Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem
Abstract
1. Introduction
2. Nitrogen Footprint in the World
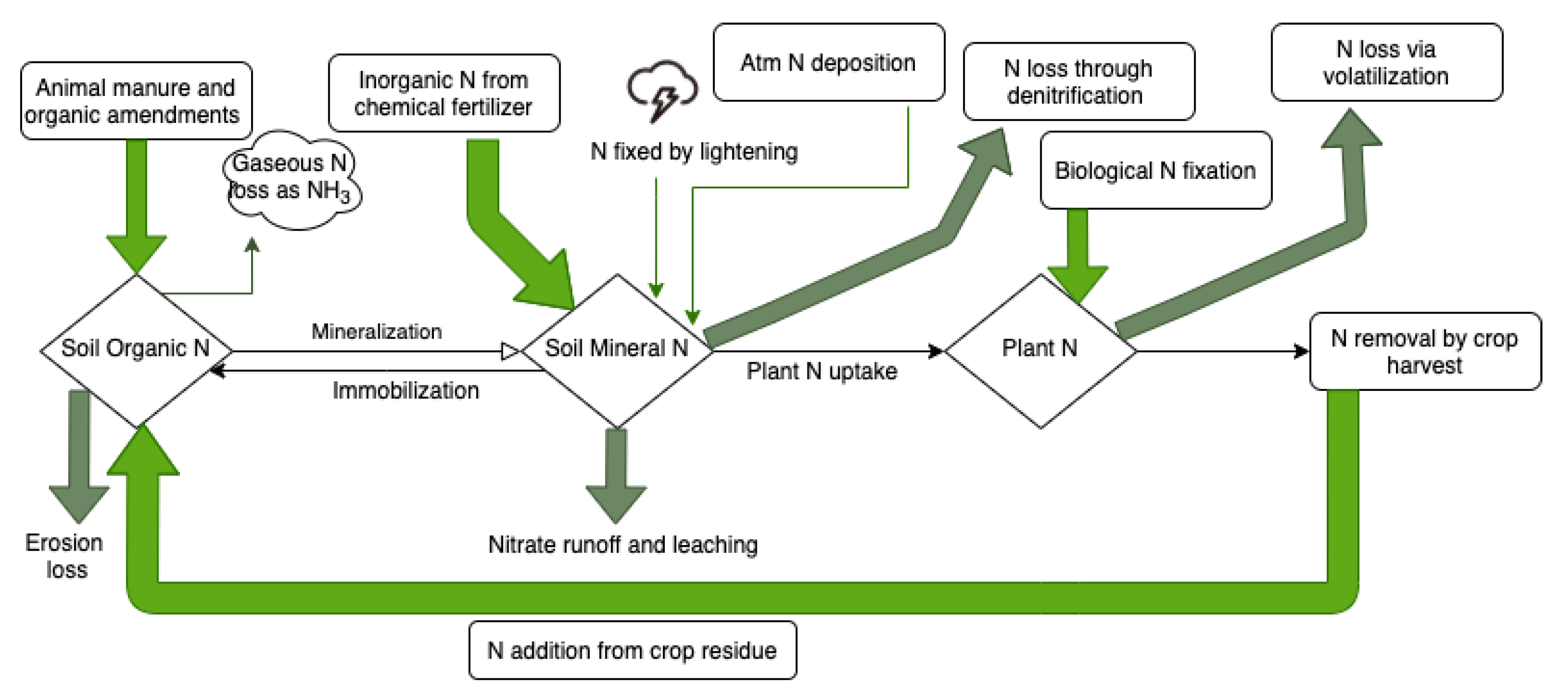
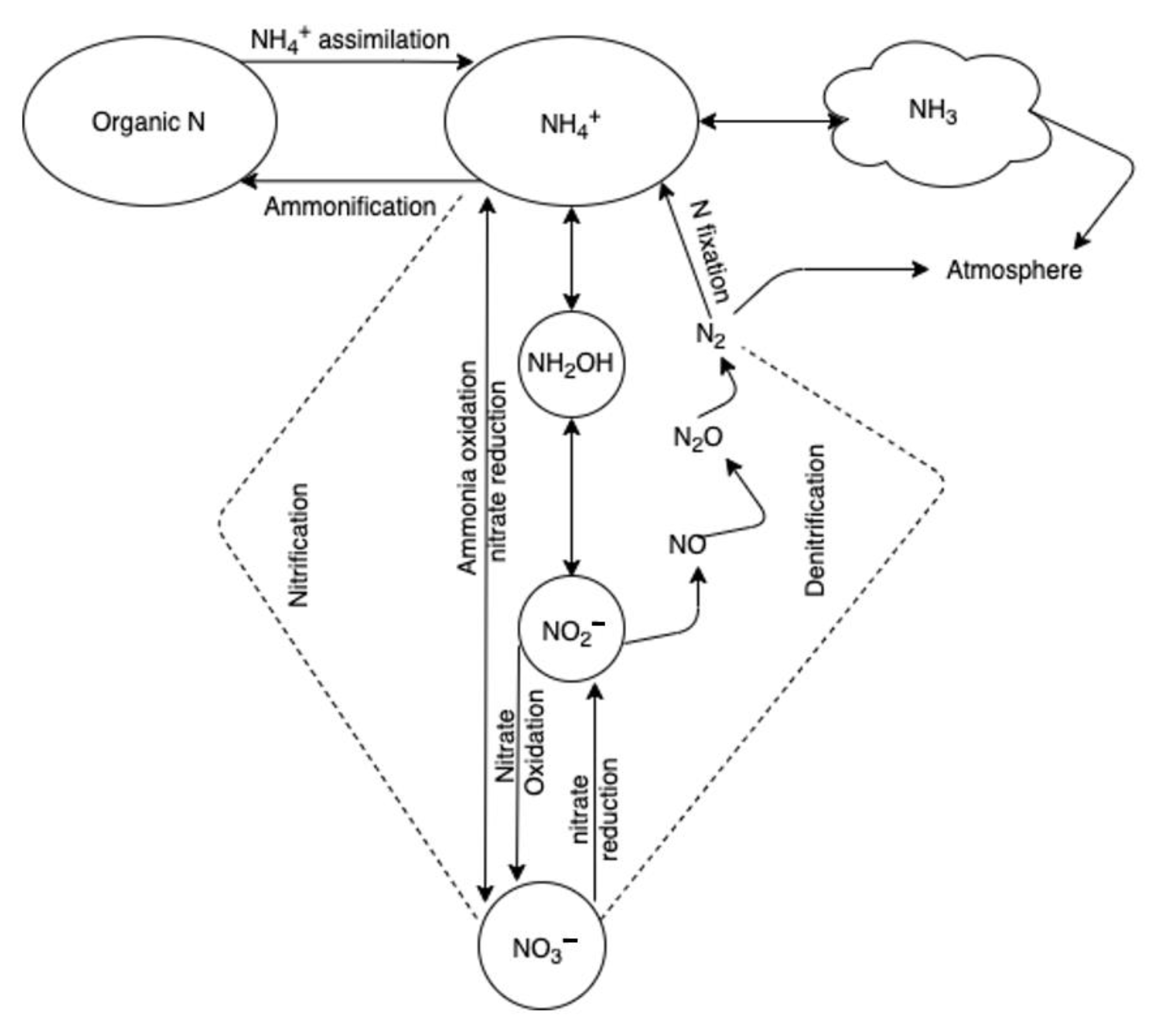
3. Nitrogen Losses in the Environment
3.1. Ammonia Volatilization
3.2. Nitrous Oxide and Oxides of Nitrogen (NOx) Emissions
3.3. Nitrate Leaching
4. Mitigation Strategies
4.1. Farming System Design
4.2. On-Farm Best Available Techniques (BATs)
4.3. Improved Nitrogen Use Efficiency (NUE)
4.3.1. Enhanced Efficiency of Fertilizer Material
4.3.2. Site-Specific Nutrient Management (SSNM) and Real-Time Nitrogen Management (RTNM)
4.3.3. Deep Placement of Urea Super Granules
4.4. Pasture and Livestock Management
4.5. Managing Livestock Wastewater
4.6. Carbon-Rich Sources
4.7. Engineering Cereal Crops for Nitrogen Fixation
4.8. Plant Growth Promoting Microbial Consortia
4.9. Phytogenic Approach and Fungal Utilization
4.10. Organic Agriculture as a Tool in Nitrogen Pollution Remediation
4.10.1. Limited External Input
4.10.2. Crop Diversification
4.11. Ecological Ditch
4.12. Genetic Improvement
4.12.1. Identifying Candidate Gene in Plants for Improved NUE
- a.
- Differentially expressed genes (DFEs) to validate their roles in NUE of different genotypes of crop species can be profiled globally for different genotypes under different N treatments. DFEs have four components and these are:
- i.
- Hybridization based transcriptome analysis to identify differentially expressed traits with low abundance [187];
- ii.
- Analyzing short sequence tags of individual mRNA and then linked to form long sequences and finally cloning them [188];
- iii.
- Probe-targeted hybridization of immobilized cDNA molecules to generate a large amount of data and analysis of the whole genome [189];
- iv.
- RNA sequencing involves the sequencing of every RNA molecule and subsequently profiling a particular gene expression [190].
- b.
- Functional validation of genes by mutation and transgenic studies.
4.12.2. Discovery of Genes by Mapping Studies
5. Nitrogen Status in Agriculture during the COVID-19 Pandemic
6. Knowledge Gaps and Questions
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grafton, R.Q.; Daugbjerg, C.; Qureshi, M.E. Towards food security by 2050. Food Secur. 2015, 7, 179–183. [Google Scholar] [CrossRef]
- Rodriguez, A.; Sanders, I.R. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015, 9, 1053–1061. [Google Scholar] [CrossRef]
- FAO. Food Wastage Footprint: Impacts on Natural Resources; Summary Report; FAO: Rome, Italy, 2013. [Google Scholar]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Pathak, H.; Jain, N.; Bhatia, A.; Kumar, A.; Chatterjee, D. Improved nitrogen management: A key to climate change adaptation and mitigation. Indian J. Fertil. 2016, 12, 151–162. [Google Scholar]
- Houlton, B.Z.; Almaraz, M.; Aneja, V.; Austin, A.T.; Bai, E.; Cassman, K.G.; Compton, J.E.; Davidson, E.A.; Erisman, J.W.; Galloway, J.N. A world of cobenefits: Solving the global nitrogen challenge. Earth’s Future 2019, 7, 865–872. [Google Scholar] [CrossRef]
- Xu, P.; Chen, A.; Houlton, B.Z.; Zeng, Z.; Wei, S.; Zhao, C.; Lu, H.; Liao, Y.; Zheng, Z.; Luan, S. Spatial Variation of Reactive Nitrogen Emissions from China’s Croplands Codetermined by Regional Urbanization and Its Feedback to Global Climate Change. Geophys. Res. Lett. 2020, 47, e2019GL086551. [Google Scholar] [CrossRef]
- Fageria, N.; Baligar, V. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Maharjan, B.; Venterea, R.T.; Rosen, C. Fertilizer and irrigation management effects on nitrous oxide emissions and nitrate leaching. Agron. J. 2014, 106, 703–714. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; Van Cleemput, O. Closing the global N2O budget: Nitrous oxide emissions through the agricultural nitrogen cycle. Nutr. Cycl. Agroecosyst. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- Gao, W.; Yang, H.; Kou, L.; Li, S. Effects of nitrogen deposition and fertilization on N transformations in forest soils: A review. J. Soils Sediments 2015, 15, 863–879. [Google Scholar] [CrossRef]
- Fesenfeld, L.P.; Schmidt, T.S.; Schrode, A. Climate policy for short-and long-lived pollutants. Nat. Clim. Chang. 2018, 8, 933–936. [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.; Song, Y.; Tang, J.; Cao, J.; Zhang, X.; Zhang, Q.; Wang, S.; Xu, T.; Kang, L. Ammonia emission control in China would mitigate haze pollution and nitrogen deposition, but worsen acid rain. Proc. Natl. Acad. Sci. USA 2019, 116, 7760–7765. [Google Scholar] [CrossRef]
- Venterea, R.T.; Halvorson, A.D.; Kitchen, N.; Liebig, M.A.; Cavigelli, M.A.; Grosso, S.J.D.; Motavalli, P.P.; Nelson, K.A.; Spokas, K.A.; Singh, B.P. Challenges and opportunities for mitigating nitrous oxide emissions from fertilized cropping systems. Front. Ecol. Environ. 2012, 10, 562–570. [Google Scholar] [CrossRef]
- Hoben, J.; Gehl, R.; Millar, N.; Grace, P.; Robertson, G. Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Glob. Chang. Biol. 2011, 17, 1140–1152. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Huddell, A.M.; Galford, G.L.; Tully, K.L.; Crowley, C.; Palm, C.A.; Neill, C.; Hickman, J.E.; Menge, D.N. Meta-analysis on the potential for increasing nitrogen losses from intensifying tropical agriculture. Glob. Chang. Biol. 2020, 26, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Leach, A.M.; Galloway, J.N.; Bleeker, A.; Erisman, J.W.; Kohn, R.; Kitzes, J. A nitrogen footprint model to help consumers understand their role in nitrogen losses to the environment. Environ. Dev. 2012, 1, 40–66. [Google Scholar] [CrossRef]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.R.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef]
- Westhoek, H.; Lesschen, J.P.; Leip, A.; Rood, T.; Wagner, S.; De Marco, A.; Murphy-Bokern, D.; Pallière, C.; Howard, C.M.; Oenema, O. Nitrogen on the Table: The Influence of Food Choices on Nitrogen Emissions and the European Environment; NERC/Centre for Ecology & Hydrology: Edinburgh, UK, 2015. [Google Scholar]
- Westhoek, H.; Lesschen, J.P.; Rood, T.; Wagner, S.; de Marco, A.; Murphy-Bokern, D.; Leip, A.; van Grinsven, H.; Sutton, M.A.; Oenema, O. Food choices, health and environment: Effects of cutting Europe’s meat and dairy intake. Glob. Environ. Chang. 2014, 26, 196–205. [Google Scholar] [CrossRef]
- Mohanty, S.; Swain, C.K.; Kumar, A.; Nayak, A. Nitrogen Footprint: A Useful Indicator of Agricultural Sustainability. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 135–156. [Google Scholar]
- Oita, A.; Nagano, I.; Matsuda, H. An improved methodology for calculating the nitrogen footprint of seafood. Ecol. Indic. 2016, 60, 1091–1103. [Google Scholar] [CrossRef]
- Galloway, J.N.; Winiwarter, W.; Leip, A.; Leach, A.M.; Bleeker, A.; Erisman, J.W. Nitrogen footprints: Past, present and future. Environ. Res. Lett. 2014, 9, 115003. [Google Scholar] [CrossRef]
- Shibata, H.; Cattaneo, L.R.; Leach, A.M.; Galloway, J.N. First approach to the Japanese nitrogen footprint model to predict the loss of nitrogen to the environment. Environ. Res. Lett. 2014, 9, 115013. [Google Scholar] [CrossRef]
- Gu, B.; Leach, A.M.; Ma, L.; Galloway, J.N.; Chang, S.X.; Ge, Y.; Chang, J. Nitrogen footprint in China: Food, energy, and nonfood goods. Environ. Sci. Technol. 2013, 47, 9217–9224. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Ju, X.; Chang, J.; Ge, Y.; Vitousek, P.M. Integrated reactive nitrogen budgets and future trends in China. Proc. Natl. Acad. Sci. USA 2015, 112, 8792–8797. [Google Scholar] [CrossRef]
- Gu, B.; Ge, Y.; Ren, Y.; Xu, B.; Luo, W.; Jiang, H.; Gu, B.; Chang, J. Atmospheric reactive nitrogen in China: Sources, recent trends, and damage costs. Environ. Sci. Technol. 2012, 46, 9420–9427. [Google Scholar] [CrossRef]
- Smil, V. Detonator of the population explosion. Nature 1999, 400, 415. [Google Scholar] [CrossRef]
- Oita, A.; Malik, A.; Kanemoto, K.; Geschke, A.; Nishijima, S.; Lenzen, M. Substantial nitrogen pollution embedded in international trade. Nat. Geosci. 2016, 9, 111–115. [Google Scholar] [CrossRef]
- San-Martín, W. Global Nitrogen in Sustainable Development: Four Challenges at the Interface of Science and Policy. Buildings 2012, 2, 300–325. [Google Scholar]
- Black, A.; Sherlock, R.; Cameron, K.; Smith, N.; Goh, K. Comparison of three field methods for measuring ammonia volatilization from urea granules broadcast on to pasture. J. Soil Sci. 1985, 36, 271–280. [Google Scholar] [CrossRef]
- Black, A.; Sherlock, R.; Smith, N.; Cameron, K.; Goh, K. Effects of form of nitrogen, season, and urea application rate on ammonia volatilisation from pastures. N. Z. J. Agric. Res. 1985, 28, 469–474. [Google Scholar] [CrossRef]
- McGarry, S.; O’Toole, P.; Morgan, M. Effects of soil temperature and moisture content on ammonia volatilization from urea-treated pasture and tillage soils. Ir. J. Agric. Res. 1987, 26, 173–182. [Google Scholar]
- Cameron, K.; Di, H.J.; Moir, J. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Acton, S.D. The Effect of Fertiliser Application Rate and Soil PH on Methane Oxidation and Nitrous Oxide Production; University of Aberdeen: Aberdeen, UK, 2007. [Google Scholar]
- Mundepi, A.; Cabrera, M.; Norton, J.; Habteselassie, M. Ammonia Oxidizers as Biological Health Indicators of Elevated Zn and Cu in Poultry Litter Amended Soil. Water Air Soil Pollut. 2019, 230, 239. [Google Scholar] [CrossRef]
- Cassity-Duffey, K.; Cabrera, M.; Franklin, D.; Gaskin, J.; Kissel, D. Effect of soil texture on nitrogen mineralization from organic fertilizers in four common southeastern soils. Soil Sci. Soc. Am. J. 2020, 84, 534–542. [Google Scholar] [CrossRef]
- Bouwman, A.; Boumans, L.; Batjes, N. Modeling global annual N2O and NO emissions from fertilized fields. Glob. Biogeochem. Cycles 2002, 16, 28-1–28-9. [Google Scholar] [CrossRef]
- Paulot, F.; Jacob, D.J.; Pinder, R.; Bash, J.; Travis, K.; Henze, D. Ammonia emissions in the United States, European Union, and China derived by high-resolution inversion of ammonium wet deposition data: Interpretation with a new agricultural emissions inventory (MASAGE_NH3). J. Geophys. Res. Atmos. 2014, 119, 4343–4364. [Google Scholar] [CrossRef]
- Sutton, M.A.; Erisman, J.W.; Dentener, F.; Möller, D. Ammonia in the environment: From ancient times to the present. Environ. Pollut. 2008, 156, 583–604. [Google Scholar] [CrossRef]
- Carlson, J.; Daehler, K.R. The refined consensus model of pedagogical content knowledge in science education. In Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science; Springer: Singapore, 2019; pp. 77–92. [Google Scholar]
- Pathak, H.; Bhatia, A.; Prasad, S.; Singh, S.; Kumar, S.; Jain, M.; Kumar, U. Emission of nitrous oxide from rice-wheat systems of Indo-Gangetic plains of India. Environ. Monit. Assess. 2002, 77, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Caranto, J.D.; Lancaster, K.M. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc. Natl. Acad. Sci. USA 2017, 114, 8217–8222. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel On Climate Change. Climate change 2007: The physical science basis. Agenda 2007, 6, 333. [Google Scholar]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Sarabia, L.; Solorio, F.J.; Ramírez, L.; Ayala, A.; Aguilar, C.; Ku, J.; Almeida, C.; Cassador, R.; Alves, B.J.; Boddey, R.M. Improving the Nitrogen Cycling in Livestock Systems Through Silvopastoral Systems. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 189–213. [Google Scholar]
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Copenhagen, Denmark, 2006. [Google Scholar]
- Avol, E.L.; Gauderman, W.J.; Tan, S.M.; London, S.J.; Peters, J.M. Respiratory effects of relocating to areas of differing air pollution levels. Am. J. Respir. Crit. Med. 2001, 164, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Bowatte, G.; Erbas, B.; Lodge, C.J.; Knibbs, L.D.; Gurrin, L.C.; Marks, G.B.; Thomas, P.S.; Johns, D.P.; Giles, G.G.; Hui, J. Traffic-related air pollution exposure over a 5-year period is associated with increased risk of asthma and poor lung function in middle age. Eur. Respir. J. 2017, 50, 1602357. [Google Scholar] [PubMed]
- Singh, B.; Sekhon, G. Nitrate pollution of groundwater from farm use of nitrogen fertilizers—A review. Agric. Environ. 1979, 4, 207–225. [Google Scholar] [CrossRef]
- Watt, W.; Tulinsky, A.; Swenson, R.P.; Watenpaugh, K.D. Comparison of the crystal structures of a flavodoxin in its three oxidation states at cryogenic temperatures. J. Mol. Biol. 1991, 218, 195–208. [Google Scholar] [CrossRef]
- Wild, A.; Cameron, K. Soil nitrogen and nitrate leaching. In Soils and Agriculture; Tinker, P.B., Ed.; Blackwell: Oxford, UK, 1980; pp. 35–70. [Google Scholar]
- Cameron, K.; Wild, A. 1984, 13, 274–278.
- Silva, R.; Cameron, K.; Di, H.; Smith, N.; Buchan, G. Effect of macropore flow on the transport of surface-applied cow urine through a soil profile. Soil Res. 2000, 38, 13–24. [Google Scholar] [CrossRef]
- Brender, J.D.; Weyer, P.J.; Romitti, P.A.; Mohanty, B.P.; Shinde, M.U.; Vuong, A.M.; Sharkey, J.R.; Dwivedi, D.; Horel, S.A.; Kantamneni, J. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ. Health Perspect. 2013, 121, 1083–1089. [Google Scholar] [CrossRef]
- Knobeloch, L.; Salna, B.; Hogan, A.; Postle, J.; Anderson, H. Blue babies and nitrate-contaminated well water. Environ. Health Perspect. 2000, 108, 675–678. [Google Scholar] [CrossRef]
- Folke, C.; Kautsky, N.; Berg, H.; Jansson, Å.; Troell, M. The ecological footprint concept for sustainable seafood production: A review. Ecol. Appl. 1998, 8, S63–S71. [Google Scholar] [CrossRef]
- Little, D.; Edwards, P. Integrated Livestock-Fish Farming Systems; Food & Agriculture Organization: Rome, Italy, 2003. [Google Scholar]
- Sutton, M.A.; Bleeker, A.; Howard, C.; Erisman, J.; Abrol, Y.; Bekunda, M.; Datta, A.; Davidson, E.; De Vries, W.; Oenema, O. Our Nutrient World. The Challenge to Produce More Food & Energy with Less Pollution; Centre for Ecology & Hydrology: Edinburgh, UK, 2013. [Google Scholar]
- FAO. FAOSTAT Online Statistical Service; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. [Google Scholar]
- Horneck, D.A.; Sullivan, D.M.; Owen, J.S.; Hart, J.M. Soil Test Interpretation Guide; Oregon State University: Corvallis, OR, USA, 2011. [Google Scholar]
- Di, H.; Cameron, K. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosystems 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Dungait, J.A.; Cardenas, L.M.; Blackwell, M.S.; Wu, L.; Withers, P.J.; Chadwick, D.R.; Bol, R.; Murray, P.J.; Macdonald, A.J.; Whitmore, A.P. Advances in the understanding of nutrient dynamics and management in UK agriculture. Sci. Total Environ. 2012, 434, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Goulding, K.; Jarvis, S.; Whitmore, A. Optimizing nutrient management for farm systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R. Mineral Nitrogen in the Plant-Soil System; Elsevier: Orlando, FL, USA, 2012. [Google Scholar]
- Kay, P.; Edwards, A.C.; Foulger, M. A review of the efficacy of contemporary agricultural stewardship measures for ameliorating water pollution problems of key concern to the UK water industry. Agric. Syst. 2009, 99, 67–75. [Google Scholar] [CrossRef]
- Monaghan, R.; De Klein, C.A.; Muirhead, R.W. Prioritisation of farm scale remediation efforts for reducing losses of nutrients and faecal indicator organisms to waterways: A case study of New Zealand dairy farming. J. Environ. Manag. 2008, 87, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, R.; Hedley, M.; Di, H.; McDowell, R.; Cameron, K.; Ledgard, S. Nutrient management in New Zealand pastures—recent developments and future issues. N. Z. J. Agric. Res. 2007, 50, 181–201. [Google Scholar] [CrossRef]
- Munoz, F.; Mylavarapu, R.; Hutchinson, C. Environmentally responsible potato production systems: A review. J. Plant Nutr. 2005, 28, 1287–1309. [Google Scholar] [CrossRef]
- Dahal, S.; Franklin, D.; Subedi, A.; Cabrera, M.; Hancock, D.; Mahmud, K.; Ney, L.; Park, C.; Mishra, D.J.S. Strategic Grazing in Beef-Pastures for Improved Soil Health and Reduced Runoff-Nitrate-A Step towards. Sustainability 2020, 12, 558. [Google Scholar] [CrossRef]
- Giller, K.E.; Chalk, P.; Dobermann, A.; Hammond, L.; Heffer, P.; Ladha, J.K.; Nyamudeza, P.; Maene, L.; Ssali, H.; Freney, J. Emerging technologies to increase the efficiency of use of fertilizer nitrogen. Agric. Nitrogen Cycle Assess. Impacts Fertil. Food Prod. Environ. 2004, 65, 35–51. [Google Scholar]
- Freney, J.R. Management practices to increase efficiency of fertilizer and animal nitrogen and minimize nitrogen loss to the atmosphere and groundwater. Tech. Bull. Food Fertil. Technol. Cent. 2011, 186, 22. [Google Scholar]
- Soares, J.R.; Cantarella, H.; de Campos Menegale, M.L. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K.; Meena, V.S.; Kumar, A.; Meena, R. Issues and challenges about sustainable agriculture production for management of natural resources to sustain soil fertility and health. J. Clean. Prod. 2015, 107, 793–794. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of soil phosphorus on legume production. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 487–510. [Google Scholar]
- Sanz-Cobena, A.; Misselbrook, T.H.; Arce, A.; Mingot, J.I.; Diez, J.A.; Vallejo, A. An inhibitor of urease activity effectively reduces ammonia emissions from soil treated with urea under Mediterranean conditions. Agric. Ecosyst. Environ. 2008, 126, 243–249. [Google Scholar] [CrossRef]
- Turner, D.; Edis, R.; Chen, D.; Freney, J.; Denmead, O.; Christie, R. Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide. Agric. Ecosyst. Environ. 2010, 137, 261–266. [Google Scholar] [CrossRef]
- Chambers, B.; Dampney, P. Nitrogen efficiency and ammonia emissions from urea-based and ammonium nitrate fertilisers. In Proceedings of Proceedings-International Fertiliser Society; Internationl Fertiliser Society: York, UK, 2009. [Google Scholar]
- Rodriguez, M.J.; Saggar, S.; Berben, P.; Palmada, T.; Lopez-Villalobos, N.; Pal, P. Use of a urease inhibitor to mitigate ammonia emissions from urine patches. Environ. Technol. 2021, 42, 20–31. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, J.J.; Wei, Z.; Dodla, S.K.; Fultz, L.M.; Gaston, L.A.; Xiao, R.; Park, J.-h.; Scaglia, G. Nitrification inhibitors reduce nitrogen losses and improve soil health in a subtropical pastureland. Geoderma 2021, 388, 114947. [Google Scholar] [CrossRef]
- Dobermann, A.; Witt, C.; Dawe, D.; Abdulrachman, S.; Gines, H.; Nagarajan, R.; Satawathananont, S.; Son, T.; Tan, P.; Wang, G. Site-specific nutrient management for intensive rice cropping systems in Asia. Field Crops Res. 2002, 74, 37–66. [Google Scholar] [CrossRef]
- Tagarakis, A.C.; Ketterings, Q.M. In-season estimation of corn yield potential using proximal sensing. Agron. J. 2017, 109, 1323–1330. [Google Scholar] [CrossRef]
- Shanahan, J.; Kitchen, N.; Raun, W.; Schepers, J.S. Responsive in-season nitrogen management for cereals. Comput. Electron. Agric. 2008, 61, 51–62. [Google Scholar] [CrossRef]
- Siqueira, R.; Longchamps, L.; Dahal, S.; Khosla, R. Use of fluorescence sensing to detect nitrogen and potassium variability in maize. Remote Sens. 2020, 12, 1752. [Google Scholar] [CrossRef]
- Dahal, S.; Phillippi, E.; Longchamps, L.; Khosla, R.; Andales, A. Variable Rate Nitrogen and Water Management for Irrigated Maize in the Western US. Agronomy 2020, 10, 1533. [Google Scholar] [CrossRef]
- Havránková, J. The evaluation of ground based remote sensing systems for canopy nitrogen management in winter wheat. CIGR J. 2007. Available online: https://dspace.lib.cranfield.ac.uk/handle/1826/1711 (accessed on 23 February 2021).
- Thind, H.; Kumar, A.; Vashistha, M. Calibrating the leaf colour chart for need based fertilizer nitrogen management in different maize (Zea mays L.) genotypes. Field Crop. Res. 2011, 120, 276–282. [Google Scholar]
- Fu, Y.-Q.; Zhong, X.-H.; Zeng, J.-H.; Liang, K.-M.; Pan, J.-F.; Xin, Y.-F.; Liu, Y.-Z.; Hu, X.-Y.; Peng, B.-L.; Chen, R.-B. Improving grain yield, nitrogen use efficiency and radiation use efficiency by dense planting, with delayed and reduced nitrogen application, in double cropping rice in South China. J. Integr. Agric. 2021, 20, 565–580. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, P.; Mo, Z.; Kong, L.; Tian, H.; Duan, M.; Li, L.; Wu, L.; Wang, Z.; Tang, X. Deep Placement of Nitrogen Fertilizer Affects Grain Yield, Nitrogen Recovery Efficiency, and Root Characteristics in Direct-Seeded Rice in South China. J. Plant. Growth Regul. 2020, 40, 379–387. [Google Scholar] [CrossRef]
- KhalilA, M. Physical and chemical manipulation of urea fertiliser for reducing the emission of gaseous nitrogen species. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; Congress Symposium 4: Greenhouse Gases from Soils. pp. 195–198. [Google Scholar]
- Chatterjee, D.; Mohanty, S.; Guru, P.K.; Swain, C.K.; Tripathi, R.; Shahid, M.; Kumar, U.; Kumar, A.; Bhattacharyya, P.; Gautam, P. Comparative assessment of urea briquette applicators on greenhouse gas emission, nitrogen loss and soil enzymatic activities in tropical lowland rice. Agric. Ecosyst. Environ. 2018, 252, 178–190. [Google Scholar] [CrossRef]
- Mencaroni, M.; Dal Ferro, N.; Furlanetto, J.; Longo, M.; Lazzaro, B.; Sartori, L.; Grant, B.; Smith, W.; Morari, F. Identifying N fertilizer management strategies to reduce ammonia volatilization: Towards a site-specific approach. J. Environ. Manag. 2021, 277, 111445. [Google Scholar] [CrossRef] [PubMed]
- Lessa, A.C.R.; Madari, B.E.; Paredes, D.S.; Boddey, R.M.; Urquiaga, S.; Jantalia, C.P.; Alves, B.J.J.A. Ecosystems; Environment. Bovine urine and dung deposited on Brazilian savannah pastures contribute differently to direct and indirect soil nitrous oxide emissions. Agric. Ecosyst. Environ. 2014, 190, 104–111. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 7, 1163. [Google Scholar] [CrossRef]
- Subedi, A.; Franklin, D.; Cabrera, M.; McPherson, A.; Dahal, S. Grazing Systems to Retain and Redistribute Soil Phosphorus and to Reduce Phosphorus Losses in Runoff. Soil Syst. 2020, 4, 66. [Google Scholar] [CrossRef]
- Adegbeye, M.; Reddy, P.R.K.; Obaisi, A.; Elghandour, M.; Oyebamiji, K.; Salem, A.; Morakinyo-Fasipe, O.; Cipriano-Salazar, M.; Camacho-Díaz, L. Sustainable agriculture options for production, greenhouse gasses and pollution alleviation, and nutrient recycling in emerging and transitional nations-An overview. J. Clean. Prod. 2020, 242, 118319. [Google Scholar] [CrossRef]
- Frank, S.; Havlík, P.; Stehfest, E.; van Meijl, H.; Witzke, P.; Pérez-Domínguez, I.; van Dijk, M.; Doelman, J.C.; Fellmann, T.; Koopman, J.F. Agricultural non-CO2 emission reduction potential in the context of the 1.5 °C target. Nat. Clim. Chang. 2019, 9, 66–72. [Google Scholar] [CrossRef]
- Herrero, M.; Havlík, P.; Valin, H.; Notenbaert, A.; Rufino, M.C.; Thornton, P.K.; Blümmel, M.; Weiss, F.; Grace, D.; Obersteiner, M. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20888–20893. [Google Scholar] [CrossRef]
- Takemasa, M.J.K. Nutritional strategies to reduce nutrient waste in livestock and poultry production. Food 1998, 36, 720–726. [Google Scholar]
- Stephenson, G.T. The Effects of Agricultural Waste-Based Compost Amendments in Organic Pest Management. Master’s Thesis, California Polytechnics University, San Luis Obispo, CA, USA, 2019. [Google Scholar]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V.; Cotton, J.; Cano, A. Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures. Appl. Soil Ecol. 2018, 132, 179–186. [Google Scholar] [CrossRef]
- Peoples, M.; Baldock, J. Nitrogen dynamics of pastures: Nitrogen fixation inputs, the impact of legumes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian farming systems. Aust. J. Exp. Agric. 2001, 41, 327–346. [Google Scholar] [CrossRef]
- Stockdale, E.; Lampkin, N.; Hovi, M.; Keatinge, R.; Lennartsson, E.; Macdonald, D.; Padel, S.; Tattersall, F.; Wolfe, M.; Watson, C. Agronomic and environmental implications of organic farming systems. Agronomy 2001, 70, 261–327. [Google Scholar]
- White, J.; Hodgson, J.G. New Zealand Pasture and Crop Science; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V. Assessing the role of interseeding alfalfa into grass on improving pasture soil health in semi-arid Texas High Plains. Appl. Soil Ecol. 2020, 147, 103399. [Google Scholar] [CrossRef]
- Mobin, S.; Alam, F. Biofuel production from algae utilizing wastewater. In Proceedings of the 19th Australasian Fluid Mechanics Conference, Melbourne, Australia, 8–11 December 2014. [Google Scholar]
- Dang, N.M.; Lee, K. Recent trends of using alternative nutrient sources for microalgae cultivation as a feedstock of biodiesel production. Appl. Chem. Eng. 2018, 29, 1–9. [Google Scholar]
- Aslan, S.; Kapdan, I.K. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol. Eng. 2006, 28, 64–70. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Nunes, S.; Carneiro, M.; Pereira, D. Nutrients’ removal from aquaculture wastewater using the macroalgae Gracilaria birdiae. Biomass Bioenergy 2009, 33, 327–331. [Google Scholar] [CrossRef]
- He, P.; Xu, S.; Zhang, H.; Wen, S.; Dai, Y.; Lin, S.; Yarish, C. Bioremediation efficiency in the removal of dissolved inorganic nutrients by the red seaweed, Porphyra yezoensis, cultivated in the open sea. Water Res. 2008, 42, 1281–1289. [Google Scholar] [CrossRef]
- Webb, J.R.; Hayes, N.M.; Simpson, G.L.; Leavitt, P.R.; Baulch, H.M.; Finlay, K. Widespread nitrous oxide undersaturation in farm waterbodies creates an unexpected greenhouse gas sink. Proc. Natl. Acad. Sci. USA 2019, 116, 9814–9819. [Google Scholar] [CrossRef]
- Meade, G.; Pierce, K.; O’Doherty, J.; Mueller, C.; Lanigan, G.; Mc Cabe, T. Ammonia and nitrous oxide emissions following land application of high and low nitrogen pig manures to winter wheat at three growth stages. Agric. Ecosyst. Environ. 2011, 140, 208–217. [Google Scholar] [CrossRef]
- Mahmud, K.; Chowhdhury, M.; Noor, N.; Huq, S.I. Effects of different sources of biochar application on the emission of a number of gases from soil. Can. J. Pure Appl. Sci. 2014, 8, 2813–2824. [Google Scholar]
- Brennan, R.B.; Healy, M.G.; Fenton, O.; Lanigan, G.J. The effect of chemical amendments used for phosphorus abatement on greenhouse gas and ammonia emissions from dairy cattle slurry: Synergies and pollution swapping. PLoS ONE 2015, 10, e0111965. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies: Wageningen, The Netherlands, 2006; Volume 5. [Google Scholar]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Panday, D.; Mikha, M.M.; Collins, H.P.; Jin, V.L.; Kaiser, M.; Cooper, J.; Malakar, A.; Maharjan, B. Optimum rates of surface-applied coal char decreased soil ammonia volatilization loss. J. Environ. Qual. 2020, 49, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Hollert, H. Comparison of sewage sludge toxicity to plants and invertebrates in three different soils. Chemosphere 2011, 83, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P. Evaluation of sewage sludge and slow pyrolyzed sewage sludge-derived biochar for adsorption of phenanthrene and pyrene. Bioresour. Technol. 2015, 192, 618–626. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Organic farming: Does it contribute to contaminant-free produce and ensure food safety? Sci. Total. Environ. 2021, 769, 145079. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P. Addition of biochar to sewage sludge decreases freely dissolved PAHs content and toxicity of sewage sludge-amended soil. Environ. Pollut. 2016, 218, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Zielińska, A.; Cornelissen, G. Stabilization of sewage sludge by different biochars towards reducing freely dissolved polycyclic aromatic hydrocarbons (PAHs) content. Bioresour. Technol. 2014, 156, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kończak, M.; Oleszczuk, P. Application of biochar to sewage sludge reduces toxicity and improve organisms growth in sewage sludge-amended soil in long term field experiment. Sci. Total Environ. 2018, 625, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Mahmud, K.; Chowdhury, M.T.A.; Huq, S.I. The use of biochar as ameliorator for soil arsenic. Dhaka Univ. J. Biol. Sci. 2015, 24, 111–119. [Google Scholar] [CrossRef][Green Version]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A.J.P. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.E.; Ryu, M.-H.; Ozaydin, B.; Broglie, R. Harnessing atmospheric nitrogen for cereal crop production. Curr. Opin. Biotechnol. 2020, 62, 181–188. [Google Scholar] [CrossRef]
- Rogers, C.; Oldroyd, G.E. Synthetic biology approaches to engineering the nitrogen symbiosis in cereals. J. Exp. Bot. 2014, 65, 1939–1946. [Google Scholar] [CrossRef]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010, 1, 1–12. [Google Scholar] [CrossRef]
- Maillet, F.; Poinsot, V.; André, O.; Puech-Pagès, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.; Poole, P.S. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Geddes, B.A.; Ryu, M.-H.; Mus, F.; Costas, A.G.; Peters, J.W.; Voigt, C.A.; Poole, P. Use of plant colonizing bacteria as chassis for transfer of N2-fixation to cereals. Curr. Opin. Biotechnol. 2015, 32, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.R.; Papini-Terzi, F.S.; Nishiyama, M.Y.; Vencio, R.Z.; Vicentini, R.; Duarte, R.D.; de Rosa, V.E.; Vinagre, F.; Barsalobres, C.; Medeiros, A.H. Signal transduction-related responses to phytohormones and environmental challenges in sugarcane. BMC Genom. 2007, 8, 71. [Google Scholar] [CrossRef]
- Ibort, P.; Imai, H.; Uemura, M.; Aroca, R. Proteomic analysis reveals that tomato interaction with plant growth promoting bacteria is highly determined by ethylene perception. J. Plant Physiol. 2018, 220, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Brusamarello-Santos, L.C.; Gilard, F.; Brulé, L.; Quilleré, I.; Gourion, B.; Ratet, P.; Maltempi de Souza, E.; Lea, P.J.; Hirel, B. Metabolic profiling of two maize (Zea mays L.) inbred lines inoculated with the nitrogen fixing plant-interacting bacteria Herbaspirillum seropedicae and Azospirillum brasilense. PLoS ONE 2017, 12, e0174576. [Google Scholar] [CrossRef]
- Kost, T.; Stopnisek, N.; Agnoli, K.; Eberl, L.; Weisskopf, L. Oxalotrophy, a widespread trait of plant-associated Burkholderia species, is involved in successful root colonization of lupin and maize by Burkholderia phytofirmans. Front. Microbiol. 2014, 4, 421. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.-M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Santos, O.J.; Marcelino, P.R.; Milani, K.M.; Zuluaga, M.Y.; Zucareli, C.; Gonçalves, L.S. Maize inoculation with Azospirillum brasilense Ab-V5 cells enriched with exopolysaccharides and polyhydroxybutyrate results in high productivity under low N fertilizer input. Front. Microbiol. 2017, 8, 1873. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Larsen, E.; Grossman, J.; Edgell, J.; Hoyt, G.; Osmond, D.; Hu, S. Soil biological properties, soil losses and corn yield in long-term organic and conventional farming systems. Soil Tillage Res. 2014, 139, 37–45. [Google Scholar] [CrossRef]
- Coelho, L.M.; Rezende, H.C.; Coelho, L.M.; de Sousa, P.; Melo, D.; Coelho, N. Bioremediation of polluted waters using microorganisms. Adv. Bioremedia. Wastewater Polluted Soil 2015, 10, 60770. [Google Scholar]
- Kloepper, J.W. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th International Conference on Plant Pathogenic Bacter, Station de Pathologie Vegetale et Phytobacteriologie, INRA, Angers, France, 27 August–2 September 1978; pp. 879–882. [Google Scholar]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Buee, M.; De Boer, W.; Martin, F.; Van Overbeek, L.; Jurkevitch, E. The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 2009, 321, 189–212. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, M.; Han, B.; Impraim, R.; Butterly, C.; Hu, H.; He, J.; Chen, D. Enhanced nitrogen retention by lignite during poultry litter composting. J. Clean. Prod. 2020, 277, 122422. [Google Scholar] [CrossRef]
- Morriën, E. Understanding soil food web dynamics, how close do we get? Soil Biol. Biochem. 2016, 102, 10–13. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q. Examining trophic-level nematode community structure and nitrogen mineralization to assess local effective microorganisms’ role in nitrogen availability of swine effluent to forage crops. Appl. Soil Ecol. 2018, 130, 209–218. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Fatzinger, B. Rebuilding Soil Ecosystems for Improved Productivity in Biosolarized Soils. Int. J. Agron. 2019, 2019. [Google Scholar] [CrossRef]
- Mahmud, K.; Franklin, D.; Ney, L.; Cabrera, M.; Habteselassie, M.; Hancock, D.; Newcomer, Q.; Subedi, A.; Dahal, S. Improving inorganic nitrogen in soil and nutrient density of edamame bean in three consecutive summers by utilizing a locally sourced bio-inocula. Org. Agric. 2021. [Google Scholar] [CrossRef]
- Li, X.; Guo, Q.; Wang, Y.; Xu, J.; Wei, Q.; Chen, L.; Liao, L. Enhancing Nitrogen and Phosphorus Removal by Applying Effective Microorganisms to Constructed Wetlands. Water 2020, 12, 2443. [Google Scholar] [CrossRef]
- Polyorach, S.; Wanapat, M.; Cherdthong, A.; Kang, S. Rumen microorganisms, methane production, and microbial protein synthesis affected by mangosteen peel powder supplement in lactating dairy cows. Trop. Anim. Health Prod. 2016, 48, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.P.; Dall-Orsoletta, A.C.; Ribeiro-Filho, H.M.N. The effects of supplementing Acacia mearnsii tannin extract on dairy cow dry matter intake, milk production, and methane emission in a tropical pasture. Trop. Anim. Health Prod. 2017, 49, 1663–1668. [Google Scholar] [CrossRef]
- Albores-Moreno, S.; Alayón-Gamboa, J.; Ayala-Burgos, A.; Solorio-Sánchez, F.; Aguilar-Pérez, C.; Olivera-Castillo, L.; Ku-Vera, J. Effects of feeding ground pods of Enterolobium cyclocarpum Jacq. Griseb on dry matter intake, rumen fermentation, and enteric methane production by Pelibuey sheep fed tropical grass. Trop. Anim. Health Prod. 2017, 49, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.E.S.; Duarte, E.R.; Alves, D.D.; Martinele, I.; D’Agosto, M.; Cedrola, F.; de Moura Freitas, A.A.; dos Santos Soares, F.D.; Beltran, M. Sheep fed with banana leaf hay reduce ruminal protozoa population. Trop. Anim. Health Prod. 2017, 49, 807–812. [Google Scholar] [CrossRef]
- Gerlach, K.; Schmithausen, A.J.; Sommer, A.C.; Trimborn, M.; Büscher, W.; Südekum, K.-H. Cattle diets strongly affect nitrous oxide in the rumen. Sustainability 2018, 10, 3679. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Trewavas, A. Urban myths of organic farming. Nature 2001, 410, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J. Going one better than nature? Nature 2001, 410, 633–634. [Google Scholar] [CrossRef]
- Willer, H.; Lernoud, J. The World of Organic Agriculture. Statistics and Emerging Trends 2019; Research Institute of Organic Agriculture FiBL and IFOAM Organics International: Frick, Switzerland; Bonn, Switzerland, 2019. [Google Scholar]
- Saffeullah, P.; Nabi, N.; Liaqat, S.; Anjum, N.A.; Siddiqi, T.O.; Umar, S. Organic Agriculture: Principles, Current Status, and Significance. In Microbiota and Biofertilizers; Springer: Singapore, 2021; pp. 17–37. [Google Scholar]
- Shi, W.; Norton, J.M. Microbial control of nitrate concentrations in an agricultural soil treated with dairy waste compost or ammonium fertilizer. Soil Biol. Biochem. 2000, 32, 1453–1457. [Google Scholar] [CrossRef]
- Habteselassie, M.Y.; Miller, B.E.; Thacker, S.G.; Stark, J.M.; Norton, J.M. Soil nitrogen and nutrient dynamics after repeated application of treated dairy-waste. Soil Sci. Soc. Am. J. 2006, 70, 1328–1337. [Google Scholar] [CrossRef]
- Cameron, K.C.; Rate, A.W.; Noonan, M.J.; Moore, S.; Smith, N.P.; Kerr, L.E. Lysimeter study of the fate of nutrients following subsurface injection and surface application of dairy pond sludge to pasture. Agric. Ecosyst. Environ. 1996, 58, 187–197. [Google Scholar] [CrossRef]
- Kirchmann, H.; Bergström, L. Do organic farming practices reduce nitrate leaching? Commun. Soil Sci. Plant Anal. 2001, 32, 997–1028. [Google Scholar] [CrossRef]
- Scialabba, N.E.-H.; Müller-Lindenlauf, M. Organic agriculture and climate change. Renew. Agric. Food Syst. 2010, 25, 158–169. [Google Scholar] [CrossRef]
- Chatterjee, R. Projecting the Future of Nitrogen Pollution 2009. [CrossRef][Green Version]
- Williams, A.; Audsley, E.; Sandars, D. Determining the Environmental Burdens and Resource Use in the Production of Agricultural and Horticultural Commodities: Defra Project Report IS0205. Available online: http://randd.defra.gov.uk/Default.aspx (accessed on 27 January 2021).
- Rahmann, G.; Ardakani, M.R.; Bàrberi, P.; Boehm, H.; Canali, S.; Chander, M.; David, W.; Dengel, L.; Erisman, J.W.; Galvis-Martinez, A.C. Organic Agriculture 3.0 is innovation with research. Org. Agric. 2017, 7, 169–197. [Google Scholar] [CrossRef]
- Smith, J.B.; Lenhart, S.S. Climate change adaptation policy options. Clim. Res. 1996, 6, 193–201. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L. Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant Soil 2003, 248, 305–312. [Google Scholar] [CrossRef]
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T.D. Removal of nutrients, organics and suspended solids in vegetated agricultural drainage ditch. Ecol. Eng. 2018, 118, 97–103. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, M.; Tsang, D.C.; Geng, N.; Lu, D.; Zhu, L.; Igalavithana, A.D.; Dissanayake, P.D.; Rinklebe, J.; Yang, X. Recent advances in control technologies for non-point source pollution with nitrogen and phosphorous from agricultural runoff: Current practices and future prospects. Appl. Biol. Chem. 2020, 63, 1–13. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Meng, F.; Iseyemi, O.; Moore, M.T.; Zhu, B.; Tao, W.; Liang, T.J.; Ilunga, L. Removal of non-point source pollutants from domestic sewage and agricultural runoff by vegetated drainage ditches (VDDs): Design, mechanism, management strategies, and future directions. Sci. Total Environ. 2018, 639, 742–759. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, J.; Dagès, C.; Bailly, J.-S.; Lagacherie, P.; Voltz, M. Managing ditches for agroecological engineering of landscape: A review. Agron. Sustain. Dev. 2015, 35, 999–1020. [Google Scholar] [CrossRef]
- Nsenga Kumwimba, M.; Dzakpasu, M.; Zhu, B.; Wang, T.; Ilunga, L.; Kavidia Muyembe, D. Nutrient removal in a trapezoidal vegetated drainage ditch used to treat primary domestic sewage in a small catchment of the upper Yangtze River. Water Environ. J. 2017, 31, 72–79. [Google Scholar] [CrossRef]
- Kröger, R.; Holland, M.; Moore, M.; Cooper, C. Hydrological variability and agricultural drainage ditch inorganic nitrogen reduction capacity. J. Environ. Qual. 2007, 36, 1646–1652. [Google Scholar] [CrossRef]
- Menon, R.; Holland, M.M. Phosphorus release due to decomposition of wetland plants. Wetlands 2014, 34, 1191–1196. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Xiao, R.; Zhang, S.; Liu, F.; Xiang, Z. Absorption capacity of nitrogen and phosphorus of aquatic plants and harvest management research. Acta Prataculturae Sin. 2013, 22, 294–299. [Google Scholar]
- Rounsley, S.D.; Glodek, A.; Sutton, G.; Adams, M.D.; Somerville, C.R.; Venter, J.C.; Kerlavage, A.R. The construction of Arabidopsis expressed sequence tag assemblies (a new resource to facilitate gene identification). Plant Physiol. 1996, 112, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Velculescu, V.E.; Zhang, L.; Vogelstein, B.; Kinzler, K.W. Serial analysis of gene expression. Science 1995, 270, 484–487. [Google Scholar] [CrossRef]
- Katagiri, F.; Glazebrook, J. Overview of mRNA expression profiling using DNA microarrays. Curr. Protoc. Mol. Biol. 2009, 85, 22.4.1–22.4.13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Zhao, P.; Zhang, Z.; Liang, W.; Tian, Z.; Zheng, Y. Mining of candidate maize genes for nitrogen use efficiency by integrating gene expression and QTL data. Plant Mol. Biol. Report. 2012, 30, 297–308. [Google Scholar] [CrossRef]
- Bordes, J.; Ravel, C.; Jaubertie, J.; Duperrier, B.; Gardet, O.; Heumez, E.; Pissavy, A.; Charmet, G.; Le Gouis, J.; Balfourier, F. Genomic regions associated with the nitrogen limitation response revealed in a global wheat core collection. Theor. Appl. Genet. 2013, 126, 805–822. [Google Scholar] [CrossRef]
- Robertson, G.P.; Bruulsema, T.W.; Gehl, R.J.; Kanter, D.; Mauzerall, D.L.; Rotz, C.A.; Williams, C.O. Nitrogen–climate interactions in US agriculture. Biogeochemistry 2013, 114, 41–70. [Google Scholar] [CrossRef]
- Suddick, E.C.; Whitney, P.; Townsend, A.R.; Davidson, E.A. The role of nitrogen in climate change and the impacts of nitrogen–climate interactions in the United States: Foreword to thematic issue. Biogeochemistry 2013, 114, 1–10. [Google Scholar] [CrossRef]
- Townsend, A.R.; Vitousek, P.M.; Houlton, B.Z. The climate benefits of better nitrogen and phosphorus management. Issues Sci. Technol. 2012, 28, 85–91. [Google Scholar]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Jebril, N. World Health Organization declared a pandemic public health menace: A systematic review of the coronavirus disease 2019 “COVID-19”, up to 26th March 2020. SSRN . 2020. [CrossRef]
- Ogen, Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to the coronavirus (COVID-19) fatality rate. Sci. Total Environ. 2020, 726, 138605. [Google Scholar] [CrossRef]
- Karaer, A.; Balafkan, N.; Gazzea, M.; Arghandeh, R.; Ozguven, E.E. Analyzing COVID-19 Impacts on Vehicle Travels and Daily Nitrogen Dioxide (NO2) Levels among Florida Counties. Energies 2020, 13, 6044. [Google Scholar] [CrossRef]
- Dasgupta, D.; Rajeev, M. Paradox of a Supply Constrained Keynesian Equilibrium. Econ. Political Week. 2020, 55, 23. [Google Scholar]
- Sampath, P.V.; Jagadeesh, G.S.; Bahinipati, C.S. Sustainable Intensification of Agriculture in the Context of the COVID-19 Pandemic: Prospects for the Future. Water 2020, 12, 2738. [Google Scholar] [CrossRef]
- Lal, R.; Brevik, E.C.; Dawson, L.; Field, D.; Glaser, B.; Hartemink, A.E.; Hatano, R.; Lascelles, B.; Monger, C.; Scholten, T. Managing soils for recovering from the COVID-19 pandemic. Soil Syst. 2020, 4, 46. [Google Scholar] [CrossRef]
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Chang. 2020, 10, 647–653. [Google Scholar] [CrossRef]
- Tirado, M.C.; Crahay, P.; Mahy, L.; Zanev, C.; Neira, M.; Msangi, S.; Brown, R.; Scaramella, C.; Coitinho, D.C.; Müller, A. Climate change and nutrition: Creating a climate for nutrition security. Food Nutr. Bull. 2013, 34, 533–547. [Google Scholar] [CrossRef]
- Porter, S.D.; Reay, D.S.; Higgins, P.; Bomberg, E. A half-century of production-phase greenhouse gas emissions from food loss & waste in the global food supply chain. Sci. Total Environ. 2016, 571, 721–729. [Google Scholar] [PubMed]
- Nemecek, T.; Jungbluth, N.; i Canals, L.M.; Schenck, R. Environmental impacts of food consumption and nutrition: Where are we and what is next. Int. J. Life Cycle Assess. 2016, 5, 607–620. [Google Scholar] [CrossRef]
- Clonan, A.; Roberts, K.E.; Holdsworth, M. Socioeconomic and demographic drivers of red and processed meat consumption: Implications for health and environmental sustainability. Proc. Nutr. Soc. 2016, 75, 367–373. [Google Scholar] [CrossRef]
- Jones, A.D.; Hoey, L.; Blesh, J.; Miller, L.; Green, A.; Shapiro, L.F. A systematic review of the measurement of sustainable diets. Adv. Nutr. 2016, 7, 641–664. [Google Scholar] [CrossRef]
- Tirado, M.; Hunnes, D.; Cohen, M.; Lartey, A. Climate change and nutrition in Africa. J. Hunger Environ. Nutr. 2015, 10, 22–46. [Google Scholar] [CrossRef]
- Niles, M.T.; Ahuja, R.; Barker, T.; Esquivel, J.; Gutterman, S.; Heller, M.C.; Mango, N.; Portner, D.; Raimond, R.; Tirado, C. Climate change mitigation beyond agriculture: A review of food system opportunities and implications. Renew. Agric. Food Syst. 2018, 33, 297–308. [Google Scholar] [CrossRef]
- Fischer, C.G.; Garnett, T. Plates, Pyramids, and Planets: Developments in National Healthy and Sustainable Dietary Guidelines: A State of Play Assessment; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Rockström, J.; Stordalen, G.A.; Horton, R. Acting in the anthropocene: The EAT–Lancet Commission; Lancet: London, UK, 2016. [Google Scholar]
- Marinangeli, C.P.; Mansilla, W.D.; Shoveller, A.-K. Navigating protein claim regulations in North America for foods containing plant-based proteins. Cereal Foods World 2018, 63, 207–216. [Google Scholar]
- Springmann, M.; Mason-D’Croz, D.; Robinson, S.; Wiebe, K.; Godfray, H.C.J.; Rayner, M.; Scarborough, P. Mitigation potential and global health impacts from emissions pricing of food commodities. Nat. Clim. Chang. 2017, 7, 69–74. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Kanter, D.R.; Lassaletta, L.; Móring, A.; Raghuram, N.; Read, N. The nitrogen decade: Mobilizing global action on nitrogen to 2030 and beyond. One Earth 2021, 4, 10–14. [Google Scholar] [CrossRef]
- De Notaris, C.; Olesen, J.E.; Sørensen, P.; Rasmussen, J. Input and mineralization of carbon and nitrogen in soil from legume-based cover crops. Nutr. Cycl. Agroecosyst. 2020, 116, 1–18. [Google Scholar] [CrossRef]
- Lu, J.; Hu, T.; Zhang, B.; Wang, L.; Yang, S.; Fan, J.; Yan, S.; Zhang, F. Nitrogen fertilizer management effects on soil nitrate leaching, grain yield and economic benefit of summer maize in Northwest China. Agric. Water Manag. 2021, 247, 106739. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, X.; Pan, H.; Zhang, X.; Cao, H.; Ulgiati, S.; Wu, J.; Zhang, Y.; Wang, G.; Xiao, Y. Impact of fertilization schemes with different ratios of urea to controlled release nitrogen fertilizer on environmental sustainability, nitrogen use efficiency and economic benefit of rice production: A study case from Southwest China. J. Clean. Prod. 2021, 293, 126198. [Google Scholar] [CrossRef]
- De Laporte, A.; Banger, K.; Weersink, A.; Wagner-Riddle, C.; Grant, B.; Smith, W. Economic and environmental consequences of nitrogen application rates, timing and methods on corn in Ontario. Agric. Syst. 2021, 188, 103018. [Google Scholar] [CrossRef]
- Wiggering, H.; Dalchow, C.; Glemnitz, M.; Helming, K.; Müller, K.; Schultz, A.; Stachow, U.; Zander, P. Indicators for multifunctional land use—Linking socio-economic requirements with landscape potentials. Ecol. Indic. 2006, 6, 238–249. [Google Scholar] [CrossRef]
| Country (High-Ranked in NFP) | NFP (kg N cap−1 yr−1) | Region | Country (Low Ranked in NFP) | NFP (kg N cap−1 yr−1) | Region |
|---|---|---|---|---|---|
| Hong Kong | 225 | Southeast Asia | Liberia | 2 | West Africa |
| Luxemburg | 145 | Western Europe | Moldova | 3 | Eastern Europe |
| Kuwait | 102 | Western Asia | Côte d’Ivoire | 5 | West Africa |
| Singapore | 98 | Southeast Asia | Papua New Guinea | 7 | Oceania |
| Uruguay | 90 | South America | Tajikistan | 9 | Central Asia |
| Australia | 88 | Oceania | Malawi | 10 | South-Eastern Africa |
| UAE | 70 | Western Asia | Sri Lanka | 11 | South Asia |
| Paraguay | 67 | South America | North Korea | 12 | East Asia |
| Canada | 65 | North America | Mozambique | 15 | Southern Africa |
| USA | 62 | North America | Burundi | 17 | East Africa |
| Reactive Nitrogen (Nr) | Amount (Tg) | Source | Pathway |
|---|---|---|---|
| Nitrate (NO3−) | 161 | Industries and Agriculture | Leaching and surface runoff |
| Ammonia (NH3) | 45 | Agriculture | Volatilized |
| Nitrous Oxide (N2O) | 6.2 | Agriculture | Gaseous emission |
| NOx | 35 | Transportation and emission | Gaseous emission |
| Nitrogen emissions potentials to water mainly as NO3− | 28 | Consumer | Mainly Sewage |
| Region | Area (Mha) | Percent (%) of Total Organic Agriculture Land |
|---|---|---|
| Oceania | 17.3 | 40 |
| Europe | 11.6 | 27 |
| Latin America | 6.8 | 15 |
| North America | 3.7 | 8 |
| Asia | 3.6 | 7 |
| Africa | 1.3 | 3 |
| Mitigation Strategies | Approach |
|---|---|
| Farming System Design | Agronomic |
| On-Farm Best Available Techniques (BATs) | Agronomic |
| Improved Nitrogen Use Efficiency (NUE) | Agronomic |
| Pasture and Livestock Management | Agronomic and landscape |
| Managing Livestock Wastewater | Hydrologic |
| Carbon-rich sources | Agronomic |
| Engineering Cereal Crops for Nitrogen Fixation | Molecular |
| Plant Growth Promoting Microbial Consortia | Agronomic and molecular |
| Phytogenic Approach and Fungal Utilization | Agronomic and molecular |
| Organic Agriculture | Agronomic |
| Ecological Ditch | Landscape |
| Genetic Improvement | Molecular |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability 2021, 13, 2400. https://doi.org/10.3390/su13042400
Mahmud K, Panday D, Mergoum A, Missaoui A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability. 2021; 13(4):2400. https://doi.org/10.3390/su13042400
Chicago/Turabian StyleMahmud, Kishan, Dinesh Panday, Anaas Mergoum, and Ali Missaoui. 2021. "Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem" Sustainability 13, no. 4: 2400. https://doi.org/10.3390/su13042400
APA StyleMahmud, K., Panday, D., Mergoum, A., & Missaoui, A. (2021). Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability, 13(4), 2400. https://doi.org/10.3390/su13042400







