Development of Coloured Stoneware Bodies through the Incorporation of Industrial Cr/Ni Electroplating Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
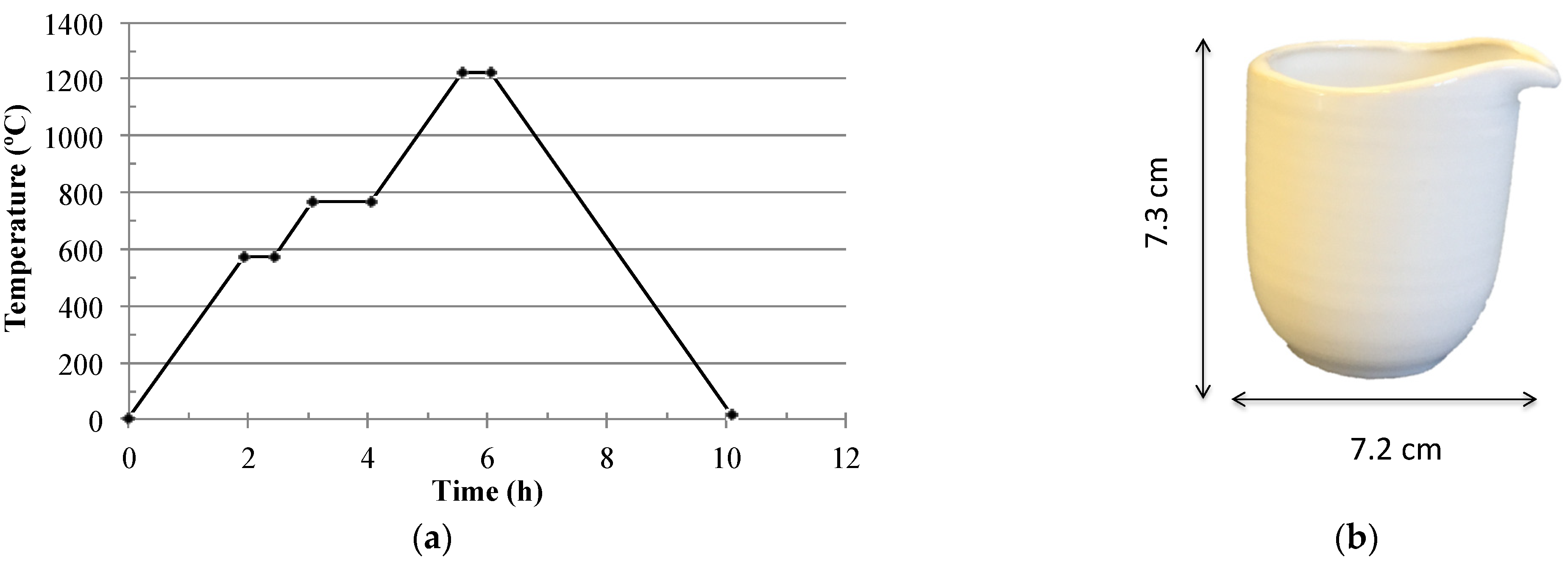
2.2. Samples Preparation
- Manual disaggregation in an agate mortar followed by sieving at 425 μm;
- Milling with zirconia balls during 10 min (proportion of 1:4, ES:balls) followed by sieving at 212 μm;
- Milling and sieving at 212 μm (as in II) + calcination at 600 °C (heating rate of 10 °C/min and a dwell time of 30 min).
2.3. Characterization Techniques
2.3.1. Physicochemical Characteristics of the Electroplating Sludge
2.3.2. Specimens Characterization
2.3.3. Leaching Experiments
3. Results and Discussion
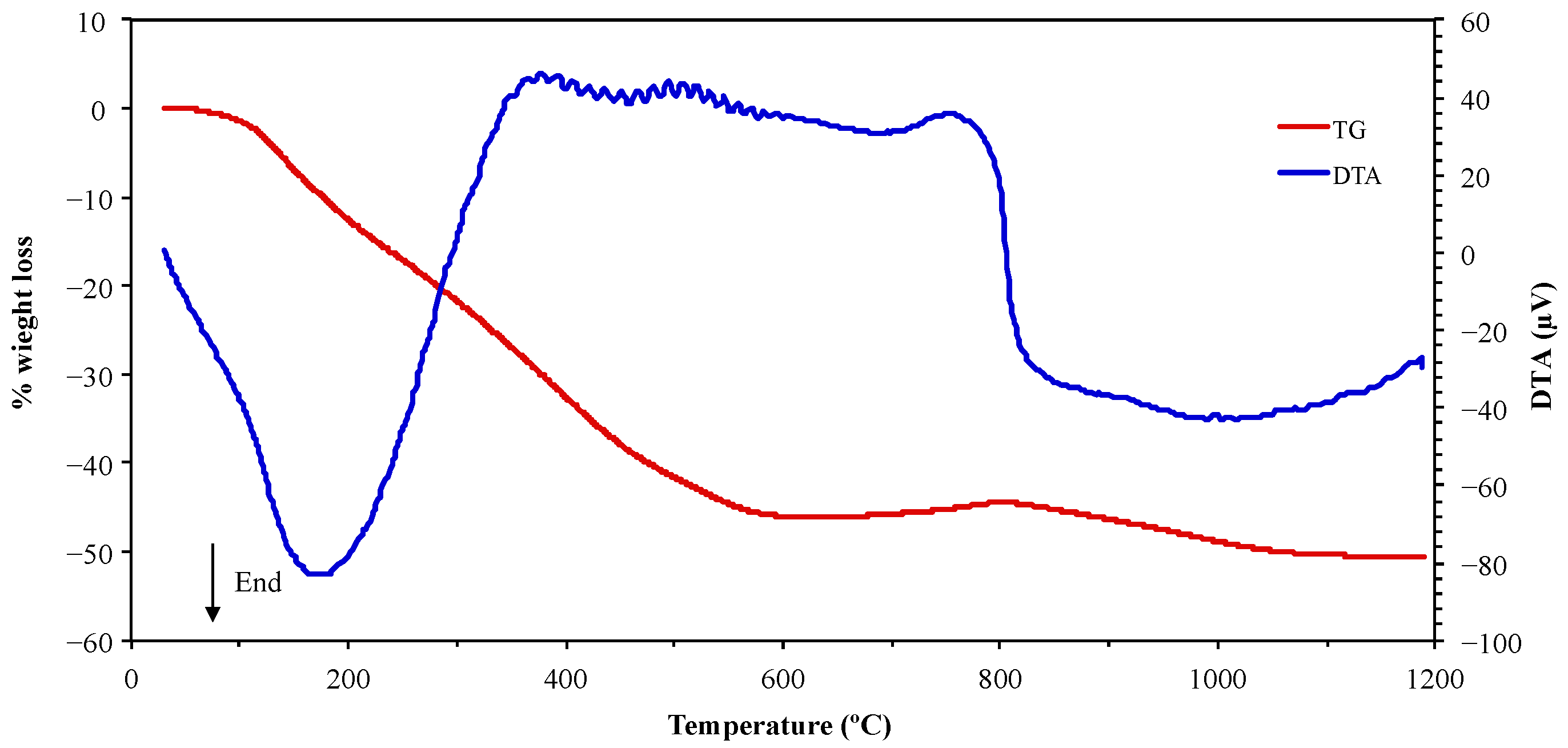
3.1. Electroplating Sludge Characterization
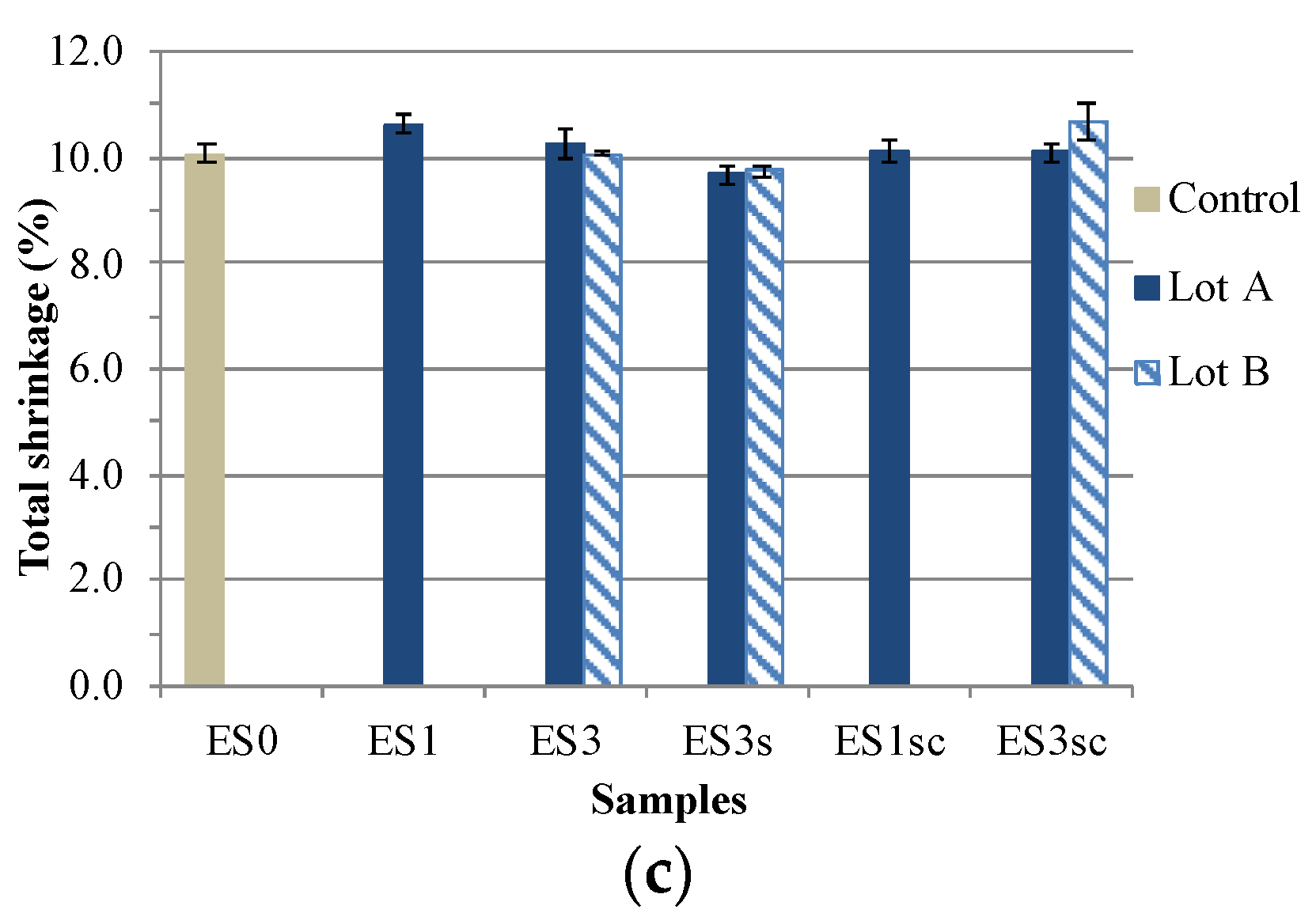
3.2. Specimen Characterisation
3.3. Leaching of the Fired Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Shi, P.; Chen, L.; Tang, Q. Utilization of Electroplating Sludge as Subgrade Backfill Materials: Mechanical and Environmental Risk Evaluation. Adv. Civ. Eng. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Magalhães, J.M.; Da Silva, J.E.; Castro, F.P.; Labrincha, J.A. Physical and chemical characterisation of metal finishing industrial wastes. J. Environ. Manag. 2005, 75, 157–166. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; de Campos Favas, P.J.; Vithanage, M.; Mohan, S.V. Industrial and Municipal Sludge: Emerging Concerns and Scope for Resource Recovery. Industrial and Municipal Sludge: Emerging Concerns and Scope for Resource Recovery; Elsevier Inc.: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Eurostat. Generation of Waste by Waste Category, Hazardousness and NACE Rev. 2 Activity. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WASGEN__custom_512986/default/table?lang=en (accessed on 9 December 2020).
- Yuan, H.; Deng, L.; Cai, X.; Zheng, T.; Zhou, S.; Chen, Y.; Yuan, Y. Recycling electroplating sludge to produce sustainable electrocatalysts for the efficient conversion of carbon dioxide in a microbial electrolysis cell. Electrochim. Acta 2016, 222, 177–184. [Google Scholar] [CrossRef]
- Tavares, C.R.G.; Franco, J.D.M. Production of concrete paving blocks (CPB) utilising electroplating residues—Evaluation of mechanical and micro-structural properties. Can. J. Chem. Eng. 2012, 90, 1092–1101. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Mugoni, C.; Guandalini, S.; Cattini, A.; Mazzini, D.; Alboni, C.; Siligardi, C. Glass recycling in the production of low-temperature stoneware tiles. J. Clean. Prod. 2018, 197, 1531–1539. [Google Scholar] [CrossRef]
- Vieira, C.; Monteiro, S. Incorporation of solid wastes in red ceramics: An updated review. Revista Matéria 2009, 14, 881–905. [Google Scholar] [CrossRef][Green Version]
- Hajjaji, W.; Costa, G.; Zanelli, C.; Ribeiro, M.; Seabra, M.P.; Dondi, M.; Labrincha, J. An overview of using solid wastes for pigment industry. J. Eur. Ceram. Soc. 2012, 32, 753–764. [Google Scholar] [CrossRef]
- Andreola, N.M.; Barbieri, L.; Lancellotti, I.; Leonelli, C.; Manfredini, T. Recycling of industrial wastes in ceramic manufacturing: State of art and glass case studies. Ceram. Int. 2016, 42, 13333–13338. [Google Scholar] [CrossRef]
- Amin, S.; Hamid, E.A.; El-Sherbiny, S.; Sibak, H.; Abadir, M. The use of sewage sludge in the production of ceramic floor tiles. HBRC J. 2018, 14, 309–315. [Google Scholar] [CrossRef]
- Ke, S.; Wang, Y.; Pan, Z.; Ning, C.; Zheng, S. Recycling of polished tile waste as a main raw material in porcelain tiles. J. Clean. Prod. 2016, 115, 238–244. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Corradi, A.; Lancellotti, I.; Manfredini, T. The possibility to recycle solid residues of the municipal waste incineration into a ceramic tile body. J. Mater. Sci. 2001, 36, 4869–4873. [Google Scholar] [CrossRef]
- Kizinievič, O.; Žurauskienė, R.; Kizinievič, V.; Žurauskas, R. Utilisation of sludge waste from water treatment for ceramic products. Constr. Build. Mater. 2013, 41, 464–473. [Google Scholar] [CrossRef]
- Cremades, L.V.; Cusidó, J.A.; Arteaga, F. Recycling of sludge from drinking water treatment as ceramic material for the manufacture of tiles. J. Clean. Prod. 2018, 201, 1071–1080. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Silva, A.; Rubio-Marcos, F.; Mello-Castanho, S.; Moya, J.; Fernández, J. High chemical stability of stoneware tiles containing waste metals. J. Eur. Ceram. Soc. 2010, 30, 2997–3004. [Google Scholar] [CrossRef]
- Bocanegra, J.J.C.; Mora, E.E.; González, G.I.C. Galvanic sludges: Effectiveness of red clay ceramics in the retention of heavy metals and effects on their technical properties. Environ. Technol. Innov. 2019, 16, 100459. [Google Scholar] [CrossRef]
- Ewais, E.M.; Besisa, N.H.; Ahmed, A. Aluminum titanate based ceramics from aluminum sludge waste. Ceram. Int. 2017, 43, 10277–10287. [Google Scholar] [CrossRef]
- Santos, P.; Martins, C.; Júlio, E. Enhancement of the thermal performance of perforated clay brick walls through the addition of industrial nano-crystalline aluminium sludge. Constr. Build. Mater. 2015, 101, 227–238. [Google Scholar] [CrossRef]
- Tucci, A.; Rambaldi, E.; Esposito, L. Use of scrap glass as raw material for porcelain stoneware tiles. Adv. Appl. Ceram. 2006, 105, 40–45. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Lye, C.Q.; Dhir, R.K. The role of glass waste in the production of ceramic-based products and other applications: A review. J. Clean. Prod. 2017, 167, 346–364. [Google Scholar] [CrossRef]
- Vieira, C.; Andrade, P.; Maciel, G.; Vernilli, F.; Monteiro, S. Incorporation of fine steel sludge waste into red ceramic. Mater. Sci. Eng. A 2006, 427, 142–147. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Wu, J.; Liu, J.; Li, C.; Ning, T.; Luo, Z.; Zhou, X.; Yang, Q.; Lu, A. The utilization of electrical insulators waste and red mud for fabrication of partially vitrified ceramic materials with high porosity and high strength. J. Clean. Prod. 2019, 223, 790–800. [Google Scholar] [CrossRef]
- He, H.; Yue, Q.; Qi, Y.; Gao, B.; Zhao, Y.; Yu, H.; Li, J.; Li, Q.; Wang, Y. The effect of incorporation of red mud on the properties of clay ceramic bodies. Appl. Clay Sci. 2012, 70, 67–73. [Google Scholar] [CrossRef]
- Silva, T.H.; Castro, A.C.M.; Neto, F.C.V.; Soares, M.M.N.S.; De Resende, D.S.; Bezerra, A.C.S. Recycling ceramic waste as a raw material in sanitary ware production. Cerâmica 2019, 65, 426–431. [Google Scholar] [CrossRef]
- Karaahmet, O.; Cicek, B. Waste recycling of cathode ray tube glass through industrial production of transparent ceramic frits. J. Air Waste Manag. Assoc. 2019, 69, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Tobaldi, D.; Capela, M.; Novais, R.; Seabra, M.; Labrincha, J. Synthesis of ceramic pigments from industrial wastes: Red mud and electroplating sludge. Waste Manag. 2018, 80, 371–378. [Google Scholar] [CrossRef]
- Magalhães, J.M.; Silva, J.E.; Castro, F.P.; Labrincha, J.A. Role of the mixing conditions and composition of galvanic sludges on the inertization process in clay-based ceramics. J. Hazard. Mater. 2004, 106, 169–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magalhães, J.M.; Silva, J.E.; Castro, F.P.; Labrincha, J.A. Kinetic study of the immobilization of galvanic sludge in clay-based matrix. J. Hazard. Mater. 2005, 121, 69–78. [Google Scholar] [CrossRef]
- Vitkalova, I.; Torlova, A.; Pikalov, E.; Selivanov, O. Development of environmentally safe acid-resistant ceramics using heavy metals containing waste. MATEC Web Conf. 2018, 193, 03035. [Google Scholar] [CrossRef]
- Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/tableware-market (accessed on 21 April 2020).
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 21 April 2020).
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/eco-friendly-tiles-market, (accessed on 2 May 2020).
- BSI Standards Publication, EN:1217 B Materials and Articles in Contact with Foodstuffs. In Test Methods for Water Absorption of Ceramic Articles; BSI: London, UK, 1998; p. 10.
- Commission Internationale d’Eclairage. Recommendations on Uniform Colour Spaces, Colour Difference Equations, Psychometrice Colour Terms. J. Oral Rehabil. 1978. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference Delta-E. A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Council of the European Union. Council Directive 84/500/EEC of 15 October 1984 on the Approximation of the Laws of the Member States Relating to Ceramic Articles Intended to Come Into Contact with Foodstuffs. Off. J. Eur. Communities 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31984L0500 (accessed on 21 April 2020).
- European Parliament and Council. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption (OJ L 330 05.12.1998). Documents in European Community Environmental Law. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 21 April 2020).


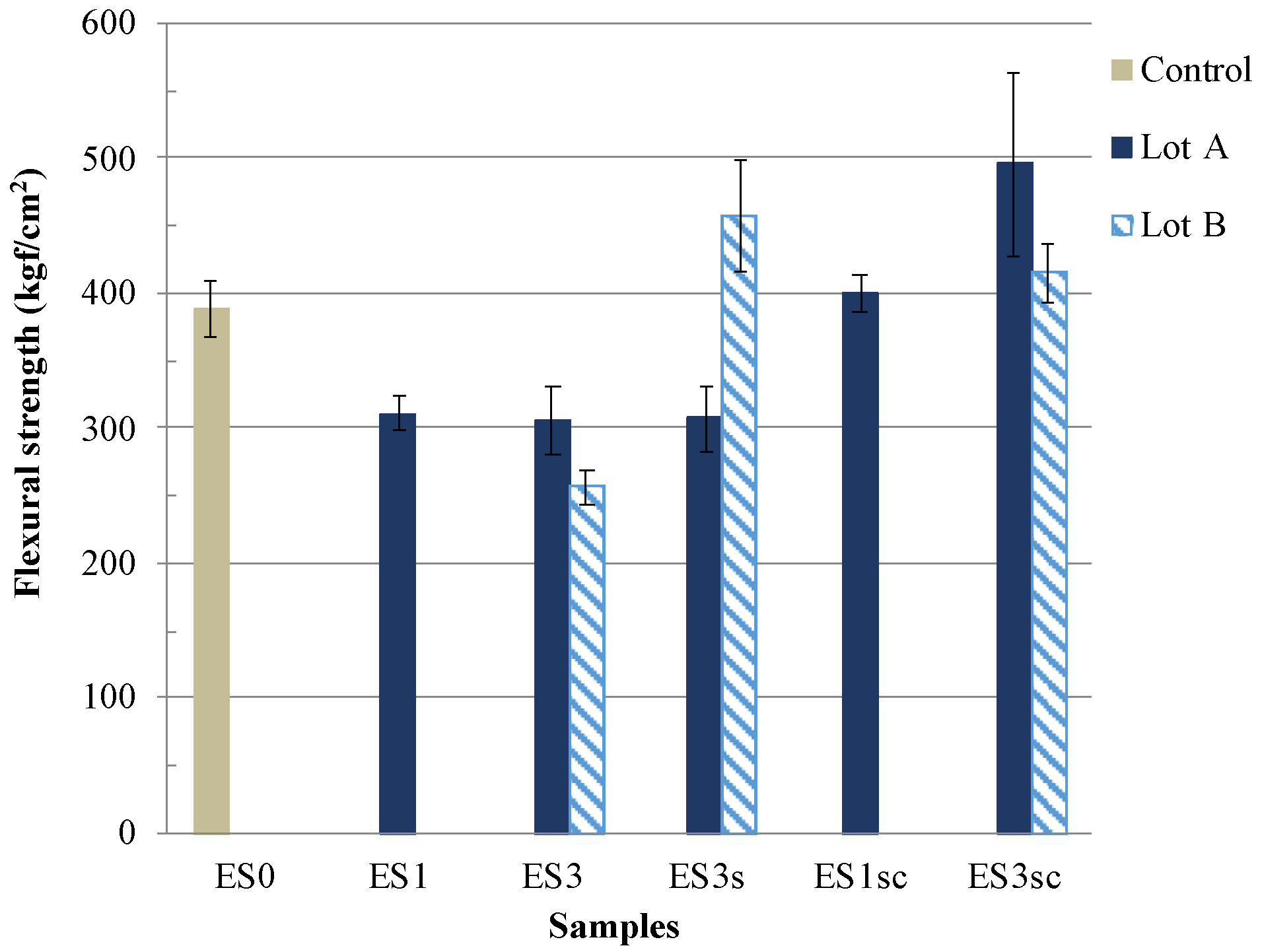
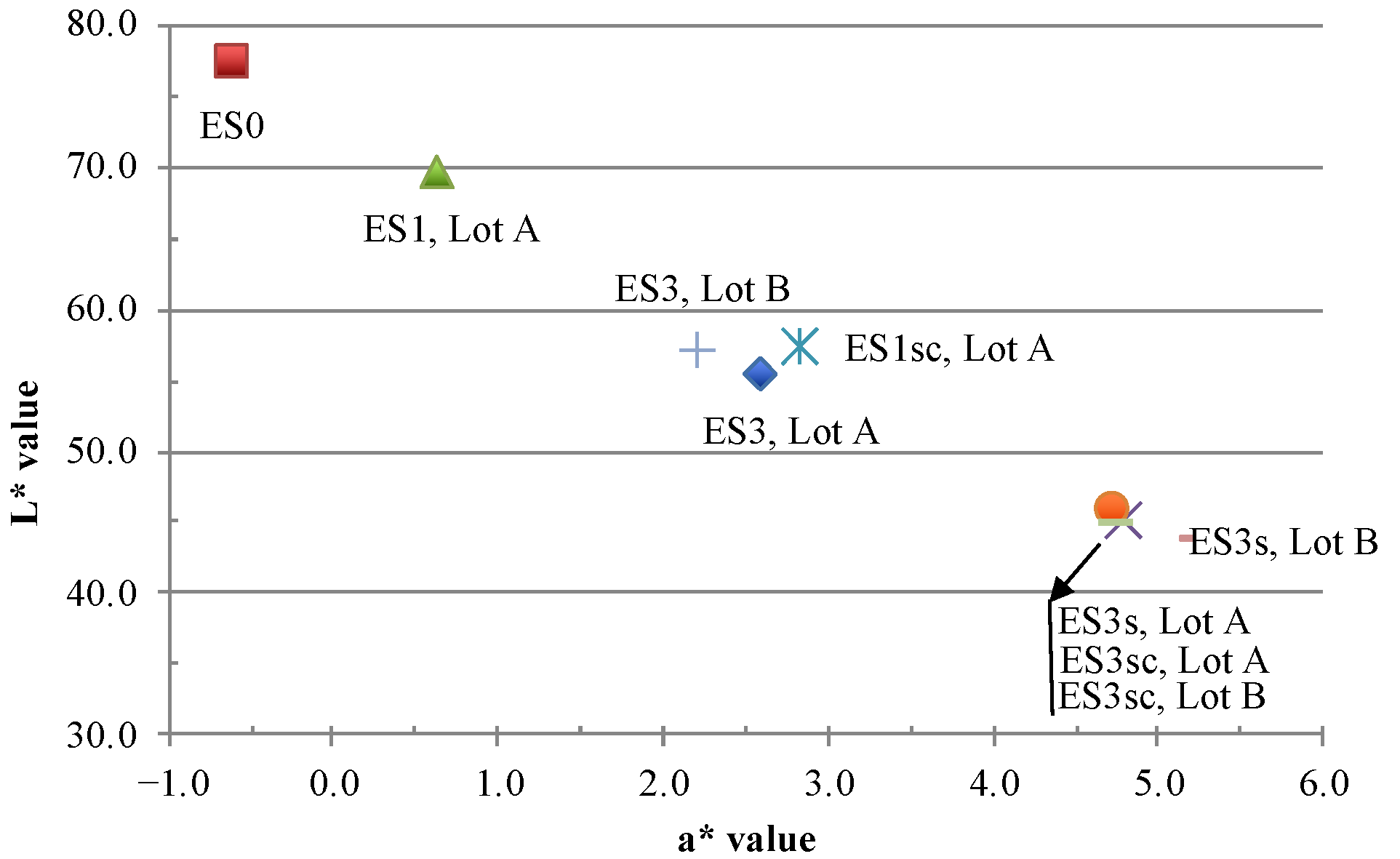
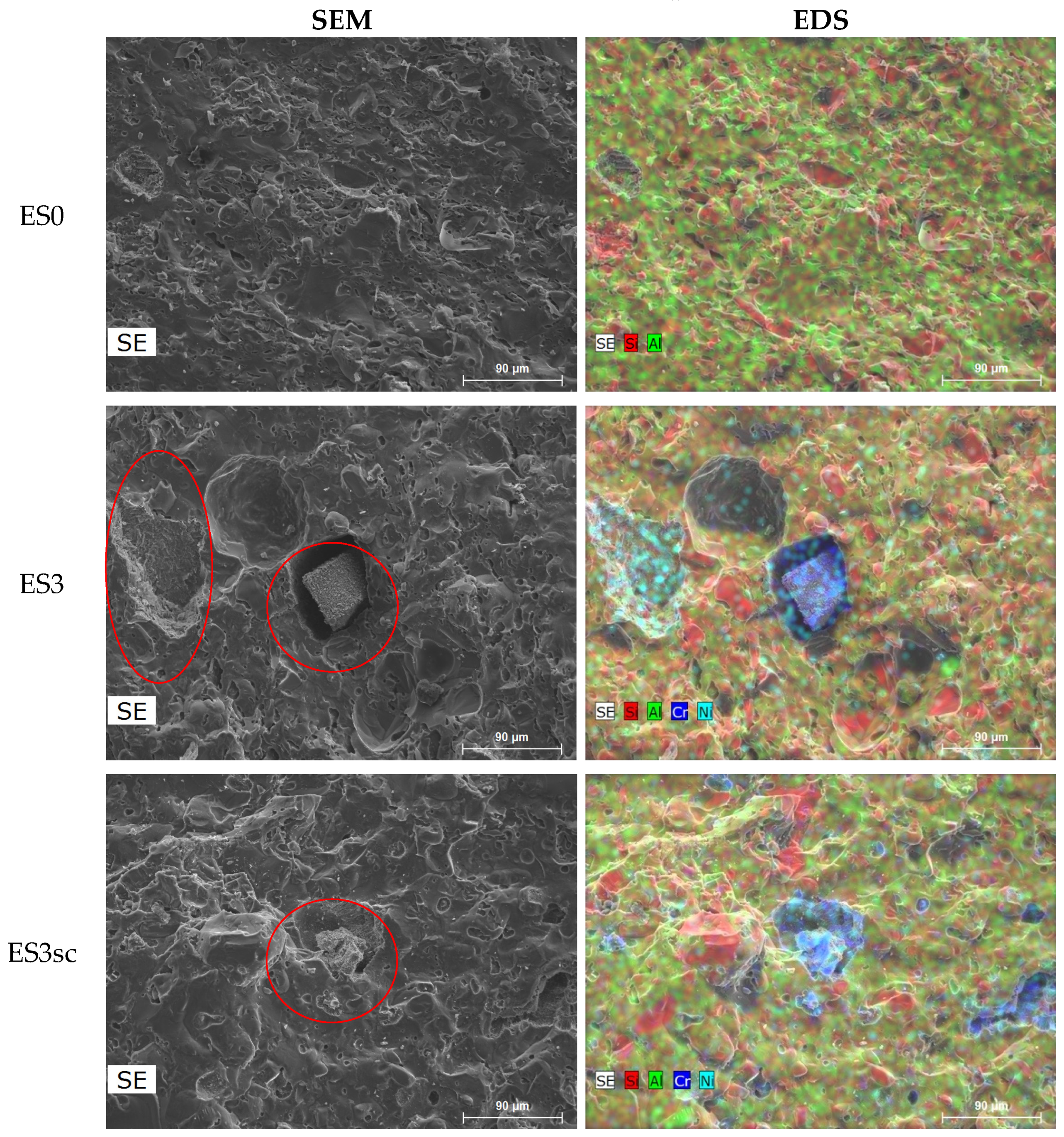
| ES0 | ES1 | ES3 | ES3s | ES1sc | ES3sc | |
|---|---|---|---|---|---|---|
| wt. (%) | ||||||
| Stoneware paste | 100 | 99 | 97 | 97 | 99 | 97 |
| ES (pre-treatment I) | - | 1 | 3 | - | - | - |
| ES (pre-treatment II) | - | - | - | 3 | - | - |
| ES (pre-treatment III) | - | - | - | - | 1 | 3 |
| Component | Lot A (wt.%) | Lot B (wt.%) |
|---|---|---|
| Ni | 25.9 | 15.5 |
| Cr | 15.3 | 9.1 |
| SO3 | 5.84 | 10.5 |
| SiO2 | 5.63 | 8.5 |
| P2O5 | 4.45 | 2.7 |
| Cu | 2.23 | 5.05 |
| Al2O3 | 1.66 | 3.27 |
| Zn | 1.58 | 2.62 |
| CaO | 1.09 | 16.1 |
| Pb | 0.88 | 0.21 |
| Fe2O3 | 0.65 | 1.15 |
| W | 0.34 | 0.19 |
| MgO | 0.16 | 0.79 |
| Ta | 0.16 | 0.11 |
| Nd | 0.09 | 0.036 |
| Ce | 0.09 | 0.038 |
| Ba | 0.07 | 0.086 |
| K2O | 0.07 | 0.119 |
| Cl | 0.06 | 0.232 |
| Sn | 0.06 | 0.024 |
| La | 0.05 | 0.645 |
| TiO2 | 0.03 | 0.174 |
| Hf | 0.02 | 0.028 |
| Zr | - | 0.025 |
| V | - | 0.062 |
| Co | - | 0.512 |
| LOI | 34.3 | 22.9 |
| ES0 | ES1 | ES3 | ES3s | ES1sc | ES3sc | ||
|---|---|---|---|---|---|---|---|
| Water absorption (%) | Lot A | 0.44 ± 0.001 | 0.21 ± 0.001 | 0.21 ± 0.001 | 0.11 ± 0.001 | 0.06 ± 0.001 | 0.0 ± 0.001 |
| Lot B | - | 0.13 ± 0.1 | 0.08 ± 0.1 | - | 0.07 ± 0.1 | ||
| Density (g/cm3) | Lot A | 2.07 ± 0.1 | 2.05 ± 0.1 | 1.96 ± 0.1 | 2.09 ± 0.1 | 2.26 ± 0.1 | 2.12 ± 0.1 |
| Lot B | - | 2.20 ± 0.1 | 2.21 ± 0.1 | - | 2.10 ± 0.1 |
| Samples | Concentration (µg/L) | |||
|---|---|---|---|---|
| Cr | Ni | Cd | Pb | |
| ≤50 (a) | ≤20 (a) | ≤20 (b) | ≤200 (b) | |
| ES0 | <20 | <5 | <0.4 | 9.9 |
| ES1 | <20 | 15 | <0.4 | 9.3 |
| ES3 | 21 | 42 | <0.4 | 6.7 |
| ES3s | <20 | <5 | <0.4 | 8.0 |
| ES1sc | <20 | <5 | <0.4 | 10 |
| ES3sc | <20 | 12 | <0.4 | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarinho, I.S.; Carneiro, J.; Pinto, C.; Labrincha, J.A.; Seabra, M.P. Development of Coloured Stoneware Bodies through the Incorporation of Industrial Cr/Ni Electroplating Sludge. Sustainability 2021, 13, 1999. https://doi.org/10.3390/su13041999
Vilarinho IS, Carneiro J, Pinto C, Labrincha JA, Seabra MP. Development of Coloured Stoneware Bodies through the Incorporation of Industrial Cr/Ni Electroplating Sludge. Sustainability. 2021; 13(4):1999. https://doi.org/10.3390/su13041999
Chicago/Turabian StyleVilarinho, Inês Silveirinha, Jorge Carneiro, Carlos Pinto, João António Labrincha, and Maria Paula Seabra. 2021. "Development of Coloured Stoneware Bodies through the Incorporation of Industrial Cr/Ni Electroplating Sludge" Sustainability 13, no. 4: 1999. https://doi.org/10.3390/su13041999
APA StyleVilarinho, I. S., Carneiro, J., Pinto, C., Labrincha, J. A., & Seabra, M. P. (2021). Development of Coloured Stoneware Bodies through the Incorporation of Industrial Cr/Ni Electroplating Sludge. Sustainability, 13(4), 1999. https://doi.org/10.3390/su13041999