A Conceptual Framework for Incorporation of Composting in Closed-Loop Urban Controlled Environment Agriculture
Abstract
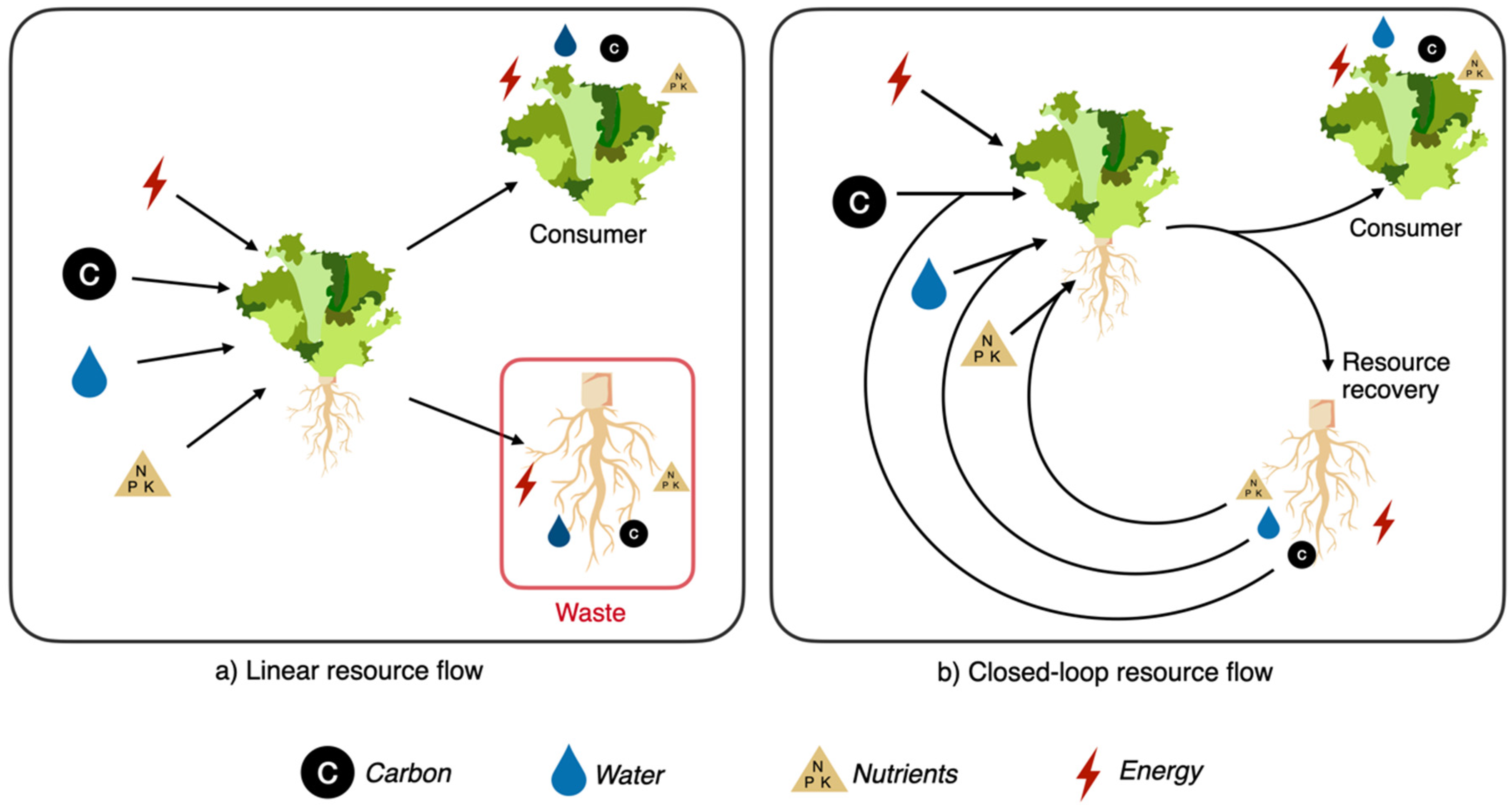
1. Introduction
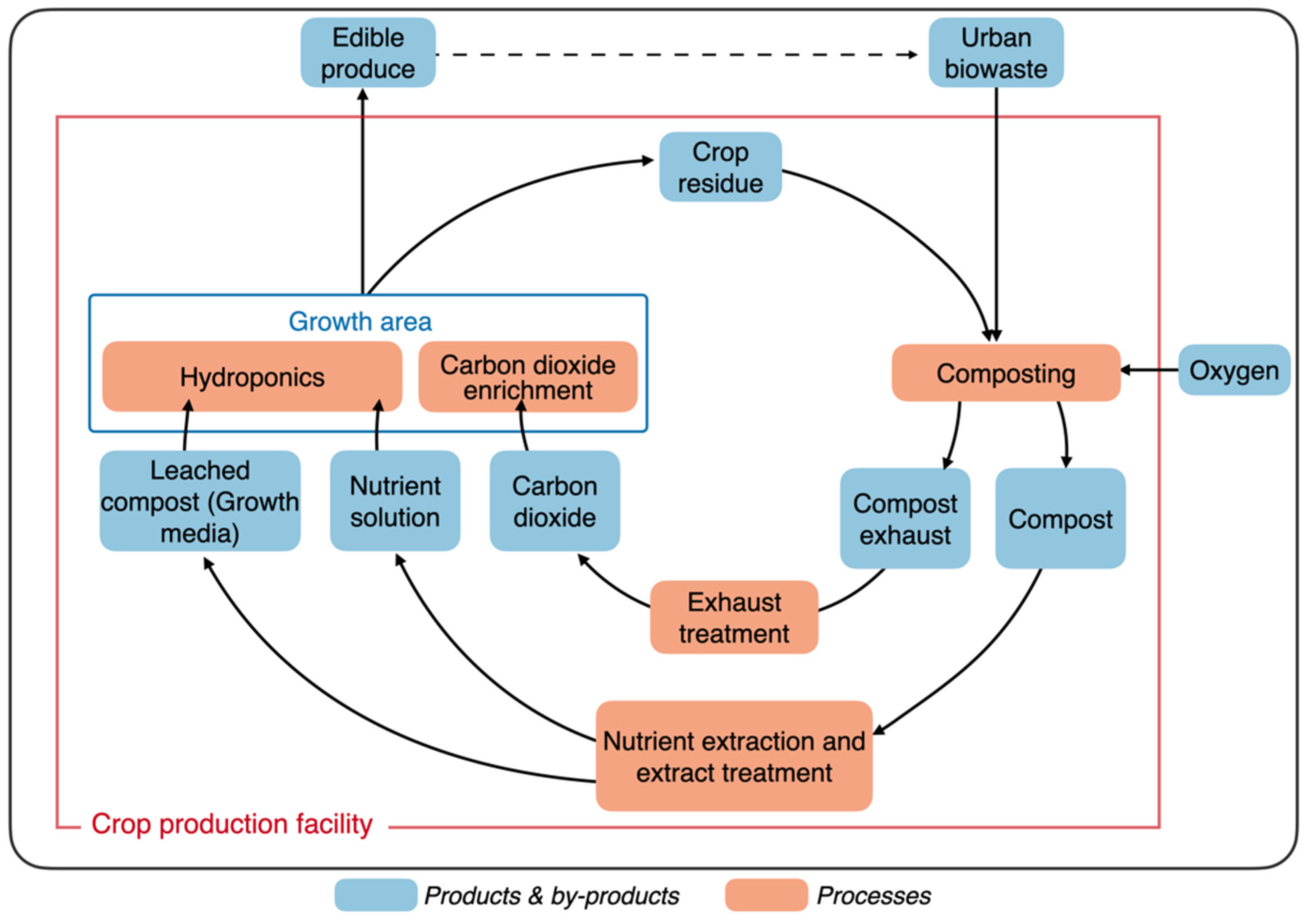
2. Composting
3. Carbon Dioxide Recovery
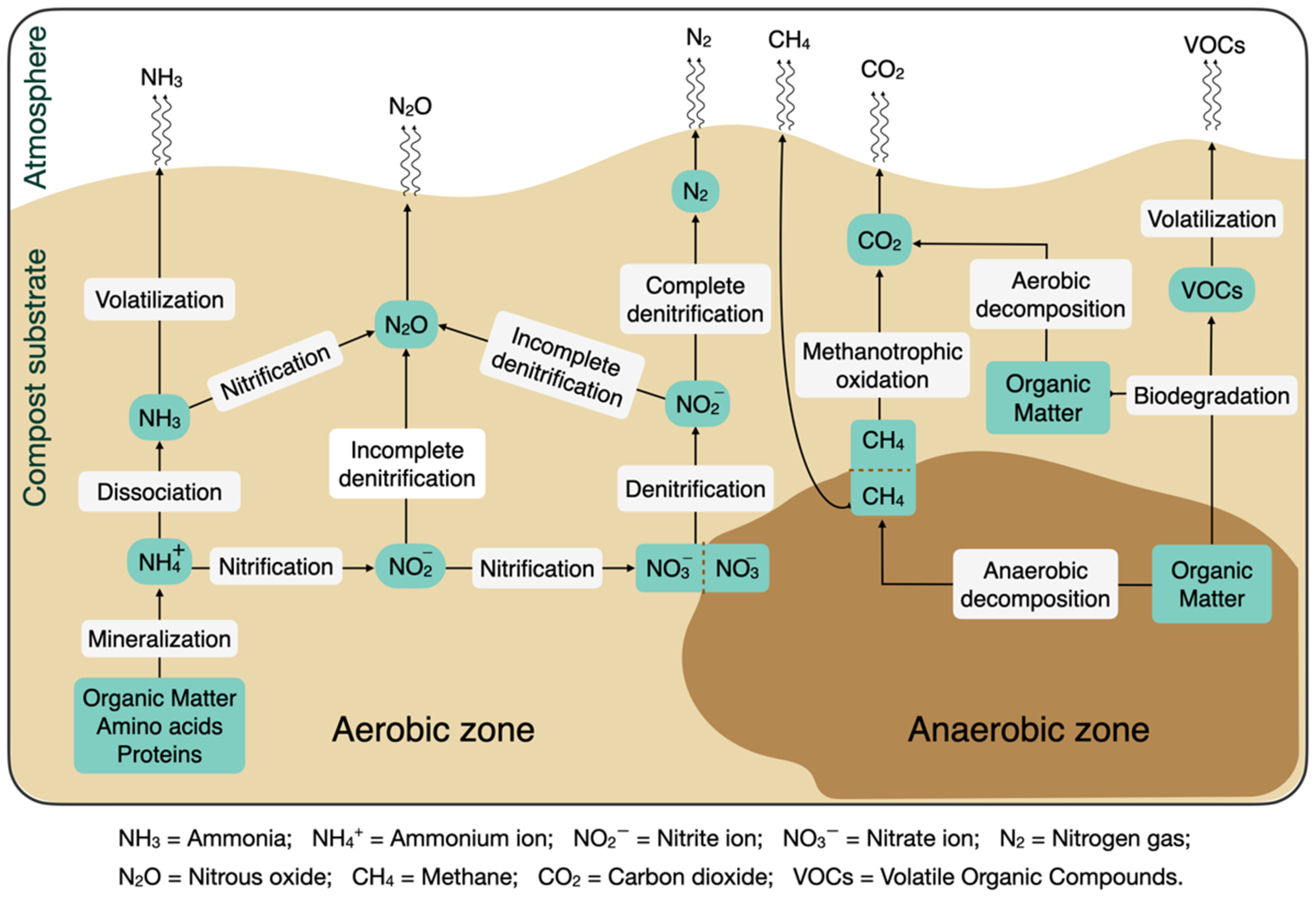
3.1. Gaseous Contaminants
3.1.1. Ammonia
3.1.2. Methane
3.1.3. Nitrous Oxide
3.1.4. Volatile Organic Compounds
3.1.5. Ethylene
3.2. Greenhouse Gas Emission Balance
3.3. Enhancing CO2 Production and Reducing Non-Target Gaseous Emissions
3.3.1. Feedstock Optimization
3.3.2. Amendments
Bulking Agents
Biochar
Mineral Additives
3.3.3. Co-Composting
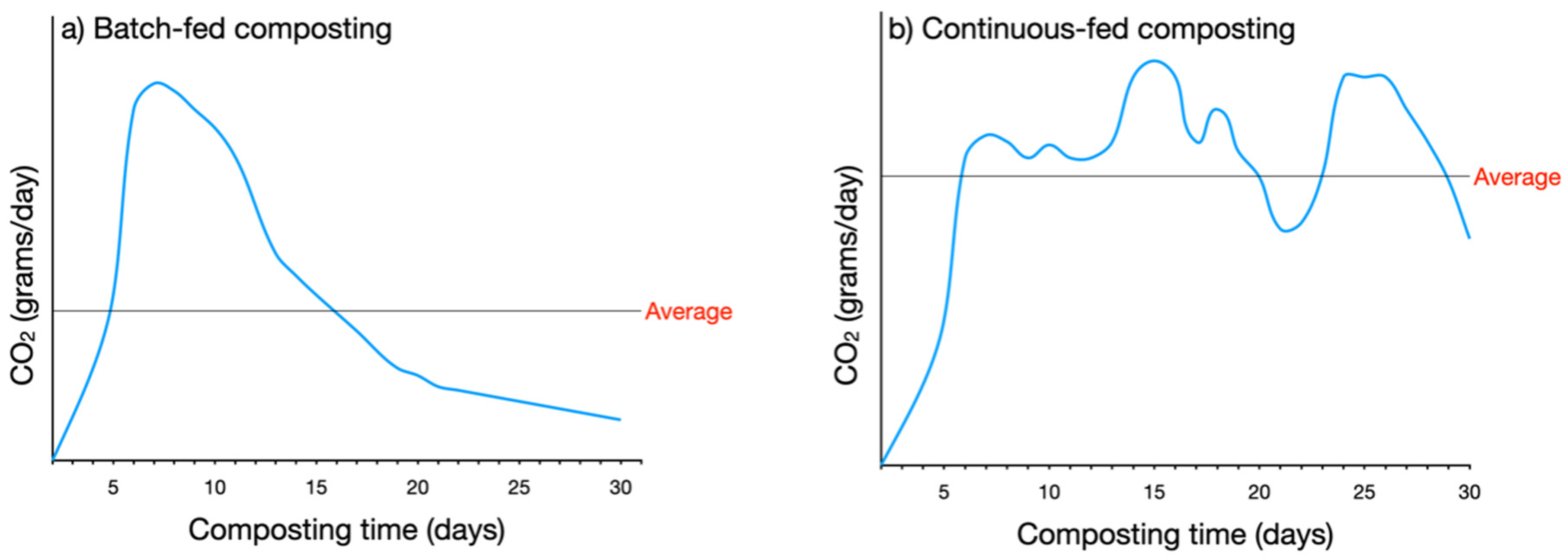
3.3.4. Process Control
Temperature
Oxygen
Moisture
3.4. Treatment of Exhaust Gases
Biofilters
3.5. CO2 Enrichment Control
4. Plant Nutrient Recovery
4.1. Nutrient Mineralization
4.2. Nutrient Solution Challenges
4.3. Phytotoxicity and Pathogenicity
4.3.1. Treatment of Nutrient Extracts
4.4. Alternate Use of Compost
5. Future Research
- Composting is a dynamic process and the optimum composting parameters are dependent on multiple factors (feedstock properties, composting methods, process control methods). Characterizing physical, chemical, and biodegradation properties of different crop residues from CEA systems is critical in determining degradation rates, optimum environmental parameters for composting, and maximizing CO2 recovery. This also includes evaluating different urban biowastes as amendments for effective composting of crop residue.
- Despite process control measures, composting can still produce small amounts of not-target gases that should be stripped off in order to use the compost exhaust for CO2 enrichment. Developing environmentally friendly treatment methods including biofiltration must be a priority.
- Developing effective and automatic enrichment control strategies in conjugation with capture, storage, and controlled release of CO2. Making these technologies low cost, low energy demand, and low environmental impact are also equally important priorities.
- Evaluating the effects of compost-based CO2 enrichment on crop growth in a completely controlled environment setup and comparing with conventional means of CO2 enrichment in terms of the crop yields and nutritional quality. This extends to detecting the presence of non-target gases (NH3, CH4, N2O, VOCs and ethylene) in compost exhaust and evaluating their effect on crop growth.
- Investigating ozonation, electrochemical oxidation, and other oxidative techniques to achieve pathogen control in conjunction with organic matter mineralization in hydroponic systems. These techniques should be tested specifically on compost extracts to be used as hydroponic nutrient solution for their effectiveness to manage pathogens and organic matter without disrupting plant functions.
- The environmental impact of these techniques must be evaluated by carrying out life cycle analysis (LCA) studies. Comparing compost based closed-loop operation to existing conventional (fossil fuel-based) technologies and also alternate technologies (anaerobic digestion, combustion and gasification) can provide insights to make economically and environmentally sound choices.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations; Department of Economic and Social Affairs; Population Division. World Population Prospects 2019: Highlights; (ST/ESA/SER.A/423); United Nations: New York, NY, USA, 2019.
- United Nations, Department of Economic and Social Affairs; Population Division. World Urbanization Prospects 2018: Highlights; (ST/ESA/SER.A/421); United Nations: New York, NY, USA, 2019.
- Sundström, J.F.; Albihn, A.; Boqvist, S.; Ljungvall, K.; Marstorp, H.; Martiin, C.; Nyberg, K.; Vågsholm, I.; Yuen, J.; Magnusson, U. Future threats to agricultural food production posed by environmental degradation, climate change, and animal and plant diseases—A risk analysis in three economic and climate settings. Food Secur. 2014, 6, 201–215. [Google Scholar] [CrossRef]
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Hazell, P.B.R.; Wood, S. Drivers of change in global agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 495–515. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Kalantari, F.; Tahir, O.M.; Joni, R.A.; Fatemi, E. Opportunities and Challenges in Sustainability of Vertical Farming: A Review. J. Landsc. Ecol. 2018, 11, 35–60. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Chapter 2—Role of the Plant Factory with Artificial Lighting (PFAL) in Urban Areas. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 7–34. ISBN 978-0-12-816691-8. [Google Scholar]
- Sarkar, A.; Majumder, M. Suitable Substrate for Crop Growth under Protected Farms—An Assessment. J. Sci. Agric. 2018, 2, 62. [Google Scholar] [CrossRef][Green Version]
- Despommier, D.; Ellingsen, E. The Vertical Farm: The Sky-Scraper as Vehicle for a Sustainable Urban Agriculture. In Proceedings of the CTBUH 2008, 8th World Congress—Tall and Green: Typology for a Sustainable Urban Future, Congress Proceedings, Dubai, United Arab Emirates, 3–5 March 2008; pp. 311–318. [Google Scholar]
- Leroy, J.L.; Ruel, M.; Frongillo, E.A.; Harris, J.; Ballard, T.J. Measuring the Food Access Dimension of Food Security: A Critical Review and Mapping of Indicators. Food Nutr. Bull. 2015, 36, 167–195. [Google Scholar] [CrossRef]
- Cole, M.B.; Augustin, M.A.; Robertson, M.J.; Manners, J.M. The science of food security. Npj Sci. Food 2018, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- FAO. Urban Food Systems and COVD-19: The Role of Cities and Local Governments in Responding to the Emergency. Policy Brief 2020. [Google Scholar] [CrossRef]
- Committee on World Food Security. Global Strategic Framework for Food Security & Nutrition; FAO: Rome, Italy, 2014.
- Santeramo, F.G. On the Composite Indicators for Food Security: Decisions Matter! Food Rev. Int. 2015, 31, 63–73. [Google Scholar] [CrossRef]
- Sweileh, W.M. Bibliometric analysis of peer-reviewed literature on food security in the context of climate change from 1980 to 2019. Agric. Food Secur. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Bakalis, S.; Valdramidis, V.P.; Argyropoulos, D.; Ahrné, L.; Chen, J.; Cullen, P.; Cummins, E.; Datta, A.K.; Emmanouilidis, C.; Foster, T.; et al. Perspectives from CO+RE: How COVID-19 changed our food systems and food security paradigms. Curr. Res. Food Sci. 2020, 3, 166–172. [Google Scholar] [CrossRef]
- Pulighe, G.; Lupia, F. Food First: COVID-19 Outbreak and Cities Lockdown a Booster for a Wider Vision on Urban Agriculture. Sustainability 2020, 12, 5012. [Google Scholar] [CrossRef]
- Kinsey, E.W.; Kinsey, D.; Rundle, A.G. COVID-19 and Food Insecurity: An Uneven Patchwork of Responses. J. Hered. 2020, 97, 332–335. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E. Will the COVID-19 pandemic make us reconsider the relevance of short food supply chains and local productions? Trends Food Sci. Technol. 2020, 99, 566–567. [Google Scholar] [CrossRef]
- Al-Kodmany, K. The Vertical Farm: A Review of Developments and Implications for the Vertical City. Buildings 2018, 8, 24. [Google Scholar] [CrossRef]
- Hadavi, E.; Ghazijahani, N. Closed and Semi-Closed Systems in Agriculture. In Sustainable Agriculture Reviews 33: Climate Impact on Agriculture; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2018; Volume 33, pp. 295–310. [Google Scholar]
- Wheeler, R. Plants for Human Life Support in Space: From Myers to Mars. Gravit. Space Biol. 2010, 23, 25–36. [Google Scholar]
- Despommier, D. The Vertical Farm: Feeding the World in the 21st Century; Thomas Dunne Books: New York, NY, USA, 2010. [Google Scholar]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef]
- Gulyas, B.; Edmondson, J. Increasing City Resilience through Urban Agriculture: Challenges and Solutions in the Global North. Sustainability 2021, 13, 1465. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Sariatli, F. Linear Economy Versus Circular Economy: A Comparative and Analyzer Study for Optimization of Economy for Sustainability. Visegr. J. Bioeconomy Sustain. Dev. 2017, 6, 31–34. [Google Scholar] [CrossRef]
- Adhikari, B.K.; Trémier, A.; Martinez, J.; Barrington, S. Home and community composting for on-site treatment of urban organic waste: Perspective for Europe and Canada. Waste Manag. Res. 2010, 28, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Chapter Four—Biological Treatment of Organic Materials for Energy and Nutrients Production—Anaerobic Digestion and Composting. In Advances in Bioenergy; Li, Y., Ge, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 121–181. [Google Scholar]
- Insam, H.; de Bertoldi, M. Microbiology of the Composting Process. In Compost Science and Technology; Diaz, L.F., de Bertoldi, M., Bidlingmaier, W., Stentiford, E., Eds.; Waste Management Series; Elsevier: Amsterdam, The Netherlands, 2007; Volume 8, pp. 25–48. [Google Scholar]
- Epstein, E. The Science of Composting; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Haug, R.T. The Practical Handbook of Compost Engineering, 1st ed.; Routledge: New York, NY, USA, 1993; ISBN 978-0-203-73623-4. [Google Scholar]
- Sanchez-Molina, J.A.; Reinoso, J.; Acién, F.; Rodriguez, F.; Lopez, J.; Fernandez, F.G.A. Development of a biomass-based system for nocturnal temperature and diurnal CO2 concentration control in greenhouses. Biomass Bioenergy 2014, 67, 60–71. [Google Scholar] [CrossRef]
- Roy, Y.; Lefsrud, M.; Orsat, V.; Filion, F.; Bouchard, J.; Nguyen, Q.; Dion, L.-M.; Glover, A.; Madadian, E.; Lee, C.P. Biomass combustion for greenhouse carbon dioxide enrichment. Biomass Bioenergy 2014, 66, 186–196. [Google Scholar] [CrossRef]
- Dion, L.-M.; Lefsrud, M.; Orsat, V. Review of CO2 recovery methods from the exhaust gas of biomass heating systems for safe enrichment in greenhouses. Biomass Bioenergy 2011, 35, 3422–3432. [Google Scholar] [CrossRef]
- Oreggioni, G.; Luberti, M.; Tassou, S. Agricultural greenhouse CO2 utilization in anaerobic-digestion-based biomethane production plants: A techno-economic and environmental assessment and comparison with CO2 geological storage. Appl. Energy 2019, 242, 1753–1766. [Google Scholar] [CrossRef]
- Jin, C.; Du, S.; Wang, Y.; Condon, J.; Lin, X.; Zhang, Y. Carbon dioxide enrichment by composting in greenhouses and its effect on vegetable production. J. Plant Nutr. Soil Sci. 2009, 172, 418–424. [Google Scholar] [CrossRef]
- Kimball, B.A. Carbon Dioxide and Agricultural Yield: An Assemblage and Analysis of 430 Prior Observations1. Agron. J. 1983, 75, 779–788. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, M.; Zhang, Y.; Wang, Q. Effect of carbon dioxide enrichment on health-promoting compounds and organoleptic properties of tomato fruits grown in greenhouse. Food Chem. 2014, 153, 157–163. [Google Scholar] [CrossRef]
- Mortensen, L.M. Review: CO2 enrichment in greenhouses. Crop responses. Sci. Hortic. 1987, 33, 1–25. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Li, D.; Miao, Z. Automatic carbon dioxide enrichment strategies in the greenhouse: A review. Biosyst. Eng. 2018, 171, 101–119. [Google Scholar] [CrossRef]
- Pierantozzi, R. Carbon Dioxide. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2003; pp. 803–822. ISBN 978-0-471-23896-6. [Google Scholar]
- Dufour, J.; Serrano, D.; Gálvez, J.; Moreno, J.; García, C. Life cycle assessment of processes for hydrogen production. Environmental feasibility and reduction of greenhouse gases emissions. Int. J. Hydrogen Energy 2009, 34, 1370–1376. [Google Scholar] [CrossRef]
- Muradov, N.; Vezirolu, T. From hydrocarbon to hydrogen-carbon to hydrogen economy. Int. J. Hydrog. Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Hendricks, P. Life Cycle Assessment of Greenhouse Tomato (Solanum lycopersicum L.) Production in Southwestern Ontario. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2012. [Google Scholar]
- Boulard, T.; Raeppel, C.; Brun, R.; Lecompte, F.; Hayer, F.; Carmassi, G.; Gaillard, G. Environmental impact of greenhouse tomato production in France. Agron. Sustain. Dev. 2011, 31, 757–777. [Google Scholar] [CrossRef]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas emissions from organic waste composting. Environ. Chem. Lett. 2015, 13, 223–238. [Google Scholar] [CrossRef]
- Hao, P.-F.; Qiu, C.-W.; Ding, G.; Vincze, E.; Zhang, G.; Zhang, Y.; Wu, F. Agriculture organic wastes fermentation CO2 enrichment in greenhouse and the fermentation residues improve growth, yield and fruit quality in tomato. J. Clean. Prod. 2020, 275, 123885. [Google Scholar] [CrossRef]
- Karim, M.F.; Hao, P.; Nordin, N.H.B.; Qiu, C.; Zeeshan, M.; Khan, A.A.; Shamsi, I.H.; Wu, F. CO2 enrichment using CRAM fermentation improves growth, physiological traits and yield of cherry tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2020, 27, 1041–1048. [Google Scholar] [CrossRef]
- Fangmeier, A.; Hadwiger-Fangmeier, A.; Van Der Eerden, L.; Jäger, H.-J. Effects of atmospheric ammonia on vegetation—A review. Environ. Pollut. 1994, 86, 43–82. [Google Scholar] [CrossRef]
- Fedoruk, M.J.; Bronstein, R.; Kerger, B.D. Ammonia exposure and hazard assessment for selected household cleaning product uses. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 534–544. [Google Scholar] [CrossRef]
- Amlinger, F.; Peyr, S.; Cuhls, C. Green house gas emissions from composting and mechanical biological treatment. Waste Manag. Res. 2008, 26, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Schuchardt, F.; Li, G.; Guo, R.; Zhao, Y. Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting. J. Environ. Sci. 2011, 23, 1754–1760. [Google Scholar] [CrossRef]
- Raviv, M.; Medina, S.; Krasnovsky, A.; Ziadna, H. Conserving Nitrogen during Composting. BioCycle 2002, 43, 48–51. [Google Scholar]
- Pagans, E.; Barrena, R.; Font, X.; Sánchez, A. Ammonia emissions from the composting of different organic wastes. Dependency on process temperature. Chemosphere 2006, 62, 1534–1542. [Google Scholar] [CrossRef]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monedero, M.Á.; Roig, A.; Paredes, C.; Bernal, M. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Komilis, D.P.; Ham, R.K.; Park, J.K. Emission of volatile organic compounds during composting of municipal solid wastes. Water Res. 2004, 38, 1707–1714. [Google Scholar] [CrossRef]
- Agapiou, A.; Vamvakari, J.P.; Andrianopoulos, A.; Pappa, A. Volatile emissions during storing of green food waste under different aeration conditions. Environ. Sci. Pollut. Res. 2016, 23, 8890–8901. [Google Scholar] [CrossRef] [PubMed]
- Büyüksönmez, F.; Evans, J. Biogenic Emissions from Green Waste and Comparison to the Emissions Resulting from Composting Part II: Volatile Organic Compounds (VOCs). Compos. Sci. Util. 2007, 15, 191–199. [Google Scholar] [CrossRef]
- Kumar, A.; Alaimo, C.P.; Horowitz, R.; Mitloehner, F.M.; Kleeman, M.J.; Green, P.G. Volatile organic compound emissions from green waste composting: Characterization and ozone formation. Atmos. Environ. 2011, 45, 1841–1848. [Google Scholar] [CrossRef]
- Smet, E.; Van Langenhove, H.; De Bo, I. The emission of volatile compounds during the aerobic and the combined anaerobic/aerobic composting of biowaste. Atmos. Environ. 1999, 33, 1295–1303. [Google Scholar] [CrossRef]
- Blount, B.C.; Kobelski, R.J.; McElprang, D.O.; Ashley, D.L.; Morrow, J.C.; Chambers, D.M.; Cardinali, F.L. Quantification of 31 volatile organic compounds in whole blood using solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. B 2006, 832, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ott, W.R.; Steinemann, A.C.; Wallace, L.A. Exposure Analysis; CRC Press: Boca Raton, FL, USA, 2006; ISBN 1-4200-1263-0. [Google Scholar]
- Dhamodharan, K.; Varma, V.S.; Veluchamy, C.; Pugazhendhi, A.; Rajendran, K. Emission of volatile organic compounds from composting: A review on assessment, treatment and perspectives. Sci. Total. Environ. 2019, 695, 133725. [Google Scholar] [CrossRef] [PubMed]
- Büyüksönmez, F. Full-Scale VOC Emissions from Green and Food Waste Windrow Composting. Compos. Sci. Util. 2012, 20, 57–62. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, M.; Ruiz-Montoya, M.; Giráldez, I.; Cabeza, I.O.; Lopez, R.; Diaz, M.J. Effect of control parameters on emitted volatile compounds in municipal solid waste and pine trimmings composting. J. Environ. Sci. Heal. Part A 2010, 45, 855–862. [Google Scholar] [CrossRef]
- Krzymien, M.; Day, M.; Shaw, K.; Zaremba, L. An Investigation of Odors and Volatile Organic Compounds Released during Composting. J. Air Waste Manag. Assoc. 1999, 49, 804–813. [Google Scholar] [CrossRef]
- Pagans, E.; Font, X.; Sánchez, A. Emission of volatile organic compounds from composting of different solid wastes: Abatement by biofiltration. J. Hazard. Mater. 2006, 131, 179–186. [Google Scholar] [CrossRef]
- Cape, J. Effects of airborne volatile organic compounds on plants. Environ. Pollut. 2003, 122, 145–157. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2002; ISBN 0-87893-823-0. [Google Scholar]
- Tholen, D.; Poorter, H.; Voesenek, L.A.C.J. Ethylene and Plant Growth. In Ethylene Action in Plants; Khan, N.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 35–49. ISBN 978-3-540-32846-9. [Google Scholar]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Dubois, M.; Broeck, L.V.D.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Daunicht, H.-J. Gas Turnover and Gas Conditions in Hermetically Closed Plant Production Systems. In Plant Production in Closed Ecosystems: The International Symposium on Plant Production in Closed Ecosystems held in Narita, Japan, August 26–29, 1996; Goto, E., Kurata, K., Hayashi, M., Sase, S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 225–244. ISBN 978-94-015-8889-8. [Google Scholar]
- Wong, M.; Cheung, Y.; Cheung, C. The effects of ammonia and ethylene oxide in animal manure and sewage sludge on the seed germination and root elongation of Brassica parachinensis. Environ. Pollut. Ser. A Ecol. Biol. 1983, 30, 109–123. [Google Scholar] [CrossRef]
- Wong, M. Phytotoxicity of refuse compost during the process of maturation. Environ. Pollut. Ser. A Ecol. Biol. 1985, 37, 159–174. [Google Scholar] [CrossRef]
- Tiquia, S. Reduction of compost phytotoxicity during the process of decomposition. Chemosphere 2010, 79, 506–512. [Google Scholar] [CrossRef]
- Schwintzer, C.R.; Tjepkema, J.D.; Seekins, B. Nitrogenase activity in composting horse bedding and leaves. Plant Soil 2002, 242, 277–282. [Google Scholar] [CrossRef]
- Rees, D.C.; Tezcan, F.A.; Haynes, C.A.; Walton, M.Y.; Andrade, S.; Einsle, O.; Howard, J.B. Structural basis of biological nitrogen fixation. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2005, 363, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, L.C.; Yang, Z.-Y.; Duval, S.; Dean, D.R. Nitrogenase reduction of carbon-containing compounds. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 1102–1111. [Google Scholar] [CrossRef]
- Hubbe, M.; Nazhad, M.; Sanchez, C. Composting as a way to convert cellulosic biomass and organic waste into high-value soil amendments: A review. Bioresources 2010, 5, 2808–2854. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Kim, S.H.; Shim, J.; Park, S.J. Composting Process and Gas Emissions during Food Waste Composting under the Effect of Different Additives. Sustainability 2020, 12, 7811. [Google Scholar] [CrossRef]
- Jovanovic, M.; Kašćelan, L.; Despotovic, A.; Kašćelan, V. The Impact of Agro-Economic Factors on GHG Emissions: Evidence from European Developing and Advanced Economies. Sustainability 2015, 7, 16290–16310. [Google Scholar] [CrossRef]
- Balafoutis, A.; Beck, B.; Fountas, S.; Vangeyte, J.; Van Der Wal, T.; Soto, I.; Gómez-Barbero, M.; Barnes, A.; Eory, V. Precision Agriculture Technologies Positively Contributing to GHG Emissions Mitigation, Farm Productivity and Economics. Sustainability 2017, 9, 1339. [Google Scholar] [CrossRef]
- Brown, S.; Kruger, C.; Subler, S. Greenhouse Gas Balance for Composting Operations. J. Environ. Qual. 2008, 37, 1396–1410. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Nair, J. The impact of landfilling and composting on greenhouse gas emissions—A review. Bioresour. Technol. 2009, 100, 3792–3798. [Google Scholar] [CrossRef]
- Boldrin, A.; Andersen, J.K.; Møller, J.; Christensen, T.H.; Favoino, E. Composting and compost utilization: Accounting of greenhouse gases and global warming contributions. Waste Manag. Res. 2009, 27, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Tsai, J.; Wu, K. Mathematical model for carbon dioxide evolution from the thermophilic composting of synthetic food wastes made of dog food. Waste Manag. 2005, 25, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Naher, U.A.; Sarkar, M.I.U.; Jahan, A.; Biswas, J.C. Co-Composting Urban Waste, Plant Residues, and Rock Phosphate: Biochemical Characterization and Evaluation of Compost Maturity. Commun. Soil Sci. Plant Anal. 2018, 49, 751–762. [Google Scholar] [CrossRef]
- Diaz, L.F.; Savage, G.M. Chapter 4 Factors that affect the process. In Compost Science and Technology; Diaz, L.F., de Bertoldi, M., Bidlingmaier, W., Stentiford, E., Eds.; Waste Management Series; Elsevier: Amsterdam, The Netherlands, 2007; Volume 8, pp. 49–65. [Google Scholar]
- Callejón-Ferre, A.; Velázquez-Martí, B.; López-Martínez, J.; Manzano-Agugliaro, F. Greenhouse crop residues: Energy potential and models for the prediction of their higher heating value. Renew. Sustain. Energy Rev. 2011, 15, 948–955. [Google Scholar] [CrossRef]
- Phyllis2 Database for (Treated) Biomass, Algae, Feedstocks for Biogas Production and Biochar. Available online: https://phyllis.nl/Biomass/View/3517 (accessed on 10 June 2020).
- Alkoaik, F.; Ghaly, A.E. Effect of Inoculum Size on the Composting of Greenhouse Tomato Plant Trimmings. Compos. Sci. Util. 2005, 13, 262–273. [Google Scholar] [CrossRef]
- Oleszek, M.; Tys, J.; Wiącek, D.; Król, A.; Kuna, J. The Possibility of Meeting Greenhouse Energy and CO2 Demands through Utilisation of Cucumber and Tomato Residues. BioEnergy Res. 2016, 9, 624–632. [Google Scholar] [CrossRef]
- Dreschel, T.W.; Wheeler, R.M.; Hinkle, C.R.; Sager, J.C.; Knott, W.M. Investigating Combustion as a Method of Processing Inedible Biomass Produced In NASA’s Biomass Production Chamber; National Aeronautics and Space Administration, John F. Kennedy Space Center: Merritt Island, FL, USA, 1991. [Google Scholar]
- Nyochembeng, L.M.; Beyl, C.A.; Pacumbaba, R. Optimizing edible fungal growth and biodegradation of inedible crop residues using various cropping methods. Bioresour. Technol. 2008, 99, 5645–5649. [Google Scholar] [CrossRef] [PubMed]
- Baggs, E.M. Nitrous Oxide from Incorporated Crop Residues and Green Manures. Doctoral Thesis, The University of Edin-burgh, Edinburgh, UK, 1997. [Google Scholar]
- Kang, S.; Hogan, J.A. Optimization of Feedstock Composition and PreProcessing for Composting in Advanced Life Support Systems; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Tuomela, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.X.; Yang, Q.Y.; Luo, W.H. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 2013, 93, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X. Influence of sugar beet pulp and paper waste as bulking agents on physical, chemical, and microbial properties during green waste composting. Bioresour. Technol. 2018, 267, 182–191. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manag. 2016, 48, 115–126. [Google Scholar] [CrossRef]
- Louhelainen, K.; Kangas, J.; Veijanen, A.; Viilos, P. Effect of in situ composting on reducing offensive odors and volatile organic compounds in swineries. AIHA J. 2001, 62, 159–167. [Google Scholar] [CrossRef]
- Barthod, J.; Rumpel, C.; Dignac, M.-F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, Q.; Ren, X.; Zhao, J.; Huang, H.; Awasthi, S.K.; Lahori, A.H.; Li, R.; Zhou, L.; Zhang, Z. Role of biochar amendment in mitigation of nitrogen loss and greenhouse gas emission during sewage sludge composting. Bioresour. Technol. 2016, 219, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the Effects of Biochar on Manure Composting: Evidence Supporting the Relationship between N2O Emission and Denitrifying Community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.; Melear, N.D.; Lakly, D. Reducing Nitrogen Loss during Poultry Litter Composting Using Biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, M.; Chen, H.; Wang, Q.; Zhao, J.; Ren, X.; Li, D.-S.; Awasthi, S.K.; Shen, F.; Li, R.; et al. Heterogeneity of biochar amendment to improve the carbon and nitrogen sequestration through reduce the greenhouse gases emissions during sewage sludge composting. Bioresour. Technol. 2017, 224, 428–438. [Google Scholar] [CrossRef]
- Czekała, W.; Malińska, K.; Cáceres, R.; Janczak, D.; Dach, J.; Lewicki, A. Co-composting of poultry manure mixtures amended with biochar—The effect of biochar on temperature and C-CO2 emission. Bioresour. Technol. 2016, 200, 921–927. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, I.; Cayuela, M.L.; Mondini, C.; Takaya, C.A.; Ross, A.B.; Sánchez-Monedero, M.A. Suitability of Different Agricultural and Urban Organic Wastes as Feedstocks for the Production of Biochar—Part 1: Physicochemical Characterisation. Sustainability 2018, 10, 2265. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, Q.; Huang, H.; Ren, X.; Lahori, A.H.; Mahar, A.; Ali, A.; Shen, F.; Li, R.; Zhang, Z. Influence of zeolite and lime as additives on greenhouse gas emissions and maturity evolution during sewage sludge composting. Bioresour. Technol. 2016, 216, 172–181. [Google Scholar] [CrossRef]
- Hao, X.; Larney, F.J.; Chang, C.; Travis, G.R.; Nichol, C.K.; Bremer, E. The Effect of Phosphogypsum on Greenhouse Gas Emissions during Cattle Manure Composting. J. Environ. Qual. 2005, 34, 774–781. [Google Scholar] [CrossRef]
- Luo, Y.; Li, G.; Luo, W.; Schuchardt, F.; Jiang, T.; Xu, D. Effect of phosphogypsum and dicyandiamide as additives on NH3, N2O and CH4 emissions during composting. J. Environ. Sci. 2013, 25, 1338–1345. [Google Scholar] [CrossRef]
- Wang, M.; Awasthi, M.K.; Wang, Q.; Chen, H.; Ren, X.; Zhao, J.; Li, R.; Zhang, Z. Comparison of additives amendment for mitigation of greenhouse gases and ammonia emission during sewage sludge co-composting based on correlation analysis. Bioresour. Technol. 2017, 243, 520–527. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Shi, H.; Wang, Y. Effects of phosphogypsum and superphosphate on compost maturity and gaseous emissions during kitchen waste composting. Waste Manag. 2015, 36, 70–76. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Addition of fish pond sediment and rock phosphate enhances the composting of green waste. Bioresour. Technol. 2017, 233, 116–126. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Chen, S.; Li, D.; Tang, H.; Chadwick, D.; Li, S.; Li, W.; Li, G. Effects of phosphogypsum, superphosphate, and dicyandiamide on gaseous emission and compost quality during sewage sludge composting. Bioresour. Technol. 2018, 270, 368–376. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Addition of seaweed and bentonite accelerates the two-stage composting of green waste. Bioresour. Technol. 2017, 243, 154–162. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Ho, W.S.; Lim, J.S.; Klemeš, J.J.; Towprayoon, S.; Ho, C.S.; Lee, C.T. A review on the global warming potential of cleaner composting and mitigation strategies. J. Clean. Prod. 2017, 146, 149–157. [Google Scholar] [CrossRef]
- Petric, I.; Helić, A.; Avdić, E.A. Evolution of process parameters and determination of kinetics for co-composting of organic fraction of municipal solid waste with poultry manure. Bioresour. Technol. 2012, 117, 107–116. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Abu Qdais, H.; Al-Widyan, M. Evaluating composting and co-composting kinetics of various agro-industrial wastes. Int. J. Recycl. Org. Waste Agric. 2016, 5, 273–280. [Google Scholar] [CrossRef]
- Puyuelo, B.; Ponsá, S.; Gea, T.; Sánchez, B.P. Determining C/N ratios for typical organic wastes using biodegradable fractions. Chemosphere 2011, 85, 653–659. [Google Scholar] [CrossRef]
- Adhikari, B.K.; Barrington, S.; Martinez, J.; King, S. Characterization of food waste and bulking agents for composting. Waste Manag. 2008, 28, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X. Using cow dung and spent coffee grounds to enhance the two-stage co-composting of green waste. Bioresour. Technol. 2017, 245, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-C.; Inoue, Y.; Yasuta, T.; Yoshida, S.; Katayama, A. Chemical and microbial properties of various compost products. Soil Sci. Plant Nutr. 2003, 49, 273–280. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting environmental risks to benefits by using spent coffee grounds (SCG) as a valuable resource. Environ. Sci. Pollut. Res. 2018, 25, 35776–35790. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef]
- Liu, K.; Price, G. Evaluation of three composting systems for the management of spent coffee grounds. Bioresour. Technol. 2011, 102, 7966–7974. [Google Scholar] [CrossRef]
- Hardgrove, S.J.; Livesley, S.J. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the variability of food waste quality: A need for efficient valorisation through anaerobic digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, R.L.; Eiteman, M.A.; Das, K. Pressure Drop through Raw Food Waste Compost containing Synthetic Bulking Agents. J. Agric. Eng. Res. 1999, 72, 375–384. [Google Scholar] [CrossRef]
- Fang, M.; Wong, J.W.C.; Ma, K.K.; Wong, M.H. Co-composting of sewage sludge and coal fly ash: Nutrient transformations. Bioresour. Technol. 1999, 67, 19–24. [Google Scholar] [CrossRef]
- Morin, S.; Lemay, S.; Barrington, S.F. An Urban Composting System. In Proceedings of the CSAE/SCGR Meeting, Montréal, QC, Canada, 6–9 July 2003; pp. 6–9. [Google Scholar]
- Sangamithirai, K.M.; Jayapriya, J.; Hema, J.; Manoj, R. Evaluation of in-vessel co-composting of yard waste and development of kinetic models for co-composting. Int. J. Recycl. Org. Waste Agric. 2015, 4, 157–165. [Google Scholar] [CrossRef]
- Benito, M.; Masaguer, A.; Moliner, A.; Arrigo, N.; Palma, R.M. Chemical and microbiological parameters for the characterisation of the stability and maturity of pruning waste compost. Biol. Fertil. Soils 2003, 37, 184–189. [Google Scholar] [CrossRef]
- De Bertoldi, M.; Vallini, G.; Pera, A. The Biology of Composting: A Review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Beck-Friis, B.; Smårs, S.; Jönsson, H.; Kirchmann, H. SE—Structures and Environment: Gaseous Emissions of Carbon Dioxide, Ammonia and Nitrous Oxide from Organic Household Waste in a Compost Reactor under Different Temperature Regimes. J. Agric. Eng. Res. 2001, 78, 423–430. [Google Scholar] [CrossRef]
- Han, Z.; Sun, D.; Wang, H.; Li, R.; Bao, Z.; Qi, F. Effects of ambient temperature and aeration frequency on emissions of ammonia and greenhouse gases from a sewage sludge aerobic composting plant. Bioresour. Technol. 2018, 270, 457–466. [Google Scholar] [CrossRef]
- Sikora, L.J.; Sowers, M.A. Effect of Temperature Control on the Composting Process. J. Environ. Qual. 1985, 14, 434–439. [Google Scholar] [CrossRef]
- Suler, D.J.; Finstein, M.S. Effect of Temperature, Aeration, and Moisture on CO2 Formation in Bench-Scale, Continuously Thermophilic Composting of Solid Waste 1. Appl. Environ. Microbiol. 1977, 33, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kulcu, R. Determination of aeration rate and kinetics of composting some agricultural wastes. Bioresour. Technol. 2004, 93, 49–57. [Google Scholar] [CrossRef]
- Stentiford, E.I. Composting Control: Principles and Practice. In The Science of Composting; de Bertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 49–59. ISBN 978-94-009-1569-5. [Google Scholar]
- Jamaludin, Z.; Rollings-Scattergood, S.; Lutes, K.; Vaneeckhaute, C. Evaluation of sustainable scrubbing agents for ammonia recovery from anaerobic digestate. Bioresour. Technol. 2018, 270, 596–602. [Google Scholar] [CrossRef]
- Joshi, J.A.; Hogan, J.A.; Cowan, R.M.; Strom, P.F.; Finstein, M.S. Biological Removal of Gaseous Ammonia in Biofilters: Space Travel and Earth-Based Applications. J. Air Waste Manag. Assoc. 2000, 50, 1647–1654. [Google Scholar] [CrossRef]
- McNevin, D.; Barford, J. Biofiltration as an odour abatement strategy. Biochem. Eng. J. 2000, 5, 231–242. [Google Scholar] [CrossRef]
- Nikiema, J.; Brzezinski, R.; Heitz, M. Elimination of methane generated from landfills by biofiltration: A review. Rev. Environ. Sci. BioTechnol. 2007, 6, 261–284. [Google Scholar] [CrossRef]
- Pagans, E.; Font, X.; Sánchez, A. Coupling composting and biofiltration for ammonia and volatile organic compound removal. Biosyst. Eng. 2007, 97, 491–500. [Google Scholar] [CrossRef]
- Hartikainen, T.; Ruuskanen, J.; Vanhatalo, M.; Martikainen, P.J. Removal of Ammonia from Air by a Peat Biofilter. Environ. Technol. 1996, 17, 45–53. [Google Scholar] [CrossRef]
- Hong, J.H.; Park, K.J. Wood Chip Biofilter Performance of Ammonia Gas from Composting Manure. Compos. Sci. Util. 2004, 12, 25–30. [Google Scholar] [CrossRef]
- Hort, C.; Gracy, S.; Platel, V.; Moynault, L. Evaluation of sewage sludge and yard waste compost as a biofilter media for the removal of ammonia and volatile organic sulfur compounds (VOSCs). Chem. Eng. J. 2009, 152, 44–53. [Google Scholar] [CrossRef]
- Joshi, J.A.; Cowan, R.M.; Hogan, J.A.; Strom, P.F.; Finstein, M.S. Gaseous Ammonia Removal in Biofilters: Effect of Biofilter Media on Products of Nitrification; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1998. [Google Scholar]
- Yasuda, T.; Kuroda, K.; Fukumoto, Y.; Hanajima, D.; Suzuki, K. Evaluation of full-scale biofilter with rockwool mixture treating ammonia gas from livestock manure composting. Bioresour. Technol. 2009, 100, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Quan, X.; Chen, J.; Chung, J.S.; Sung, J.Y.; Chen, S.; Xue, D.; Zhao, Y. Long-term results of ammonia removal and transformation by biofiltration. J. Hazard. Mater. 2000, 80, 259–269. [Google Scholar] [CrossRef]
- Bejan, D.; Graham, T.; Bunce, N.J. Chemical methods for the remediation of ammonia in poultry rearing facilities: A review. Biosyst. Eng. 2013, 115, 230–243. [Google Scholar] [CrossRef]
- Sikora, L.J.; Ramirez, M.A.; Troeschel, T.A. Laboratory Composter for Simulation Studies. J. Environ. Qual. 1983, 12, 219–224. [Google Scholar] [CrossRef]
- Janczak, D.; Malińska, K.; Czekała, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, D.J.; Ravindran, B.; Jeong, K.-H.; Wong, J.W.-C.; Selvam, A.; Karthikeyan, O.P.; Kwag, J.-H. Evaluation of integrated ammonia recovery technology and nutrient status with an in-vessel composting process for swine manure. Bioresour. Technol. 2017, 245, 365–371. [Google Scholar] [CrossRef]
- Melse, R.W.; Van Der Werf, A.W. Biofiltration for Mitigation of Methane Emission from Animal Husbandry. Environ. Sci. Technol. 2005, 39, 5460–5468. [Google Scholar] [CrossRef]
- Ergas, S.J.; Schroeder, E.D.; Chang, D.P.Y.; Morton, R.L. Control of volatile organic compound emissions using a compost biofilter. Water Environ. Res. 1995, 67, 816–821. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Martín, M.; Pagans, E.; Vera, L.; Olmo, J.G.; Chica, A. Dynamic olfactometry and GC–TOFMS to monitor the efficiency of an industrial biofilter. Sci. Total. Environ. 2015, 512, 572–581. [Google Scholar] [CrossRef]
- Van Der Heyden, C.; Demeyer, P.; Volcke, E.I. Mitigating emissions from pig and poultry housing facilities through air scrubbers and biofilters: State-of-the-art and perspectives. Biosyst. Eng. 2015, 134, 74–93. [Google Scholar] [CrossRef]
- Leson, G.; Winer, A.M. Biofiltration: An Innovative Air Pollution Control Technology for VOC Emissions. J. Air Waste Manag. Assoc. 1991, 41, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G. Chapter 5—Plant Factory as a Resource-Efficient Closed Plant Production System. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 93–115. ISBN 978-0-12-816691-8. [Google Scholar]
- Gomes, A.P.; Pereira, F.A. Mathematical modelling of a composting process, and validation with experimental data. Waste Manag. Res. 2008, 26, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Komilis, D.P.; Ham, R.K. Carbon dioxide and ammonia emissions during composting of mixed paper, yard waste and food waste. Waste Manag. 2006, 26, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, I.A.; Chowdhury, A.K.M.M.B.; Akratos, C.S.; Tekerlekopoulou, A.G.; Pavlou, S.; Vayenas, D.V. Mathematical modeling of olive mill waste composting process. Waste Manag. 2015, 43, 61–71. [Google Scholar] [CrossRef]
- Rodríguez-Mosqueda, R.; Bramer, E.A.; Brem, G. CO2 capture from ambient air using hydrated Na2CO3 supported on activated carbon honeycombs with application to CO2 enrichment in greenhouses. Chem. Eng. Sci. 2018, 189, 114–122. [Google Scholar] [CrossRef]
- Fang, W.; Chung, H. Bioponics for lettuce production in a plant factory with artificial lighting. Acta Hortic. 2018, 593–598. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Calvo, M.J.; Petit-Boix, A.; Villalba, G.; Gabarrell, X. Exploring nutrient recovery from hydroponics in urban agriculture: An environmental assessment. Resour. Conserv. Recycl. 2020, 155, 104683. [Google Scholar] [CrossRef]
- Brentrup, F.; Hoxha, A.; Christensen, B. Carbon Footprint Analysis of Mineral Fertilizer Production in Europe and Other World Regions; University College Dublin (UCD): Dublin, Ireland, 2016. [Google Scholar]
- Chen, W.; Geng, Y.; Hong, J.; Yang, D.; Ma, X. Life cycle assessment of potash fertilizer production in China. Resour. Conserv. Recycl. 2018, 138, 238–245. [Google Scholar] [CrossRef]
- Skowrońska, M.; Filipek, T. Life cycle assessment of fertilizers: A review. Int. Agrophysics 2014, 28, 101–110. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Q.; Hong, J.; Chen, W.; Qi, C.; Ye, L. Life cycle assessment of diammonium- and monoammonium-phosphate fertilizer production in China. J. Clean. Prod. 2017, 141, 1087–1094. [Google Scholar] [CrossRef]
- Mackowiak, C.; Wheeler, R.; Stutte, G.; Yorio, N.; Sager, J. Use of biologically reclaimed minerals for continuous hydroponic potato production in a CELSS. Adv. Space Res. 1997, 20, 1815–1820. [Google Scholar] [CrossRef]
- Mackowiak, C.; Garland, J.; Sager, J. Recycling crop residues for use in recirculating hydroponic crop production. Acta Hortic. 1996, 440, 19–24. [Google Scholar] [CrossRef]
- Mackowiak, C.; Garland, J.; Strayer, R.; Finger, B.; Wheeler, R. Comparison of aerobically-treated and untreated crop residue as a source of recycled nutrients in a recirculating hydroponic system. Adv. Space Res. 1996, 18, 281–287. [Google Scholar] [CrossRef]
- Atkin, K.; Nichols, M.A. Organic Hydroponics. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2004; Volume 648, pp. 121–127. [Google Scholar]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Boron, D.J.; Evans, E.W.; Peterson, J.M. An overview of peat research, utilization, and environmental considerations. Int. J. Coal Geol. 1987, 8, 1–31. [Google Scholar] [CrossRef]
- Childers, D.L.; Corman, J.; Edwards, M.; Elser, J.J. Sustainability Challenges of Phosphorus and Food: Solutions from Closing the Human Phosphorus Cycle. Bioscience 2011, 61, 117–124. [Google Scholar] [CrossRef]
- Grigatti, M.; Giorgioni, M.; Ciavatta, C. Compost-based growing media: Influence on growth and nutrient use of bedding plants. Bioresour. Technol. 2007, 98, 3526–3534. [Google Scholar] [CrossRef]
- Othman, I.; Al-Masri, M.S. Impact of phosphate industry on the environment: A case study. Appl. Radiat. Isot. 2007, 65, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Egea-Gilabert, C. Application of Directly Brewed Compost Extract Improves Yield and Quality in Baby Leaf Lettuce Grown Hydroponically. Agron. 2020, 10, 370. [Google Scholar] [CrossRef]
- Monda, H.; Cozzolino, V.; Vinci, G.; Spaccini, R.; Piccolo, A. Molecular characteristics of water-extractable organic matter from different composted biomasses and their effects on seed germination and early growth of maize. Sci. Total. Environ. 2017, 590–591, 40–49. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Aoyama, C.; Fujiwara, K.; Shinohara, M.; Takano, M. Study on the Hydroponic Culture of Lettuce with Microbially Degraded Solid Food Waste as a Nitrate Source. Jpn. Agric. Res. Q. 2014, 48, 71–76. [Google Scholar] [CrossRef]
- Martin, C.C.S.; Brathwaite, R.A. Compost and compost tea: Principles and prospects as substrates and soil-borne disease management strategies in soil-less vegetable production. Biol. Agric. Hortic. 2012, 28, 1–33. [Google Scholar] [CrossRef]
- Scheuerell, S.; Mahaffee, W. Compost Tea: Principles and Prospects for Plant Disease Control. Compos. Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil Sci. Plant Nutr. 2011, 57, 190–203. [Google Scholar] [CrossRef]
- Ikeda, H.; Osawa, T. Comparison of Adaptability to Nitrogen Source among Vegetable Crops I. Growth Response and Nitrogen Assimilation of Fruit Vegetables Cultured in Nutrient Solution Containing Nitrate, Ammonium, and Nitrite Nitrogen. J. Jpn. Soc. Hortic. Sci. 1979, 47, 454–462. [Google Scholar] [CrossRef]
- Puritch, G.S.; Barker, A.V. Structure and Function of Tomato Leaf Chloroplasts during Ammonium Toxicity. Plant Physiol. 1967, 42, 1229–1238. [Google Scholar] [CrossRef]
- Fujiwara, K.; Aoyama, C.; Takano, M.; Shinohara, M. Suppression of Ralstonia solanacearum bacterial wilt disease by an organic hydroponic system. J. Gen. Plant Pathol. 2012, 78, 217–220. [Google Scholar] [CrossRef]
- Yang, P.; Guo, Y.-Z.; Qiu, L. Effects of ozone-treated domestic sludge on hydroponic lettuce growth and nutrition. J. Integr. Agric. 2018, 17, 593–602. [Google Scholar] [CrossRef]
- Ahn, K.-H.; Yeom, I.-T.; Park, K.-Y.; Maeng, S.-K.; Lee, Y.; Song, K.-G.; Hwang, J.-H. Reduction of sludge by ozone treatment and production of carbon source for denitrification. Water Sci. Technol. 2002, 46, 121–125. [Google Scholar] [CrossRef]
- Bruguera-Casamada, C.; Sirés, I.; Brillas, E.; Araujo, R.M. Effect of electrogenerated hydroxyl radicals, active chlorine and organic matter on the electrochemical inactivation of Pseudomonas aeruginosa using BDD and dimensionally stable anodes. Sep. Purif. Technol. 2017, 178, 224–231. [Google Scholar] [CrossRef]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar] [CrossRef]
- Gonçalves, M.R.; Marques, I.P.; Correia, J.P. Electrochemical mineralization of anaerobically digested olive mill wastewater. Water Res. 2012, 46, 4217–4225. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Olive mill wastewater treatment by anodic oxidation with parallel plate electrodes. Water Res. 2006, 40, 1179–1184. [Google Scholar] [CrossRef]
- Un, U.T.; Altay, U.; Koparal, A.S.; Öğütveren, Ü.B. Complete treatment of olive mill wastewaters by electrooxidation. Chem. Eng. J. 2008, 139, 445–452. [Google Scholar] [CrossRef]
- Michitsch, R.C.; Chong, C.; Holbein, B.E.; Voroney, R.P.; Liu, H.-W. Use of Wastewater and Compost Extracts as Nutrient Sources for Growing Nursery and Turfgrass Species. J. Environ. Qual. 2007, 36, 1031–1041. [Google Scholar] [CrossRef]
- Williams, K.; Nelson, J. Challenges of using organic fertilizers in hydroponic production systems. Acta Hortic. 2016, 365–370. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Chong, C.; Voroney, R.P. Evaluation of Compost Leachates for Plant Growth in Hydroponic Culture. J. Plant Nutr. 2005, 28, 651–667. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Rufián-Henares, J.Á.; Pastoriza, S.; Montilla-Gómez, J.; Delgado, G. Phytotoxicity and chelating capacity of spent coffee grounds: Two contrasting faces in its use as soil organic amendment. Sci. Total. Environ. 2020, 717, 137247. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.; Roberts, S.J. Eradication of plant pathogens and nematodes during composting: A review. Plant Pathol. 2004, 53, 548–568. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Guidelines for Compost Quality; CCME: Winnipeg, MB, Canada, 2005; p. Cat. No. PN1341.
- Alsanius, B.W.; Wohanka, W. Chapter 5—Root Zone Microbiology of Soilless Cropping Systems. In Soilless Culture, 2nd ed.; Elsevier: Boston, MA, USA, 2019; pp. 149–194. [Google Scholar]
- Lévesque, S.; Graham, T.; Bejan, D.; Lawson, J.; Zhang, P.; Dixon, M. Inactivation of Rhizoctonia solani in fertigation water using regenerative in situ electrochemical hypochlorination. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Levesque, S.; Graham, T.; Bejan, D.; Lawson, J.; Zhang, P.; Dixon, M. An electrochemical advanced oxidation process (EAOP) for the inactivation of Rhizoctonia solani in fertigation solutions. Can. J. Plant Sci. 2020, 100, 415–424. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Bonarrigo, G.; Verheust, Y.; Roccaro, P.; Van Hulle, S. Water reuse in closed hydroponic systems: Comparison of GAC adsorption, ion exchange and ozonation processes to treat recycled nutrient solution. Aquac. Eng. 2017, 78, 190–195. [Google Scholar] [CrossRef]
- Graham, T.; Zhang, P.; Zheng, Y.; Dixon, M.A. Phytotoxicity of Aqueous Ozone on Five Container-grown Nursery Species. HortScience 2009, 44, 774–780. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Yoshii, M.; Isobe, T.; Park, J.-S.; Kurata, K.; Fujiwara, K. Nutrient Solution Prepared with Ozonated Water does not Damage Early Growth of Hydroponically Grown Tomatoes. Ozone: Sci. Eng. 2009, 31, 21–27. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, C.; Song, W. Effect of Ozonated Nutrient Solution on the Growth and Root Antioxidant Capacity of Substrate and Hydroponically Cultivated Lettuce (lactuca Sativa). Ozone: Sci. Eng. 2019, 42, 286–292. [Google Scholar] [CrossRef]
- Mokhtarani, N.; Nasiri, A.; Ganjidoust, H.; Yasrobi, S.Y. Post-Treatment of Composting Leachate by Ozonation. Ozone: Sci. Eng. 2014, 36, 540–548. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M. Plant pathogens in recycled irrigation water in commercial plant nurseries and greenhouses: Their detection and management. Irrig. Sci. 2011, 29, 267–297. [Google Scholar] [CrossRef]
- Rogers, M.A. Organic Vegetable Crop Production in Controlled Environments Using Soilless Media. HortTechnology 2017, 27, 166–170. [Google Scholar] [CrossRef]
- Benito, M.; Masaguer, A.; DeAntonio, R.; Moliner, A. Use of pruning waste compost as a component in soilless growing media. Bioresour. Technol. 2005, 96, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Spiers, T.; Fietje, G. Green Waste Compost as a Component in Soilless Growing Media. Compos. Sci. Util. 2000, 8, 19–23. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Composted Green Waste as a Substitute for Peat in Growth Media: Effects on Growth and Nutrition of Calathea insignis. PLoS ONE 2013, 8, e78121. [Google Scholar] [CrossRef]
- Boldrin, A.; Hartling, K.R.; Laugen, M.; Christensen, T.H. Environmental inventory modelling of the use of compost and peat in growth media preparation. Resour. Conserv. Recycl. 2010, 54, 1250–1260. [Google Scholar] [CrossRef]
- Saer, A.; Lansing, S.; Davitt, N.H.; Graves, R.E. Life cycle assessment of a food waste composting system: Environmental impact hotspots. J. Clean. Prod. 2013, 52, 234–244. [Google Scholar] [CrossRef]

| Factor | Desirable Range |
|---|---|
| C/N | 25:1–30:1 |
| Moisture | 50–60% wet basis |
| Particle size | 0.5–5 cm |
| pH | 5.5–8.0 |
| O2 | >15% |
| Temperature | 45–55 °C |
| Crop | C% (Dry Weight) | N% (Dry Weight) | C/N | Reference |
|---|---|---|---|---|
| Tomato residue | 38.17 | 2.30 | 16.59 | [94] |
| 30.00 | 3.40 | 8.82 | [95] | |
| - | - | 9.34 | [96] | |
| - | - | 15.27 | [97] | |
| Soybean residue | 42.30 | 2.47 | 17.12 | [98] |
| 38.6 | 4.4 | 8.7 | [99] | |
| Lettuce roots | 38.55 | 5.41 | 7.12 | [98] |
| Lettuce residue | 39.9 | 5.1 | 7.8 | [100] |
| Cucumber residue | - | - | 12.01 | [97] |
| 33.81 | 3.00 | 11.27 | [94] | |
| Basil residue | 36 | 4.6 | 7.7 | [99] |
| Aubergine residue | 42.09 | 2.18 | 19.3 | [94] |
| Bean residue | 42.86 | 3.62 | 11.83 | |
| Pepper residue | 39.27 | 3.28 | 11.97 |
| Waste | C/N | pH | Reference |
|---|---|---|---|
| Spent coffee grounds | 21.3 | 7.64 | [130] |
| 22 | - | [131] | |
| 20.2 ± 0.09 | - | [132] | |
| 21.5 | 6.0 | [133] | |
| 23.11 | 5.48 | [134] | |
| Food waste | 18.5 | 5.1 | [135] |
| Lettuce waste (from grocery store) | 10.3 | - | [136] |
| Sawdust | 211 | 5.55 | [137] |
| 792 | - | [136] | |
| Food waste: restaurant | 4.3–9.2 | 3.8–5.2 | [138] |
| Food waste: Grocery | 2.8–20.5 | 4–5 | [138] |
| Food waste: University Residence | 22.8 | 4.6 | [138] |
| Yard wastes (dried fall leaves: grass clippings: wooden debris, 1:1:1) | 10.8 ± 0.1 | 4.8±0.1 | [139] |
| Pruning waste | 46.8 | 6.9 | [140] |
| Woodchips | 98.2, 107.5 | 4.8, 4.9 | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dsouza, A.; Price, G.W.; Dixon, M.; Graham, T. A Conceptual Framework for Incorporation of Composting in Closed-Loop Urban Controlled Environment Agriculture. Sustainability 2021, 13, 2471. https://doi.org/10.3390/su13052471
Dsouza A, Price GW, Dixon M, Graham T. A Conceptual Framework for Incorporation of Composting in Closed-Loop Urban Controlled Environment Agriculture. Sustainability. 2021; 13(5):2471. https://doi.org/10.3390/su13052471
Chicago/Turabian StyleDsouza, Ajwal, Gordon W. Price, Mike Dixon, and Thomas Graham. 2021. "A Conceptual Framework for Incorporation of Composting in Closed-Loop Urban Controlled Environment Agriculture" Sustainability 13, no. 5: 2471. https://doi.org/10.3390/su13052471
APA StyleDsouza, A., Price, G. W., Dixon, M., & Graham, T. (2021). A Conceptual Framework for Incorporation of Composting in Closed-Loop Urban Controlled Environment Agriculture. Sustainability, 13(5), 2471. https://doi.org/10.3390/su13052471






