trampling by tourists on a high altitudinal grassland in the Tyrolean Alps, Austria. Vegetatio 1982, 48, 209–217. [Google Scholar]
- Talbot, L.M.; Turton, S.M.; Graham, A.W. Trampling resistance of tropical rainforest soils and vegetation in the wet tropics of north east Australia. J. Environ. Manag. 2003, 69, 63–69. [Google Scholar] [CrossRef]
- Zachar, D. Soil Erosion; Elsevier: New York, NY, USA, 2011. [Google Scholar]
- Marion, J.L.; Cole, D.N. Spatial and Temporal Variation in Soil and Vegetation Impacts on Campsites. Ecol. Appl. 1996, 6, 520–530. [Google Scholar] [CrossRef]
- Cole, D.; Spildie, D. Hiker, horse and llama trampling effects on native vegetation in Montana, USA. J. Environ. Manag. 1998, 53, 61–71. [Google Scholar] [CrossRef]
- Monz, C.A. The response of two arctic tundra plant communities to human trampling disturbance. J. Environ. Manag. 2002, 64, 207–217. [Google Scholar] [CrossRef]
- Sun, D.; Liddle, M. Field occurrence, recovery, and simulated trampling resistance and recovery of two grasses. Biol. Conserv. 1991, 57, 187–203. [Google Scholar] [CrossRef]
- Chardon, N.I.; Wipf, S.; Rixen, C.; Beilstein, A.; Doak, D.F. Local trampling disturbance effects on alpine plant populations and communities: Negative implications for climate change vulnerability. Ecol. Evol. 2018, 8, 7921–7935. [Google Scholar] [CrossRef]
- Leug, Y.; Marion, J.L. Recreation Impacts and Management in Wilderness: A State-of-Knowledge Reviwe. In Proceedings of the Wilderness Science in a Time of Change Conference, Missoula, MT, USA, 23–27 May 1999; Volume 15. [Google Scholar]
- Li, Z.; Siemann, E.; Deng, B.; Wang, S.; Gao, Y.; Liu, X.; Zhang, X.; Guo, X.; Zhang, L. Soil microbial community responses to soil chemistry modifications in alpine meadows following human trampling. Catena 2020, 194, 104717. [Google Scholar] [CrossRef]
- Cole, D.N. Minimizing conflict between recreation and nature conservation. In Ecology of Greenways: Design and Function of Linear Conservation Areas; Smith, D.S., Hellmund, P.C., Eds.; University of Minnesota Press: Minneapolis, MN, USA, 1993; pp. 105–122. [Google Scholar]
- Klug, B.; Scharfetter-Lehrl, G.; Scharfetter, E. Effects of trampling on vegetation above the timberline in the eastern Alps, Austria. Arct. Antarct. Alp. Res. 2002, 34, 377–388. [Google Scholar] [CrossRef]
- Marion, J.L.; Olive, N. Assessing and Understanding Trail Degradation: Results from Big South Fork National River and Recreational Area. US Geological Survey. 2006. Available online: www.pwrc.usgs.gov (accessed on 10 April 2020).
- Fidelus, J. Slope transformations within tourist footpaths in the northern and southern parts of the Western Tatra Mountains (Poland, Slovakia). Z. Geomorphol. 2016, 60, 139–162. [Google Scholar] [CrossRef]
- Barros, A.; Pickering, C.M. Impacts of experimental trampling by hikers and pack animals on a high-altitude alpine sedge meadow in the Andes. Plant Ecol. Divers. 2014, 8, 265–276. [Google Scholar] [CrossRef]
- Barros, A.; Pickering, C.M.; Renison, D. Short-Term Effects of Pack Animal Grazing Exclusion from Andean Alpine Meadows. Arct. Antarct. Alp. Res. 2014, 46, 333–343. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Sánchez, J.R.; Fillat, F.; Aguirre, J.; Gomez, D.; Badía-Villas, D. Influence of livestock soil eutrophication on floral composition in the Pyrenees mountains. J. Mt. Sci. 2008, 5, 63–72. [Google Scholar] [CrossRef]
- Hiltbrunner, D.; Schulze, S.; Hagedorn, F.; Schmidt, M.W.; Zimmmermann, S. Cattle trampling alters soil properties and changes soil microbial communities in a Swiss sub-alpine pasture. Geoderma 2012, 170, 369–377. [Google Scholar] [CrossRef]
- Tomczyk, A.M.; Ewertowski, M. Planning of recreational trails in protected areas: Application of regression tree analysis and geographic information systems. Appl. Geogr. 2013, 40, 129–139. [Google Scholar] [CrossRef]
- Arnberger, A.; Eder, R.; Allex, B.; Sterl, P.; Burns, R.C. Relationships between national-park affinity and attitudes towards protected area management of visitors to the Gesaeuse National Park, Austria. For. Policy Econ. 2012, 19, 48–55. [Google Scholar] [CrossRef]

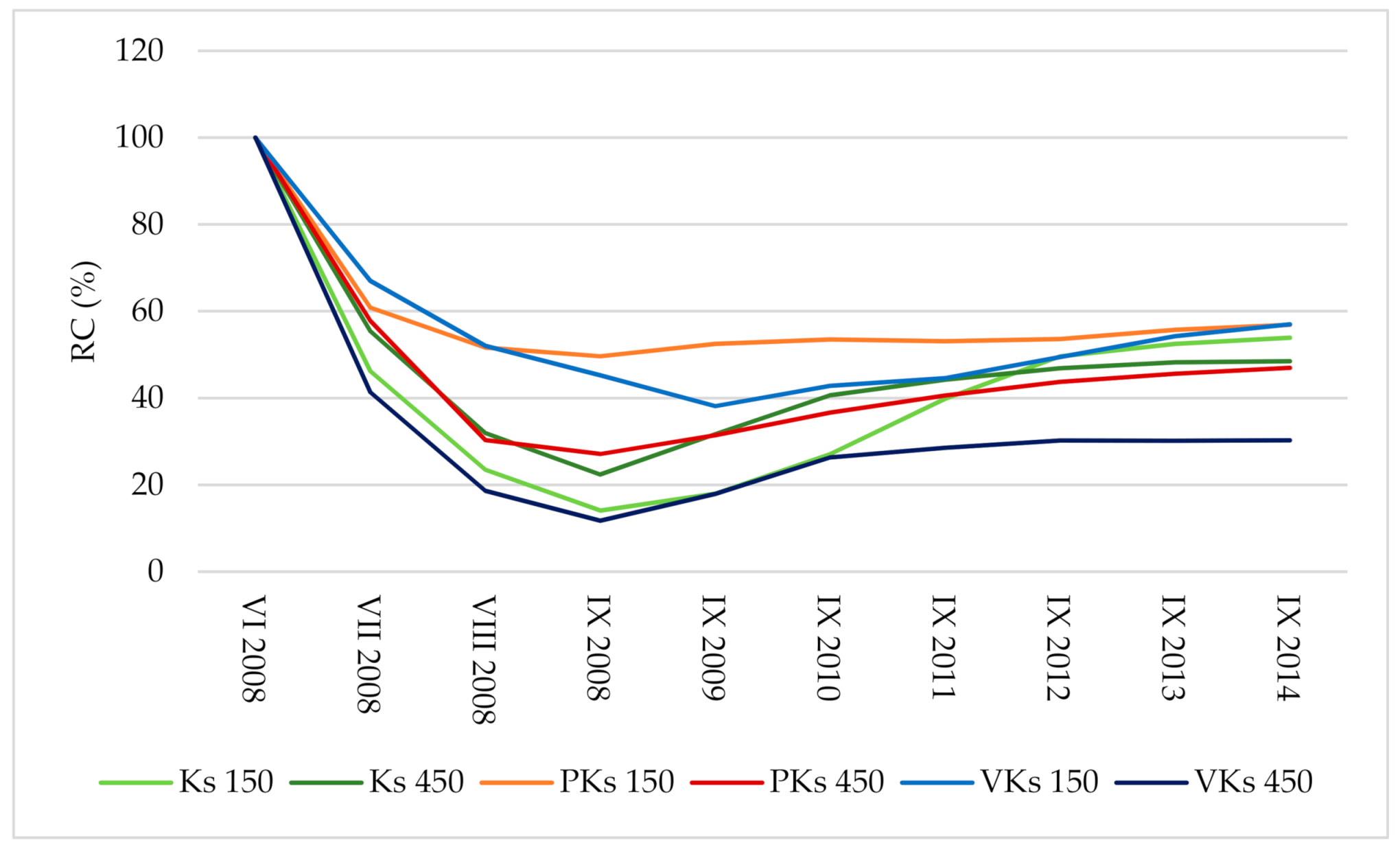
| Date/Plot | Ks150 | Ks450 | PKs150 | PKs450 | VKs150 | VKs450 |
|---|---|---|---|---|---|---|
| June/July 2008 | 6.67 | 0.00 | −15.78 | −18.12 | −13.64 | −38.10 |
| June/August 2008 | 0.00 | 0.00 | −20.89 | −33.88 | −31.82 | −57.14 |
| June/September 2008 | 6.67 | −5.88 | −26.89 | −41.76 | −45.45 | −61.90 |
| June 2008/September 2009 | 6.67 | −5.88 | −15.44 | −35.88 | −31.82 | −47.62 |
| June 2008/September 2010 | 6.67 | −5.88 | −12.33 | −31.76 | −18.18 | −33.33 |
| June 2008/September 2011 | 6.67 | 0.00 | −6.56 | −29.41 | −4.55 | −23.81 |
| June 2008/September 2012 | 6.67 | 0.00 | −4.89 | −25.41 | −4.55 | −19.05 |
| June 2008/September 2013 | 6.67 | 0.00 | −4.00 | −26.82 | −9.09 | −19.05 |
| June 2008/September 2014 | 6.67 | 0.00 | −3.11 | −26.82 | −13.64 | −19.05 |
| Plot | July 2008 | August 2008 | September 2008 | |||
|---|---|---|---|---|---|---|
| Mosses | Lichens | Mosses | Lichens | Mosses | Lichens | |
| Ks150 | 61.32 | 70.57 | 38.16 | 65.08 | 22.47 | 52.09 |
| Ks450 | 43.32 | 59.47 | 42.06 | 40.84 | 31.14 | 25.26 |
| PKs150 | 66.6 | 86.60 | 55.84 | 81.09 | 33.09 | 78.08 |
| PKs450 | 56.46 | 77.59 | 15.21 | 66.86 | 12.28 | 65.64 |
| VKs150 | 80.75 | 96.43 | 75.69 | 68.63 | 60.88 | 67.9 |
| VKs450 | 40.65 | 71.48 | 34.09 | 2.91 | 31.95 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscová, V.; Ševčík, M.; Hreško, J.; Petrovič, F. Effects of a Short-Term Trampling Experiment on Alpine Vegetation in the Tatras, Slovakia. Sustainability 2021, 13, 2750. https://doi.org/10.3390/su13052750
Piscová V, Ševčík M, Hreško J, Petrovič F. Effects of a Short-Term Trampling Experiment on Alpine Vegetation in the Tatras, Slovakia. Sustainability. 2021; 13(5):2750. https://doi.org/10.3390/su13052750
Chicago/Turabian StylePiscová, Veronika, Michal Ševčík, Juraj Hreško, and František Petrovič. 2021. "Effects of a Short-Term Trampling Experiment on Alpine Vegetation in the Tatras, Slovakia" Sustainability 13, no. 5: 2750. https://doi.org/10.3390/su13052750
APA StylePiscová, V., Ševčík, M., Hreško, J., & Petrovič, F. (2021). Effects of a Short-Term Trampling Experiment on Alpine Vegetation in the Tatras, Slovakia. Sustainability, 13(5), 2750. https://doi.org/10.3390/su13052750






