Soil Respiration May Overestimate or Underestimate in Forest Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site
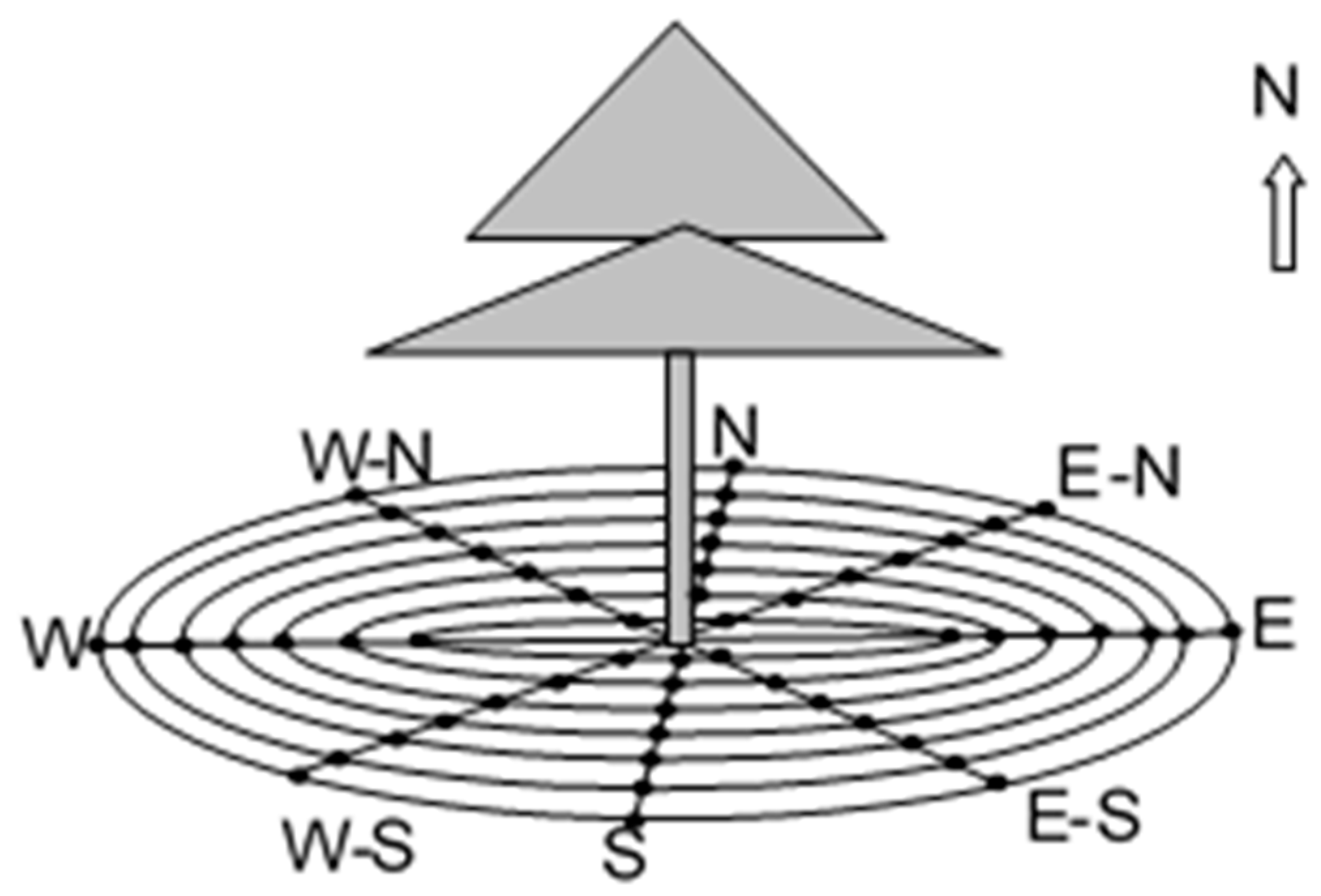
2.2. Experimental Design
2.3. Data Analysis
3. Results
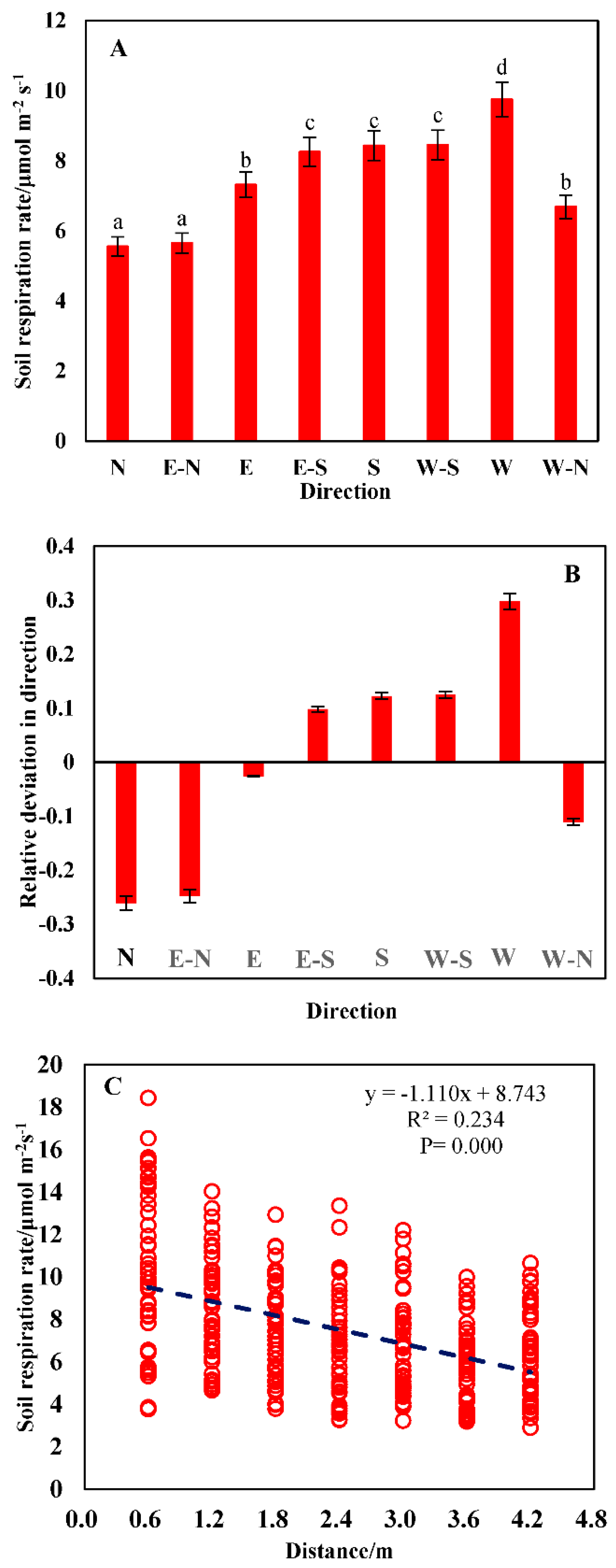
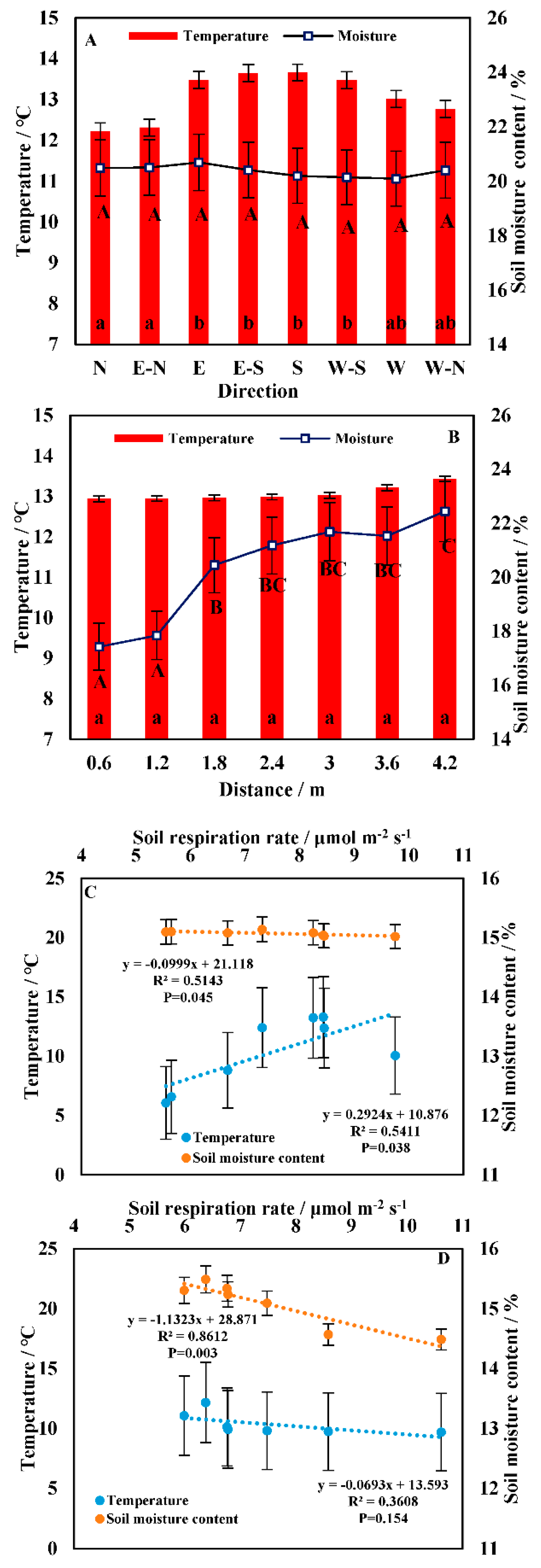
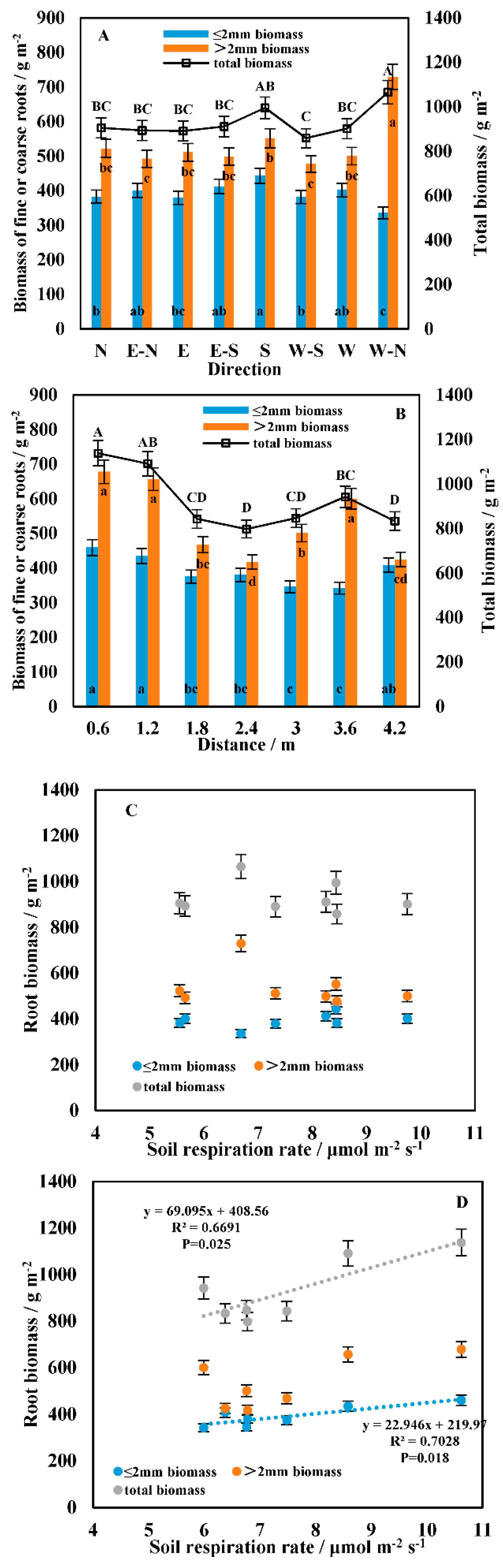
3.1. Spatial Heterogeneity in the Soil Respiration Rate
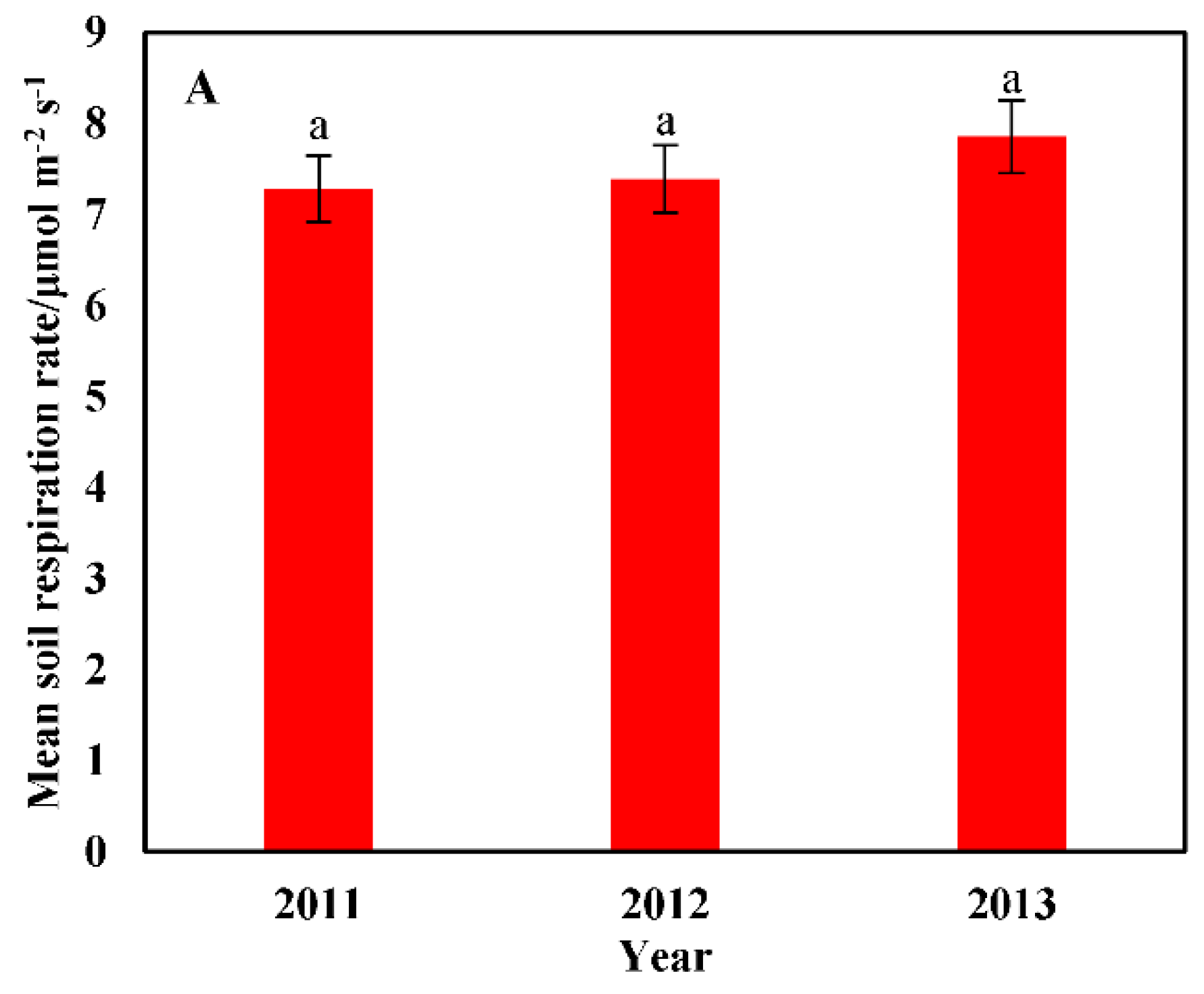
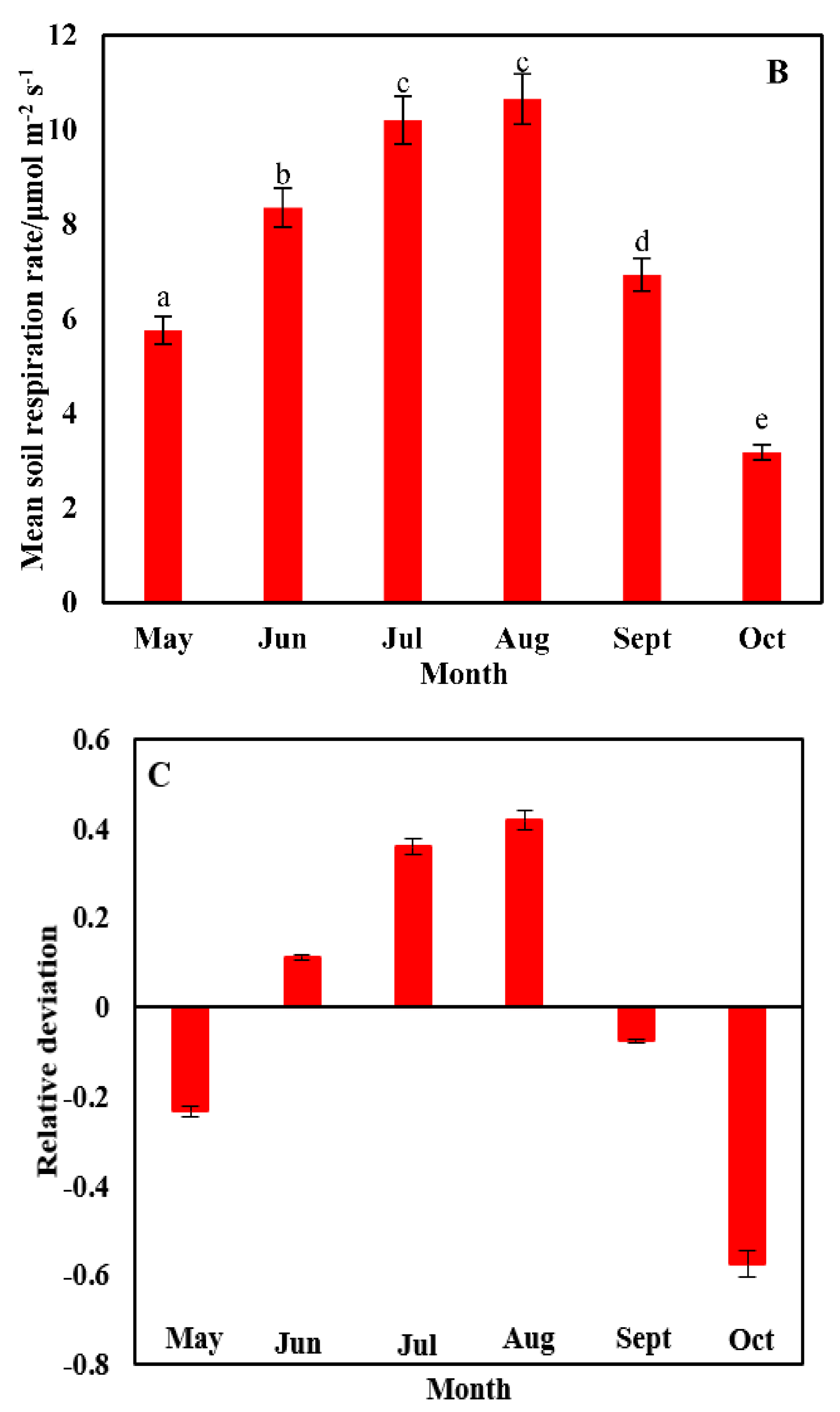
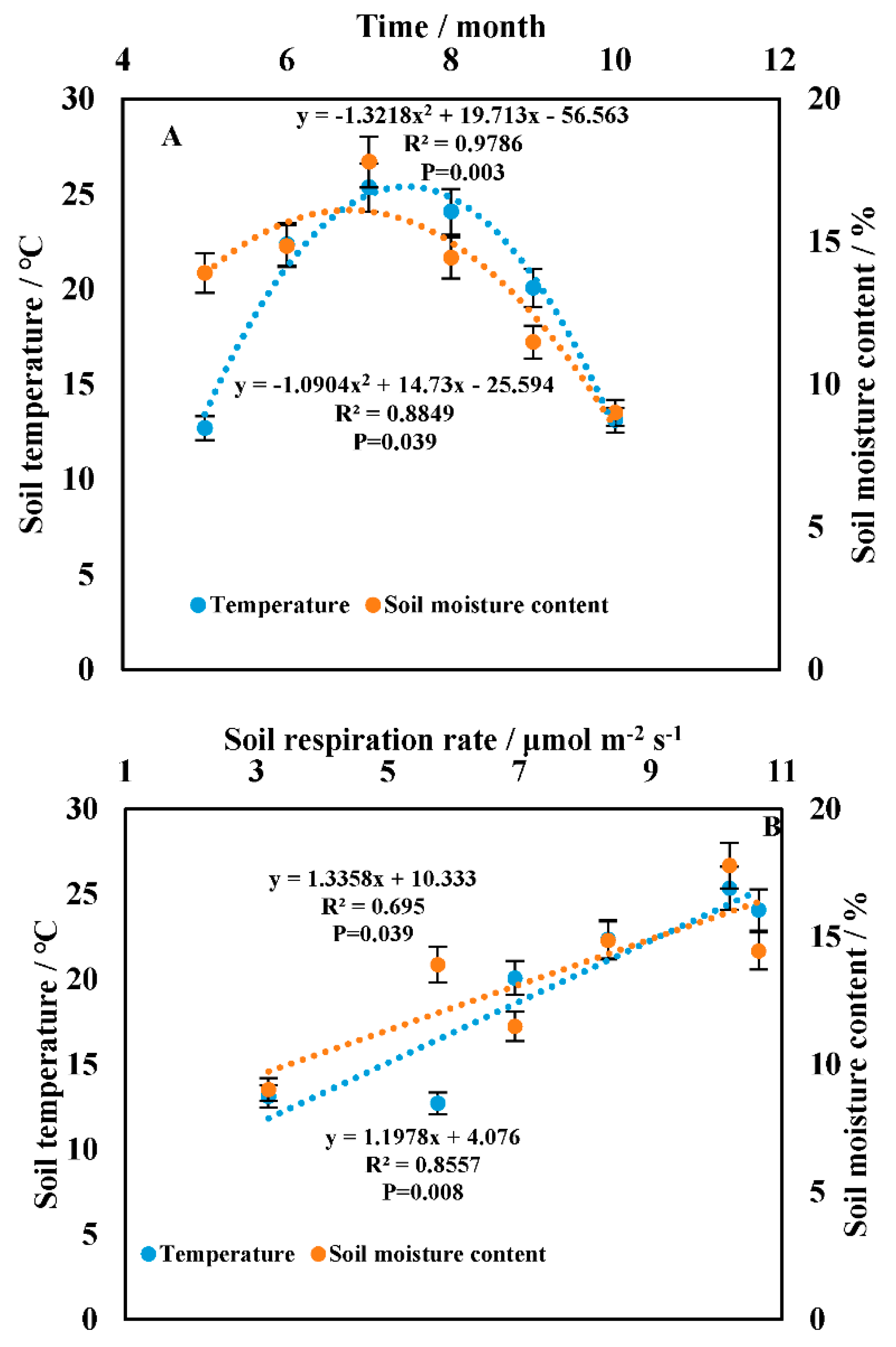
3.2. Temporal Heterogeneity in the Soil Respiration Rate
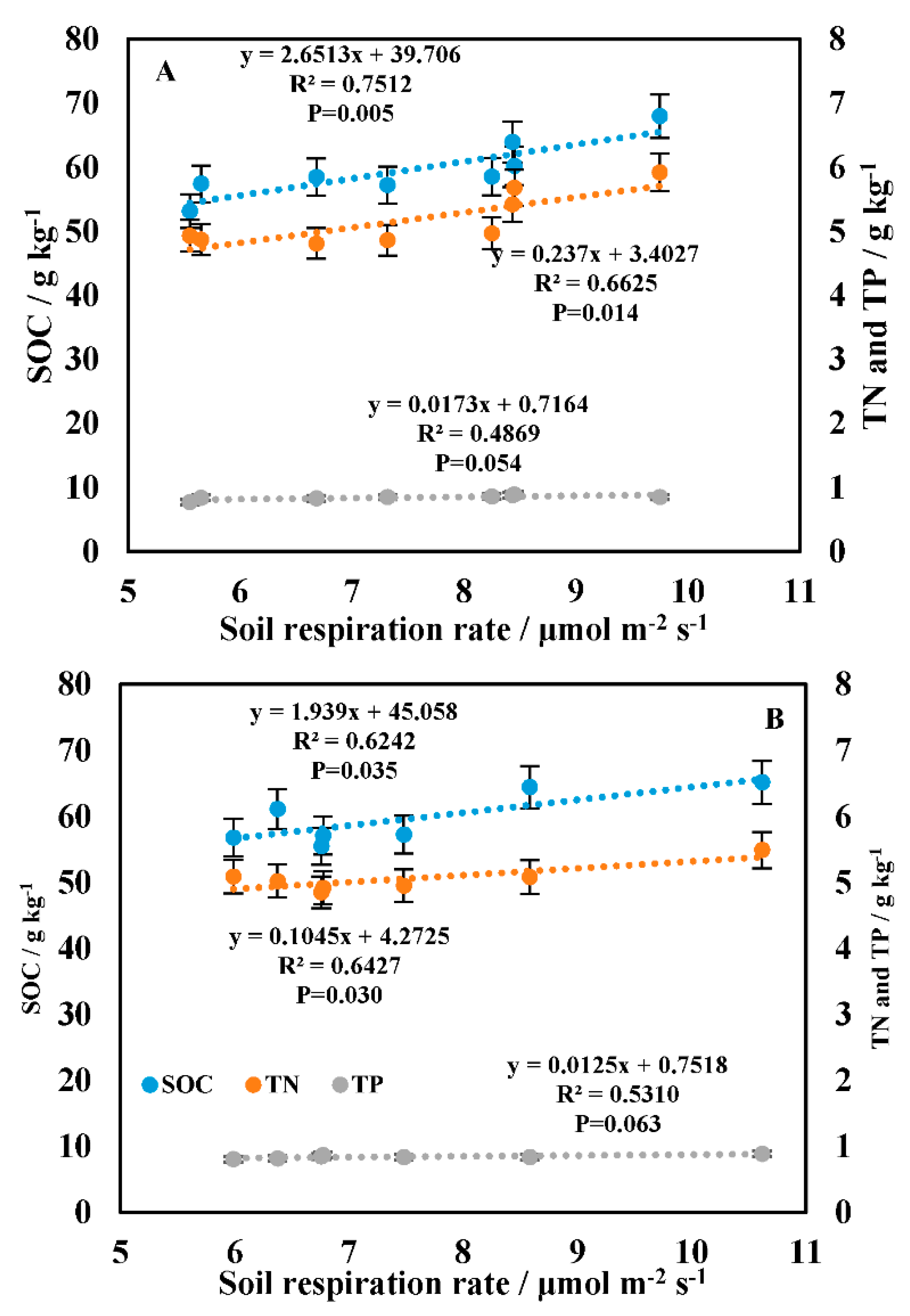
3.3. Correlation between Soil Respiration and the Driving Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrence, M.G.; Schafer, S.; Muri, H.; Scott, V.; Oschlies, A.; Vaughan, N.E.; Boucher, O.; Schmidt, H.; Haywood, J.; Scheffran, J. Evaluating climate geoengineering proposals in the context of the Paris Agreement temperature goals. Nat. Commun. 2018, 9, 3734. [Google Scholar] [CrossRef]
- Borken, W.; Xu, Y.J.; Davidson, E.A.; Beese, A. Site and temporal variation of soil respiration in European beech, Norway spruce, and Scots pine forests. Global Chang. Biol. 2002, 8, 1205–1216. [Google Scholar] [CrossRef]
- Chen, S.P.; Lin, G.H.; Huang, J.H.; Jenerette, G.D. Dependence of carbon sequestration on the differential responses of ecosystem photosynthesis and respiration to rain pulses in a semiarid steppe. Global Chang. Biol. 2009, 15, 2450–2461. [Google Scholar] [CrossRef]
- Chimner, R.A.; Welker, J.M.; Morgan, J.; Lecain, D.; Reeder, J. Experimental manipulations of winter snow and summer rain influence ecosystem carbon cycling in a mixed-grass prairie, Wyoming, USA. Ecohydrology 2010, 3, 284–293. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joos, O. Experimental summer drought reduces soil CO2 effluxes and DOC leaching in Swiss grassland soils along an elevational gradient. Biogeochemistry 2014, 117, 395–412. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Francaviglia, R.; Renzi, G. A cost and time-effective method to evaluate soil microbial respiration for soil quality assessment. Appl. Soil Ecol. 2019, 140, 121–125. [Google Scholar] [CrossRef]
- Yim, M.H.; Joo, S.J.; Nakane, K. Comparison of field methods for measuring soil respiration: A static alkali absorption method and two dynamic closed chamber methods. Forest Ecol. Manag. 2002, 170, 189–197. [Google Scholar] [CrossRef]
- Vargas, R.; Collins, S.L.; Thomey, M.; Johnson, J.E.; Brown, R.F.; Natvig, D.O.; Friggens, M.T. Precipitation variability and fire influence the temporal dynamics of soil CO2 efflux in an arid grassland. Global Chang. Biol. 2012, 18, 1401–1411. [Google Scholar] [CrossRef]
- Xu, X.; Shi, Z.; Chen, X.; Lin, Y.; Niu, S.; Jiang, L.; Luo, R.; Luo, Y. Unchanged carbon balance driven by equivalent responses of production and respiration to climate change in a mixed-grass prairie. Global Chang. Biol. 2016, 22, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Peñuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Global Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Sponseller, R.A. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Chang. Biol. 2007, 13, 426–436. [Google Scholar] [CrossRef]
- Smith, N.G.; Rodgers, V.L.; Brzostek, E.R.; Kulmatiski, A.; Avolio, M.L.; Hoover, D.L.; Koerner, S.E.; Grant, K.; Jentsch, A.; Fatichi, S.; et al. Toward a better integration of biological data from precipitation manipulation experiments into Earth system models. Rev. Geophys. 2014, 52, 412–434. [Google Scholar] [CrossRef]
- Suseela, A.; Dukes, J. The responses of soil and rhizosphere respiration to simulated climatic changes vary by season. Ecology 2013, 94, 403–413. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Global Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Agren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and soil organic matter decomposition rates—Synthesis of current knowledge and a way forward. Global Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Gomez-Casanovas, N.; Matamala, R.; Cook, D.R.; Gonzalez-Meler, M.A. Net ecosystem exchange modifies the relationship between the autotrophic and heterotrophic components of soil respiration with abiotic factors in prairie grasslands. Global Chang. Biol. 2012, 18, 2532–2545. [Google Scholar] [CrossRef]
- Chu, X.J.; Han, G.X.; Xing, Q.H.; Xia, J.Y.; Sun, B.Y.; Li, X.G.; Yu, J.B.; Li, D.J.; Song, W.M. Changes in plant biomass induced by soil moisture variability drive interannual variation in the net ecosystem CO2 exchange over a reclaimed coastal wetland. Agric. For. Meteorol. 2019, 264, 138–148. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Horwath, W.R.; Dorodnikov, M.; Blagodatskaya, E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: No changes in pools, but increased fluxes and accelerated cycles. Soil Biol. Biochem. 2019, 128, 66–78. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Mukumbuta, I.; Shimizu, M.; Hatano, R. Short-term land-use change from grassland to cornfield increases soil organic carbon and reduces total soil respiration. Soil Till. Res. 2019, 186, 1–10. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Baldocchi, D.; Chu, H.S.; Reichstein, M. Inter-annual variability of net and gross ecosystem carbon fluxes: A review. Agric. For. Meteorol. 2018, 249, 520–533. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekcioglu, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Yu, S.Q.; Chen, Y.Q.; Zhao, J.; Fu, S.L.; Li, Z.; Xia, H.P.; Zhou, L.X. Temperature sensitivity of total soil respiration and its heterotrophic and autotrophic components in six vegetation types of subtropical China. Sci. Total Environ. 2017, 607, 160–167. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Meir, P.; Aragao, L.E.O.C.; Malhi, Y.; da Costa, A.C.L.; Braga, A.; Goncalves, P.H.L.; de Athaydes, J.; de Almeida, S.S.; Williams, M. Factors controlling spatio-temporal variation in carbon dioxide efflux from surface litter, roots, and soil organic matter at four rain forest sites in the eastern Amazon. J. Geophys. Res-Biogeo. 2007, 112, G04001. [Google Scholar] [CrossRef]
- Kupers, J.J.; van Gelderen, K.; Pierik, R. Location matters: Canopy light responses over spatial scales. Trends Plant Sci. 2018, 23, 865–873. [Google Scholar] [CrossRef]
- Crockford, R.H.; Richardson, D.P. Partitioning of rainfall into throughfall, stemflow and interception: Effect of forest type, ground cover and climate. Hydrol. Process. 2000, 14, 2903–2920. [Google Scholar] [CrossRef]
- Sheng, H.C.; Cai, T.J. Influence of Rainfall on Canopy Interception in Mixed Broad-Leaved-Korean Pine Forest in Xiaoxing’an Mountains, Northeastern China. Forests 2019, 10, 248. [Google Scholar] [CrossRef]
- Zhang, S.L.; Liang, C.P. Effect of a native forest canopy on rainfall chemistry in China’s Qinling Mountains. Environ. Earth Sci. 2012, 67, 1503–1513. [Google Scholar] [CrossRef]
- Kosugi, Y.; Ltoh, M.; Tani, M.; Matsuo, N.; Takanashi, S.; Ohkubo, S.; Nik, A.R. Spatial and temporal variation in soil respiration in a Southeast Asian tropical rainforest. Agric. For. Meteorol. 2007, 147, 35–47. [Google Scholar] [CrossRef]
- Chen, Q.S.; Wang, Q.B.; Han, X.G.; Wan, S.Q.; Li, L.H. Temporal and spatial variability and controls of soil respiration in a temperate steppe in northern China. Global Biogeochem. Cycles 2010, 24, GB2010. [Google Scholar] [CrossRef]
- Raich, J.W. Temporal variability of soil respiration in experimental tree plantations in lowland Costa Rica. Forests 2017, 8, 40. [Google Scholar] [CrossRef]
- Mellado, A.; Morillas, L.; Gallardo, A.; Zamora, R. Temporal dynamic of parasite-mediated linkages between the forest canopy and soil processes and the microbial community. New Phytol. 2016, 211, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, P.; Hogberg, M.N.; Gottlicher, S.G.; Betson, N.R.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Schindlbacher, A.; Hurry, V.; Lundmark, T.; et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol. 2008, 177, 220–228. [Google Scholar] [CrossRef]
- Yang, X.; Xu, M. Biodiversity conservation in Changbai Mountain Biosphere Reserve, northeastern China: Status, problem, and strategy. Biodivers. Conserv. 2003, 12, 883–903. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Martin, J.G.; Bolstad, P.V. Variation of soil respiration at three spatial scales: Components within measurements, intra-site variation and patterns on the landscape. Soil Biol. Biochem. 2012, 41, 530–543. [Google Scholar] [CrossRef]
- Vose, J.M.; Ryan, M.G. Seasonal respiration of foliage, fine roots, and woody tissues in relation to growth, tissue N, and photosynthesis. Global Chang. Biol. 2002, 8, 182–193. [Google Scholar] [CrossRef]
- Desrochers, A.; Landhausser, S.M.; Lieffers, V.J. Coarse and fine root respiration in aspen (Populus tremuloides). Tree Physiol. 2002, 22, 725–732. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Dannoura, M.; Kominami, Y.; Mizoguchi, T.; Ishii, H.; Kanazawa, Y. Fine root morphological traits determine variation in root respiration of Quercus serrata. Tree Physiol. 2009, 29, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Anderson, C.G.; Bond-Lamberty, B.; Crump, A.R.; Chen, X.; Hess, N. Soil respiration across a permafrost transition zone: Spatial structure and environmental correlates. Biogeosciences 2017, 14, 4341–4354. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Xiao, H.; Wang, B.; Zhang, Y.; Wu, H.; Wang, X.; Yang, Y.; Wei, T. Soil Respiration May Overestimate or Underestimate in Forest Ecosystems. Sustainability 2021, 13, 2716. https://doi.org/10.3390/su13052716
Cao Y, Xiao H, Wang B, Zhang Y, Wu H, Wang X, Yang Y, Wei T. Soil Respiration May Overestimate or Underestimate in Forest Ecosystems. Sustainability. 2021; 13(5):2716. https://doi.org/10.3390/su13052716
Chicago/Turabian StyleCao, Yuanbo, Huijie Xiao, Baitian Wang, Yunlong Zhang, Honghui Wu, Xijing Wang, Yadong Yang, and Tingting Wei. 2021. "Soil Respiration May Overestimate or Underestimate in Forest Ecosystems" Sustainability 13, no. 5: 2716. https://doi.org/10.3390/su13052716
APA StyleCao, Y., Xiao, H., Wang, B., Zhang, Y., Wu, H., Wang, X., Yang, Y., & Wei, T. (2021). Soil Respiration May Overestimate or Underestimate in Forest Ecosystems. Sustainability, 13(5), 2716. https://doi.org/10.3390/su13052716




