The Hydration, Mechanical, Autogenous Shrinkage, Durability, and Sustainability Properties of Cement–Limestone–Slag Ternary Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Mixtures
2.1.1. Material
2.1.2. Mixture Ratio
2.2. Experimental Method
2.2.1. Compressive Strength Tests
2.2.2. Autogenous Shrinkage Tests
2.2.3. Isothermal Calorimetry Tests
2.2.4. Non-Destructive Techniques (NDT)
2.2.5. XRD and ATR-FTIR
3. Results and Discussion
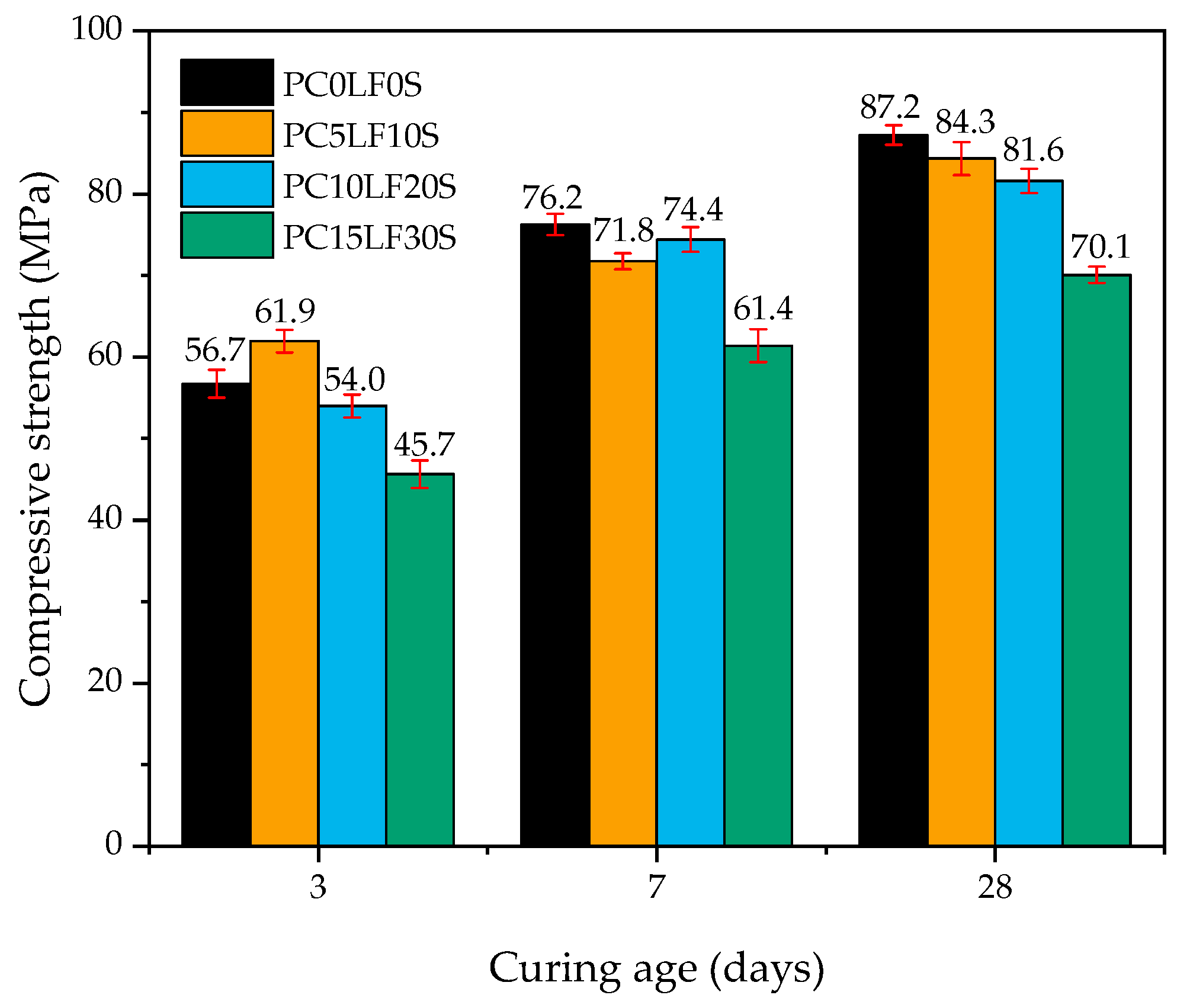
3.1. Compressive Strength
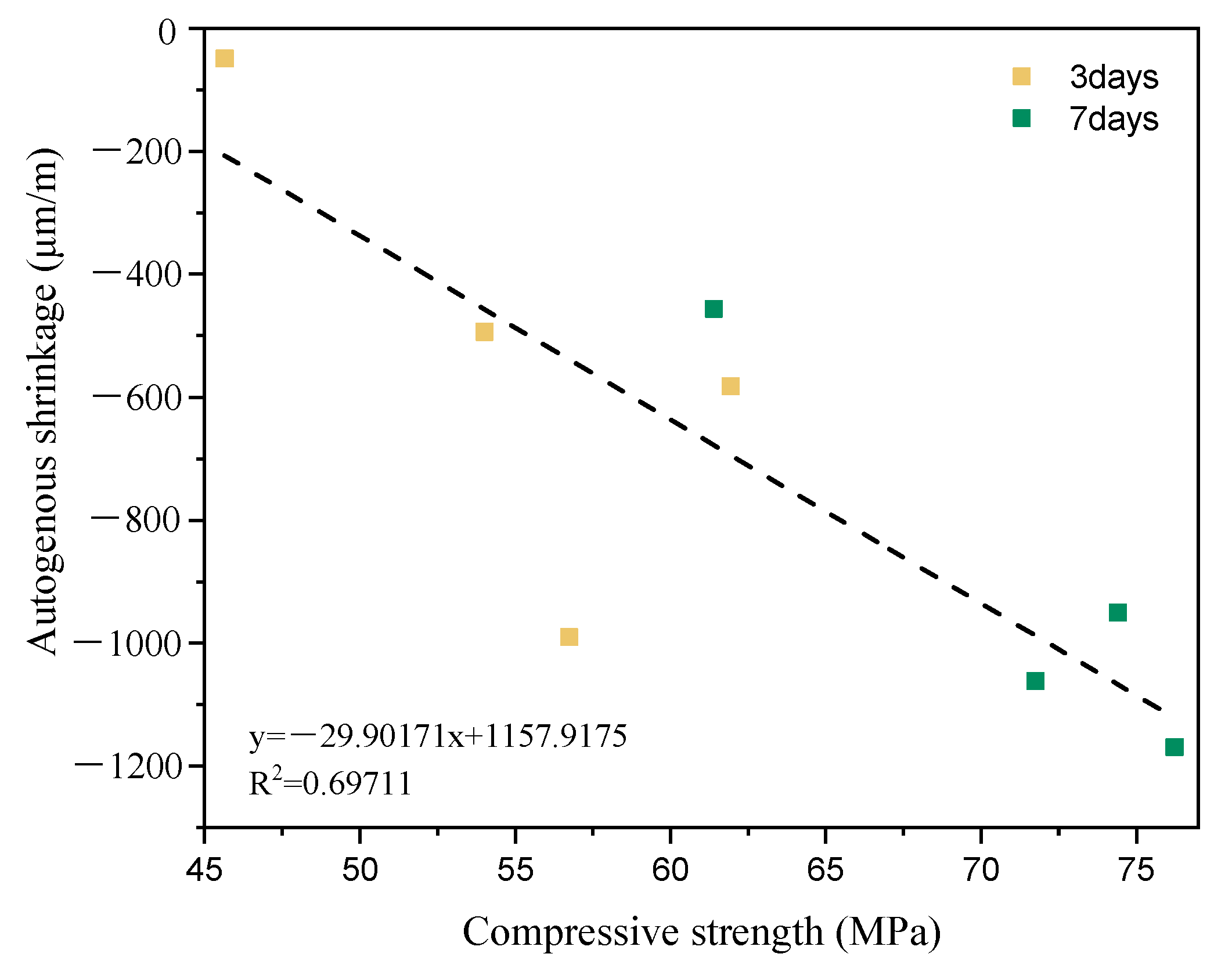
3.2. Effect of LF and BFS on AS
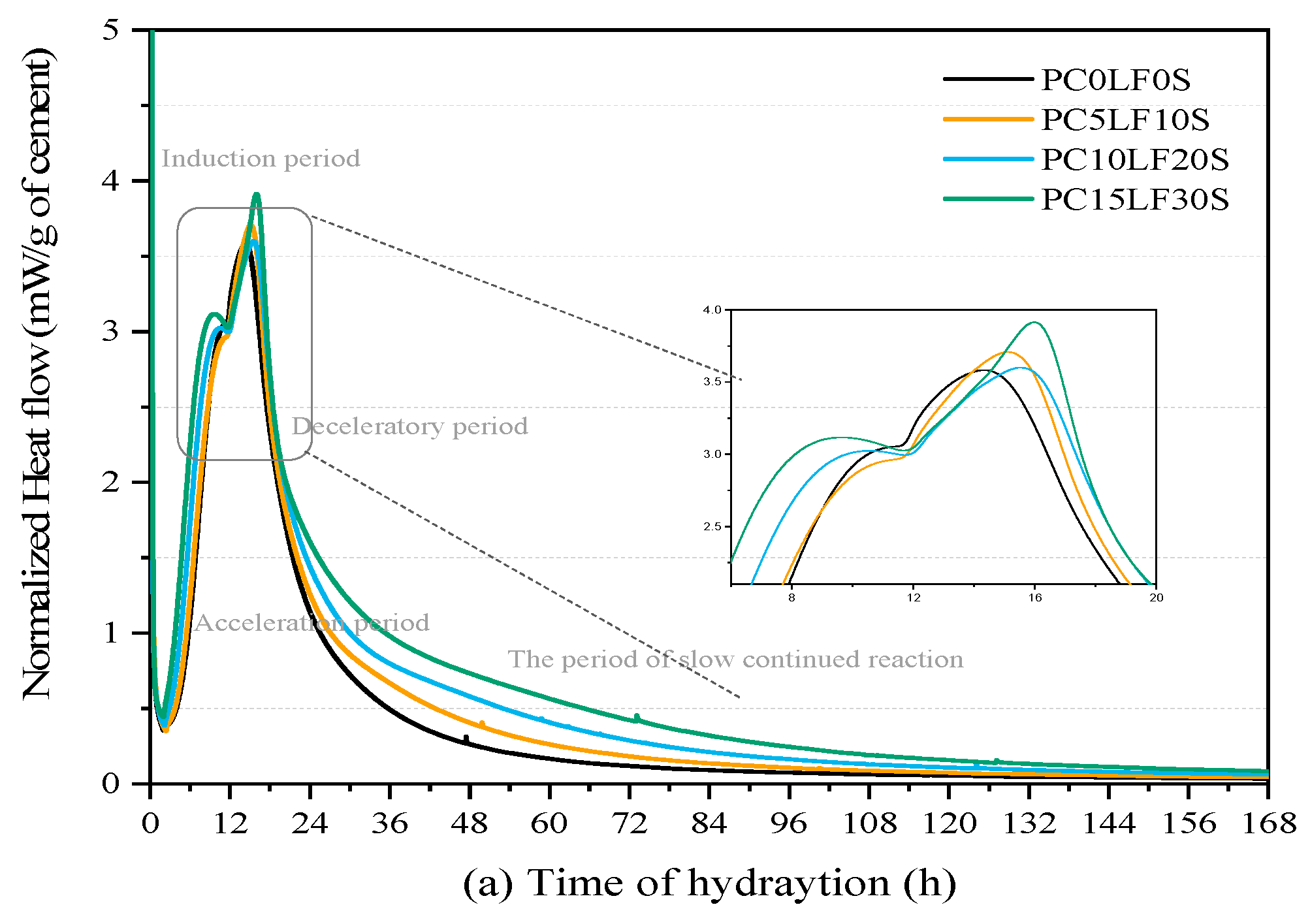
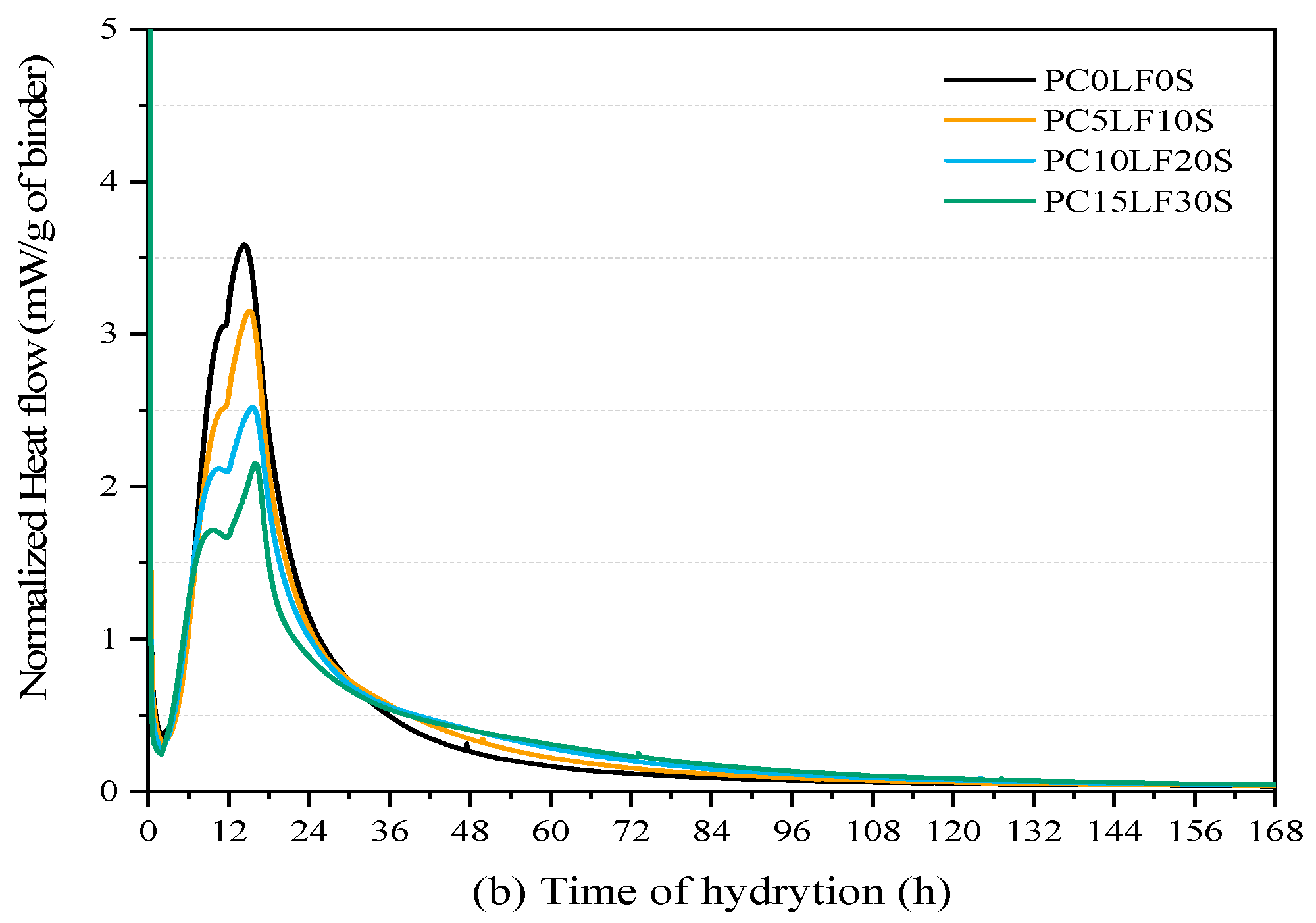
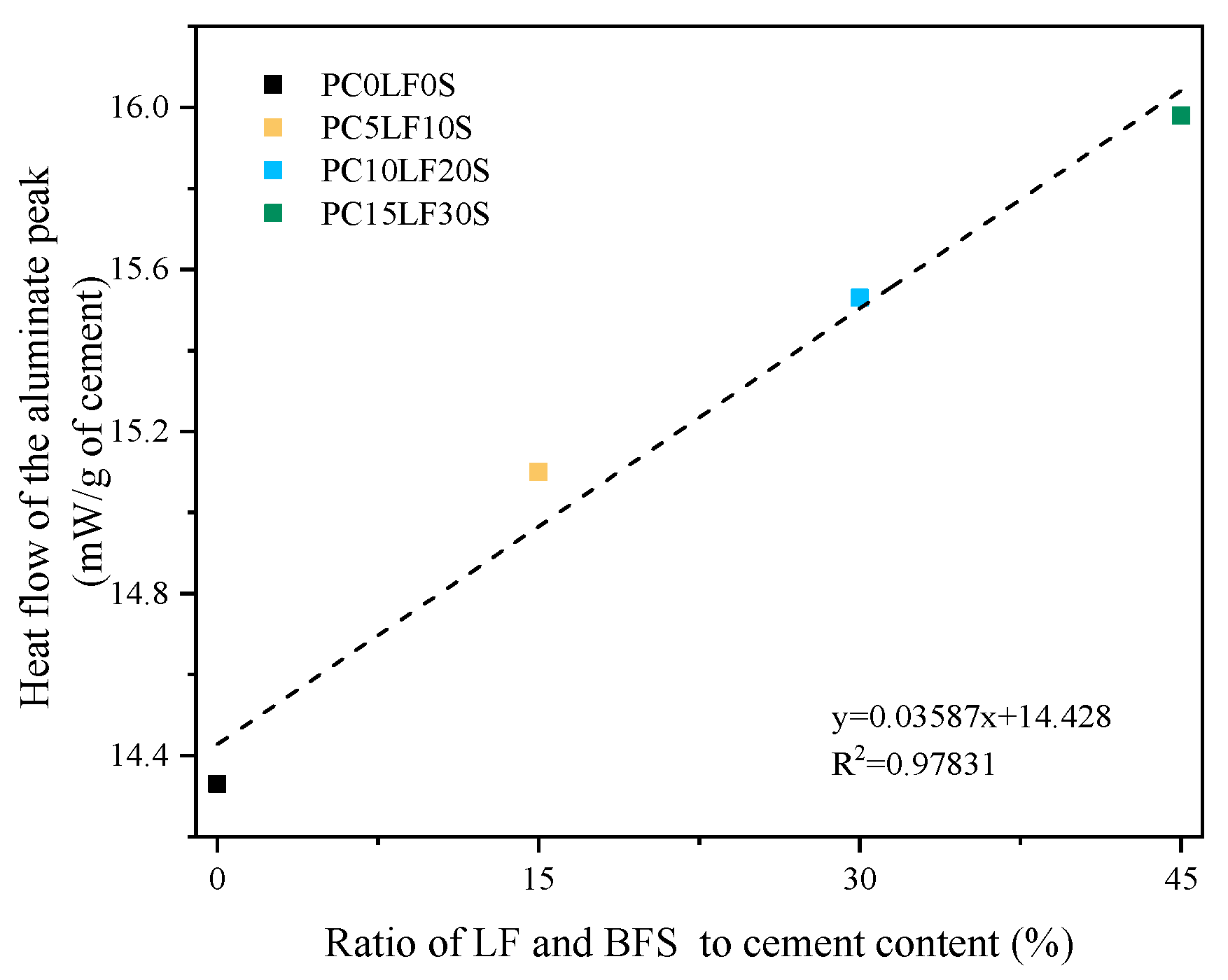
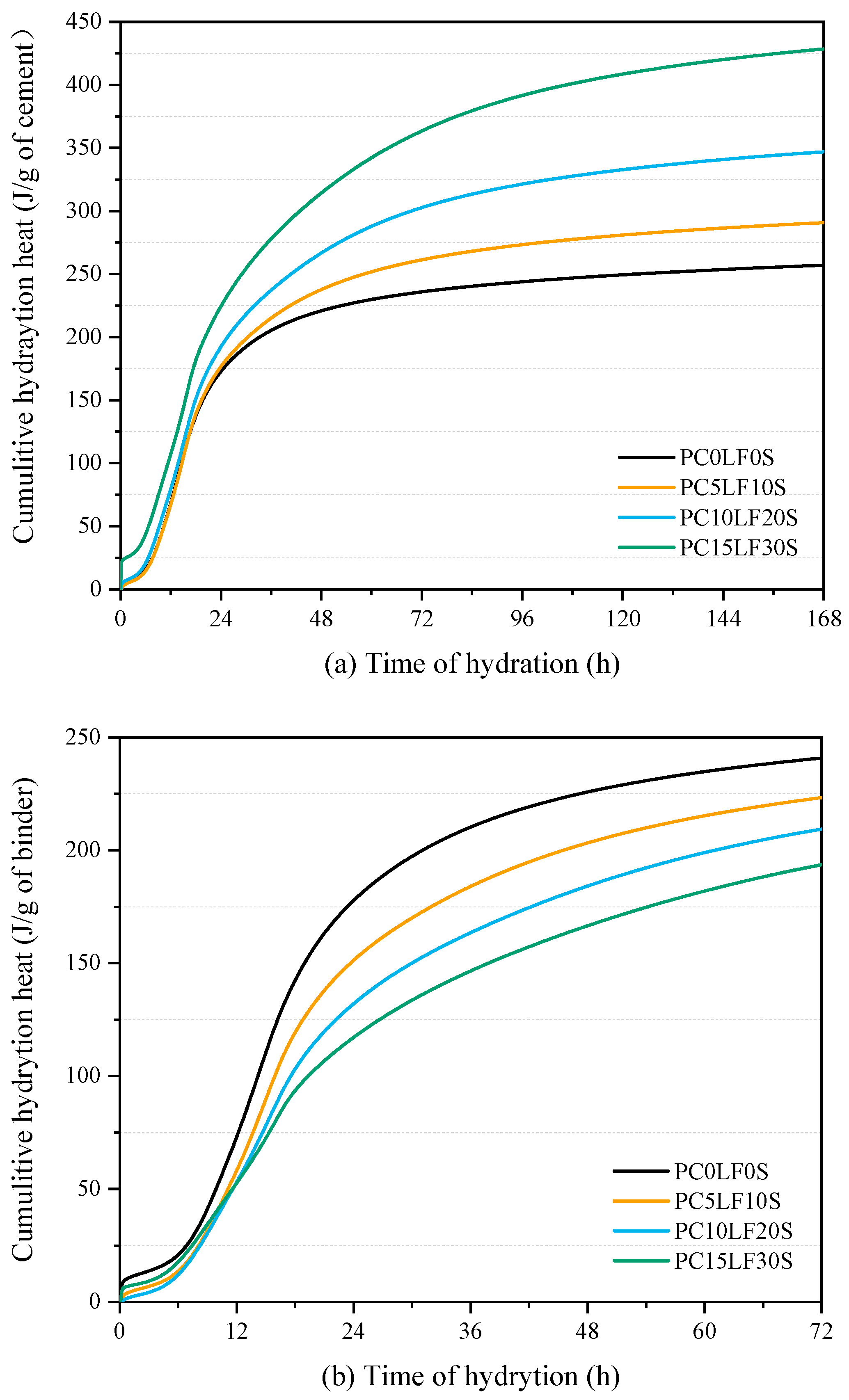
3.3. Hydration Reaction and Released Curve
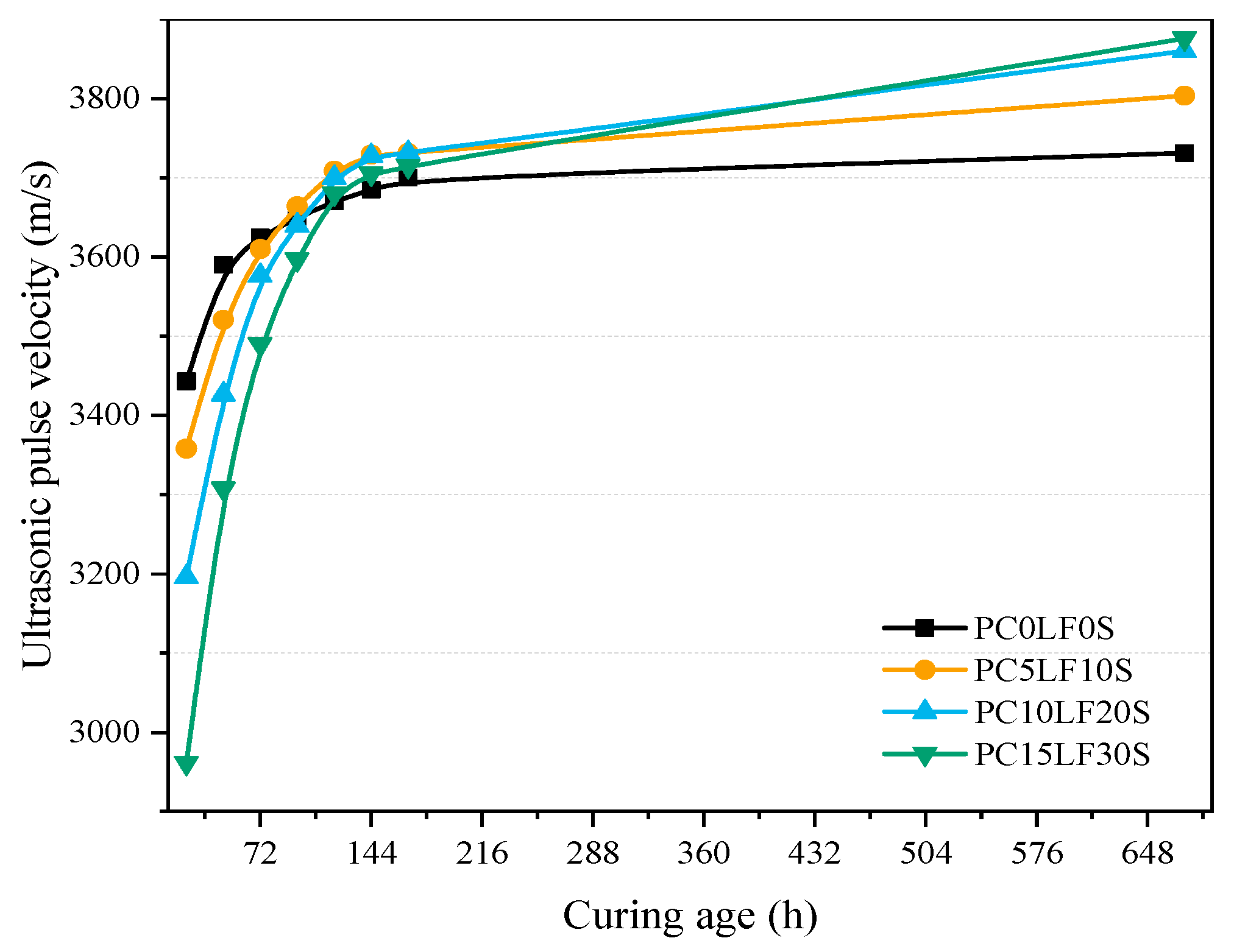
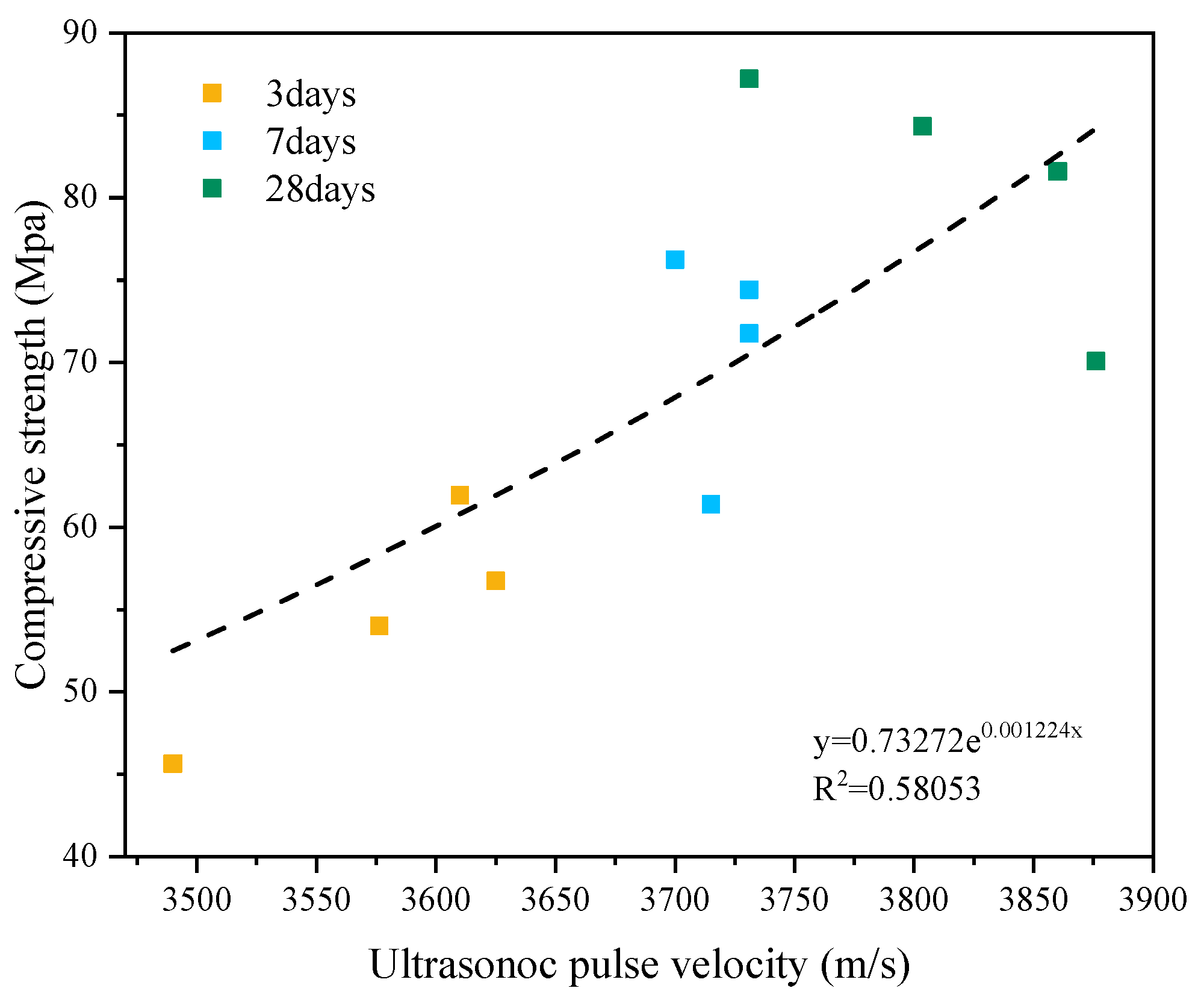
3.4. UPV
3.5. Electrical Resistivity
3.6. XRD
3.7. Fourier-Transform Infrared Spectroscopy (FTIR)
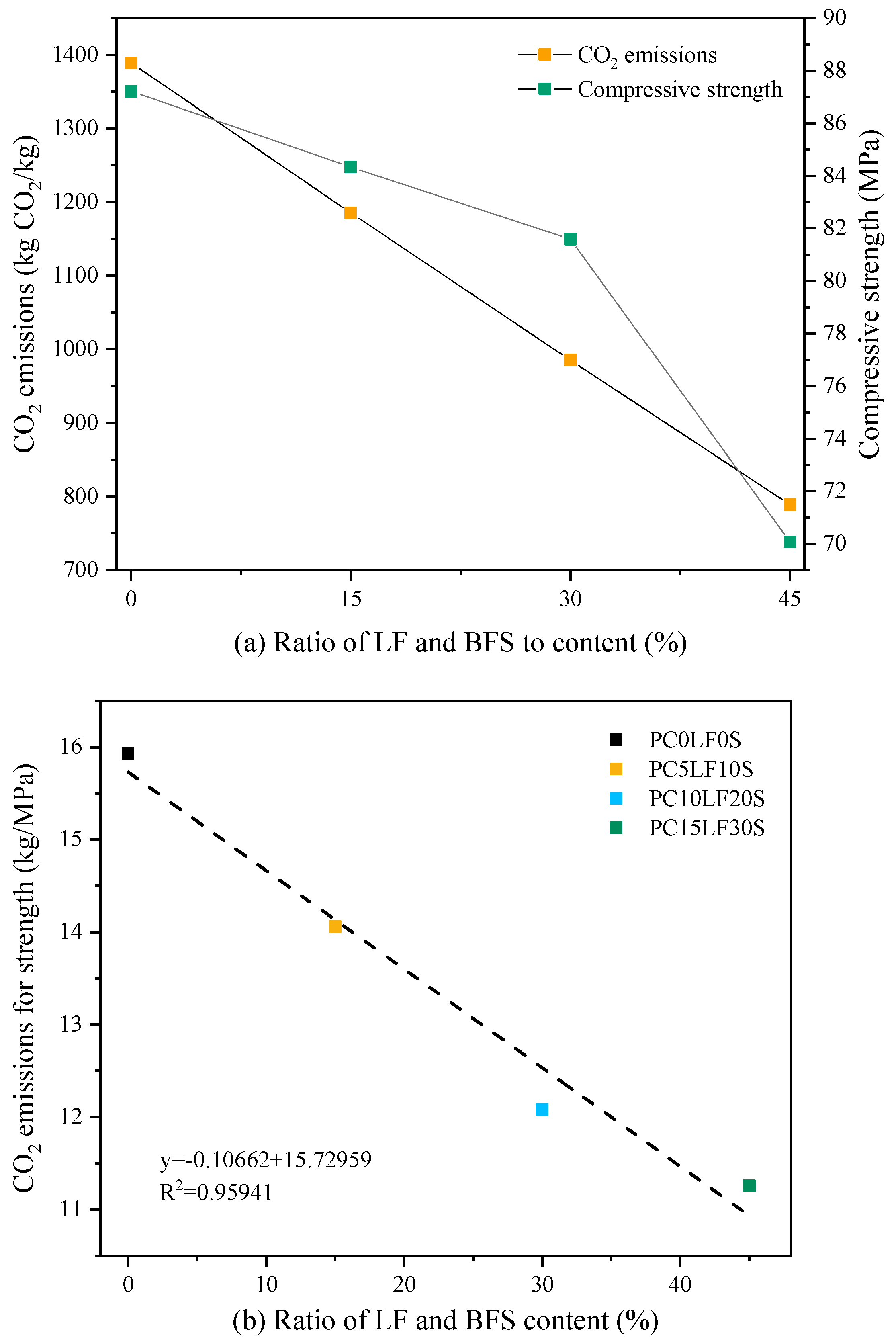
3.8. CO2 Emissions
4. Conclusions
- (1)
- In terms of early-stage strength (at 3 days), the compressive strength of PC5LF10S was 5.2 MPa higher than that of the control PC0LF0S. This can be attributed to the nucleation effect of LF and formation of Hc. The nucleation effect effectively promotes the hydration reaction and Hc can fill the porosity.
- (2)
- In terms of late-stage (28 days) strength, specimens PC5LF10S and PC10LF20S exhibited a small reduction in strength. This was due to the BFS reaction and synergistic effect of LF and BFS. However, the compressive strength of PC15LF30S was significantly lower due to the dilution effect and the lower reactivity of LF and BFS compared with cement.
- (3)
- The autogenous shrinkage of the ternary composites decreased with increasing cement substitution. In addition, the PC15LF30S specimen exhibited a slight swelling phenomenon in the early stage. A linear regression revealed a linear relationship between compressive strength and AS.
- (4)
- In terms of heat of hydration, when heat flow is normalized to the cement content, the main and shoulder peaks of the ternary composites were higher than those of the control PC0LF0S. As the replacement rate increased, the main peak shifted forward, and the peak of the silicate reaction peak appeared earlier. At 3 and 7 days, there was a linear relationship between compressive strength and cumulative hydration heat.
- (5)
- The experimental results for UPV showed a rapid increase in the early stage and a slow increase in the later stage, which was similar to the development of the hydration reaction. UPV showed a crossover phenomenon among various specimens, which was similar to the compressive strength development phenomenon.
- (6)
- In terms of electrical resistivity, the ternary composite specimen had increased the electrical resistivity, which was 91%, 160%, and 321% higher than the plain specimen at 28 days. This was due to the pore refinement due to BFS secondary reactions and the synergic effect between LF and BFS.
- (7)
- XRD analysis revealed the variation of Hc and Mc in the mixture. In the early stage, only Hc production was found. As the reaction proceeded, Hc transformed into Mc. The presence of LF prevented the decomposition of ettringite to monosulfate, while the formation of Hc and Mc indirectly stabilizes ettringite. A significant reduction in CH content was observed from the FTIR results.
- (8)
- For ternary composites, as the replacement ratio increased, the strength and total CO2 emissions decreased. CO2 emissions per unit strength were determined as the ratio of total CO2 emission to strength. For ternary composites, as the replacement ratio increased, CO2 emissions per unit strength reduced significantly. Therefore, the use of OPC–LF–BFS ternary concrete has a positive impact on sustainable development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, P.; Mi, G.; Wang, Q. A comparison of early hydration properties of cement–steel slag binder and cement–limestone powder binder. J. Therm. Anal. Calorim. 2013, 115, 193–200. [Google Scholar] [CrossRef]
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon dioxide reduction potential in the global cement industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H.; Ou, Z. A review on use of limestone powder in cement-based materials: Mechanism, hydration and microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- De Weerdt, K.; Kjellsen, K.; Sellevold, E.; Justnes, H. Synergy between fly ash and limestone powder in ternary cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.A.; Costa, H.; Júlio, E.; Portugal, A.; Durães, L. Effect of supplementary cementitious materials on autogenous shrinkage of ultra-high performance concrete. Constr. Build. Mater. 2016, 127, 43–48. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Courard, L.; Michel, F. Limestone fillers cement based composites: Effects of blast furnace slags on fresh and hardened properties. Constr. Build. Mater. 2014, 51, 439–445. [Google Scholar] [CrossRef]
- Carrasco, M.; Menéndez, G.; Bonavetti, V.; Irassar, E. Strength optimization of “tailor-made cement” with limestone filler and blast furnace slag. Constr. Build. Res. 2005, 35, 1324–1331. [Google Scholar] [CrossRef]
- Bouasker, M.; Khalifa, N.E.H.; Mounanga, P.; Kahla, N.B. Early-age deformation and autogenous cracking risk of slag–limestone filler-cement blended binders. Constr. Build. Mater. 2014, 55, 158–167. [Google Scholar] [CrossRef]
- Elgalhud, A.A.; Dhir, R.K.; Ghataora, G. Limestone addition effects on concrete porosity. Cem. Concr. Compos. 2016, 72, 222–234. [Google Scholar] [CrossRef]
- Celik, K.; Hay, R.; Hargis, C.W.; Moon, J. Effect of volcanic ash pozzolan or limestone replacement on hydration of Portland cement. Constr. Build. Mater. 2019, 197, 803–812. [Google Scholar] [CrossRef]
- Arora, A.; Sant, G.; Neithalath, N. Ternary blends containing slag and interground/blended limestone: Hydration, strength, and pore structure. Constr. Build. Mater. 2016, 102, 113–124. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Li, C.; Jiang, L. Utilization of limestone powder as an activator for early-age strength improvement of slag concrete. Constr. Build. Mater. 2020, 253, 119257. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Zajac, M.; Stabler, C.; Lothenbach, B.; Black, L. Influence of limestone on the hydration of ternary slag cements. Cem. Concr. Res. 2017, 100, 96–109. [Google Scholar] [CrossRef]
- Itim, A.; Ezziane, K.; Kadri, E.-H. Compressive strength and shrinkage of mortar containing various amounts of mineral additions. Constr. Build. Mater. 2011, 25, 3603–3609. [Google Scholar] [CrossRef]
- ASTM C349-14. Standard Test Method for Compressive Strength of Hydraulic–Cement Mortars (Using Portions of Prisms Broken in Flexure); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM C1698-09. Standard Test Method for Autogenous Strain of Cement Paste and Mortar; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Kumarappa, D.B.; Peethamparan, S.; Ngami, M. Autogenous shrinkage of alkali activated slag mortars: Basic mechanisms and mitigation methods. Cem. Concr. Res. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xiao, J.; Zhong, P. Internal curing effect of saturated recycled fine aggregates in early-age mortar. Cem. Concr. Compos. 2020, 108, 103444. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Concu, G.; Trulli, N. Concrete defects sizing by means of ultrasonic velocity maps. Buildings 2018, 8, 176. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R. Electrical resistivity of concrete for durability evaluation: A review. Adv. Mater. Sci. Eng. 2017, 2017, 8453095. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Wang, L.; Gao, X.; Huang, Y.; Song, Q.; Liu, K. Alumina-rich pozzolan modification on Portland-limestone cement concrete: Hydration kinetics, formation of hydrates and long-term performance evolution. Constr. Build. Mater. 2020, 258, 119712. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Black, L.; Skocek, J.; Haha, M.B.; Zajac, M. Effect of sulfate additions on hydration and performance of ternary slag-limestone composite cements. Constr. Build. Mater. 2018, 164, 451–462. [Google Scholar] [CrossRef]
- Craeye, B.; De Schutter, G.; Desmet, B.; Vantomme, J.; Heirman, G.; Vandewalle, L.; Cizer, Ö.; Aggoun, S.; Kadri, E. Effect of mineral filler type on autogenous shrinkage of self-compacting concrete. Cem. Concr. Res. 2010, 40, 908–913. [Google Scholar] [CrossRef]
- Duran-Herrera, A.; De-León-Esquivel, J.; Bentz, D.P.; Valdez-Tamez, P. Self-compacting concretes using fly ash and fine limestone powder: Shrinkage and surface electrical resistivity of equivalent mortars. Constr. Build. Mater. 2019, 199, 50–62. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V.; Mounanga, P.; Khelidj, A.; Loukili, A.; Rafai, N. Autogenous deformations of cement pastes: Part II. W/C effects, micro–macro correlations, and threshold values. Cem. Concr. Res. 2006, 36, 123–136. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Cho, H.-K.; Wang, X.-Y. Effect of Nano-Silica on the Autogenous Shrinkage, Strength, and Hydration Heat of Ultra-High Strength Concrete. Appl. Sci. 2020, 10, 5202. [Google Scholar] [CrossRef]
- Wu, L.; Farzadnia, N.; Shi, C.; Zhang, Z.; Wang, H. Autogenous shrinkage of high performance concrete: A review. Constr. Build. Mater. 2017, 149, 62–75. [Google Scholar] [CrossRef]
- Darquennes, A.; Staquet, S.; Delplancke-Ogletree, M.-P.; Espion, B. Effect of autogenous deformation on the cracking risk of slag cement concretes. Cem. Concr. Compos. 2011, 33, 368–379. [Google Scholar] [CrossRef]
- Acharya, P.K.; Patro, S.K. Effect of lime and ferrochrome ash (FA) as partial replacement of cement on strength, ultrasonic pulse velocity and permeability of concrete. Constr. Build. Mater. 2015, 94, 448–457. [Google Scholar] [CrossRef]
- Li, Y.; Bao, J.; Guo, Y. The relationship between autogenous shrinkage and pore structure of cement paste with mineral admixtures. Constr. Build. Mater. 2010, 24, 1855–1860. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Puerta-Falla, G.; Balonis, M.; Le Saout, G.; Falzone, G.; Zhang, C.; Neithalath, N.; Sant, G. Elucidating the role of the aluminous source on limestone reactivity in cementitious materials. J. Am. Ceram. Soc. 2015, 98, 4076–4089. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Interactions between alite and C3A-gypsum hydrations in model cements. Cem. Concr. Res. 2013, 44, 46–54. [Google Scholar] [CrossRef]
- Bergold, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem. Concr. Res. 2017, 93, 32–44. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.-I.; Sharp, J. The chemical composition and microstructure of hydration products in blended cements. Cem. Concr. Compos. 2004, 26, 967–976. [Google Scholar] [CrossRef]
- Ye, G.; Lura, P.; Van Breugel, K.; Fraaij, A. Study on the development of the microstructure in cement-based materials by means of numerical simulation and ultrasonic pulse velocity measurement. Cem. Concr. Compos. 2004, 26, 491–497. [Google Scholar] [CrossRef]
- Afrakoti, M.T.P.; Choobbasti, A.J.; Ghadakpour, M.; Kutanaei, S.S. Investigation of the effect of the coal wastes on the mechanical properties of the cement-treated sandy soil. Constr. Build. Mater. 2020, 239, 117848. [Google Scholar] [CrossRef]
- Estévez, E.; Martín, D.A.; Argiz, C.; Sanjuán, M.Á. Ultrasonic Pulse Velocity—Compressive Strength Relationship for Portland Cement Mortars Cured at Different Conditions. Crystals 2020, 10, 133. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; Shin, H.-O.; Yoon, Y.-S. Ultrasonic monitoring of setting and strength development of ultra-high-performance concrete. Materials 2016, 9, 294. [Google Scholar] [CrossRef]
- Demirboğa, R.; Türkmen, İ.; Karakoc, M.B. Relationship between ultrasonic velocity and compressive strength for high-volume mineral-admixtured concrete. Cem. Concr. Res. 2004, 34, 2329–2336. [Google Scholar] [CrossRef]
- Godinho, J.P.; De Souza Júnior, T.; Medeiros, M.H.F.; SILVA, M.A. Factors influencing ultrasonic pulse velocity in concrete. Rev. IBRACON Estrut. Mater. 2020, 13, 222–247. [Google Scholar] [CrossRef]
- Shen, D.; Wen, C.; Zhu, P.; Wu, Y.; Yuan, J. Influence of Barchip fiber on early-age autogenous shrinkage of high strength concrete. Constr. Build. Mater. 2020, 256, 119223. [Google Scholar] [CrossRef]
- Cyr, M.; Lawrence, P.; Ringot, E. Efficiency of mineral admixtures in mortars: Quantification of the physical and chemical effects of fine admixtures in relation with compressive strength. Cem. Concr. Res. 2006, 36, 264–277. [Google Scholar] [CrossRef]
- Ramezanianpour, A.M.; Hooton, R.D. A study on hydration, compressive strength, and porosity of Portland-limestone cement mixes containing SCMs. Cem. Concr. Compos. 2014, 51, 1–13. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Assessment of pore structure evolution in the limestone calcined clay cementitious system and its implications for performance. Cem. Concr. Compos. 2017, 84, 36–47. [Google Scholar] [CrossRef]
- Huang, W.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Effect of cement substitution by limestone on the hydration and microstructural development of ultra-high performance concrete (UHPC). Cem. Concr. Compos. 2017, 77, 86–101. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Mo, Z.; Gao, X.; Su, A. Mechanical performances and microstructures of metakaolin contained UHPC matrix under steam curing conditions. Constr. Build. Mater. 2021, 268, 121112. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Su, A.; Li, Q. Effect of carbonation curing on sulfate resistance of cement-coal gangue paste. J. Clean. Prod. 2021, 278, 123897. [Google Scholar] [CrossRef]
- Silva, D.; Roman, H.; Gleize, P. Evidences of chemical interaction between EVA and hydrating Portland cement. Cem. Concr. Res. 2002, 32, 1383–1390. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Ren, M.; Wen, X.; Gao, X.; Liu, Y. Thermal and mechanical properties of ultra-high performance concrete incorporated with microencapsulated phase change material. Constr. Build. Mater. 2020, 121714. [Google Scholar] [CrossRef]
- Lin, R.-S.; Lee, H.-S.; Han, Y.; Wang, X.-Y. Experimental studies on hydration–strength–durability of limestone-cement-calcined Hwangtoh clay ternary composite. Constr. Build. Mater. 2020, 269, 121290. [Google Scholar] [CrossRef]






| Oxides | Ordinary Portland Cement (OPC) | Limestone Filler (LF) | Blast Furnace Slag (BFS) |
|---|---|---|---|
| CaO | 63.28 | 57.62 | 38.30 |
| SiO2 | 22.12 | – | 36.10 |
| Al2O3 | 5.67 | 0.20 | 14.80 |
| Fe2O3 | 2.60 | – | 0.48 |
| MgO | 2.90 | 0.52 | 6.47 |
| Na2O | 0.05 | – | 0.05 |
| TiO2 | 0.24 | – | 0.61 |
| SO3 | 2.34 | – | 1.60 |
| Loss on ignition | 0.54 | 41.30 | 1.17 |
| Labels | Binders (%) | w/b | Superplasticizer (wt % of Binder) | ||
|---|---|---|---|---|---|
| OPC | LF | BFS | |||
| PC0LF0S | 100 | – | – | 0.3 | 0.25 |
| PC5LF10S | 85 | 5 | 10 | 0.3 | 0.25 |
| PC10LF20S | 70 | 10 | 20 | 0.3 | 0.25 |
| PC15LF30S | 55 | 15 | 30 | 0.3 | 0.25 |
| Method | Test Samples | Test Time |
|---|---|---|
| Compressive strength | All samples | 3, 7, and 28 days |
| Autogenous shrinkage | All samples | 1–7 days |
| Isothermal calorimetry | All samples | 168 h |
| Ultrasonic pulse velocity | All samples | 1–28 days |
| Electrical resistivity | All samples | 1–28 days |
| X-ray diffraction | All samples | 3 and 28 days |
| ATR-FTIR spectra | All samples | 28 days |
| Days | Ratio (%) | Compressive Strength (Mpa) | Experiment-AS (μm/m) | Regression-AS (μm/m) |
|---|---|---|---|---|
| 3 D | 0 | 56.74 | −990.02 | −538.68 |
| 15 | 61.95 | −582.05 | −694.47 | |
| 30 | 54.02 | −493.84 | −457.35 | |
| 45 | 45.65 | −49.14 | −207.08 | |
| 7 D | 0 | 76.23 | −1168.51 | −1121.45 |
| 15 | 71.75 | −1061.74 | −987.50 | |
| 30 | 74.41 | −950.23 | −1067.03 | |
| 45 | 61.40 | −456.26 | −678.02 |
| Days | Ratio (%) | Heat (J/g) | Experiment-Compressive Strength (MPa) | Regression-Compressive Strength (MPa) |
|---|---|---|---|---|
| 3 D | 0 | 240.90 | 56.74 | 67.07 |
| 15 | 223.37 | 61.95 | 59.48 | |
| 30 | 209.41 | 54.02 | 53.44 | |
| 45 | 193.66 | 45.65 | 46.62 | |
| 7 D | 0 | 262.03 | 76.23 | 76.21 |
| 15 | 248.43 | 71.75 | 70.33 | |
| 30 | 240.38 | 74.41 | 66.84 | |
| 45 | 229.41 | 61.40 | 62.10 |
| Days | Ratio (%) | UPV (m/s) | Experiment-Compressive Strength (MPa) | Regression-Compressive Strength (MPa) |
|---|---|---|---|---|
| 3 D | 0 | 3625 | 56.74 | 58.87 |
| 15 | 3610 | 61.95 | 57.65 | |
| 30 | 3576 | 54.02 | 54.97 | |
| 45 | 3490 | 45.65 | 48.73 | |
| 7 D | 0 | 3700 | 76.23 | 65.39 |
| 15 | 3731 | 71.75 | 68.29 | |
| 30 | 3731 | 74.41 | 68.29 | |
| 45 | 3715 | 61.40 | 66.77 | |
| 28 D | 0 | 3731 | 87.22 | 68.29 |
| 15 | 3804 | 84.34 | 75.64 | |
| 30 | 3860 | 81.59 | 81.80 | |
| 45 | 3876 | 70.08 | 83.66 |
| Region | Frequency cm−1 | Group |
|---|---|---|
| ① | 3640 | ν OH− |
| ② | 3395 | ν3 H2O |
| ③ | 1644 | ν2 H2O |
| ④ | 1416 | ν3 CO32− |
| ⑤ | 1109 | ν3 SO42− |
| ⑥ | 952 | ν3 SiO44− |
| ⑦ | 873 | ν2 CO32−, ν4 SiO44− |
| ⑧ | 713 | ν4 CO32− |
| ⑨ | 657 | ν4 SiO44− |
| Component | Unit CO2 Emissions (kg/kg) | Density (kg/m3) |
|---|---|---|
| Water | 0.000196 | 1000 |
| SP | 0.25 | 1220 |
| OPC | 0.86 | 3150 |
| LF | 0.008 | 2710 |
| BFS | 0.09 | 2880 |
| Type | Mass of Binder (kg/m3) | CO2 Emission (kg/kg) | Total (kg/m3) | CO2 Emission per Unit Strength | ||||
|---|---|---|---|---|---|---|---|---|
| OPC | LF | BFS | OPC | LF | BFS | |||
| PC0LF0S | 1614.18 | – | – | 1388.20 | – | – | 1389.30 | 15.93 |
| PC5LF10S | 1359.86 | 79.99 | 159.98 | 1169.48 | 0.64 | 14.40 | 1185.61 | 14.06 |
| PC10LF20S | 1110.03 | 158.58 | 317.15 | 954.62 | 1.27 | 28.54 | 985.52 | 12.08 |
| PC15LF30S | 864.55 | 235.79 | 471.57 | 743.51 | 1.89 | 42.44 | 788.92 | 11.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, M.-Y.; Han, Y.; Wang, X.-Y. The Hydration, Mechanical, Autogenous Shrinkage, Durability, and Sustainability Properties of Cement–Limestone–Slag Ternary Composites. Sustainability 2021, 13, 1881. https://doi.org/10.3390/su13041881
Xuan M-Y, Han Y, Wang X-Y. The Hydration, Mechanical, Autogenous Shrinkage, Durability, and Sustainability Properties of Cement–Limestone–Slag Ternary Composites. Sustainability. 2021; 13(4):1881. https://doi.org/10.3390/su13041881
Chicago/Turabian StyleXuan, Mei-Yu, Yi Han, and Xiao-Yong Wang. 2021. "The Hydration, Mechanical, Autogenous Shrinkage, Durability, and Sustainability Properties of Cement–Limestone–Slag Ternary Composites" Sustainability 13, no. 4: 1881. https://doi.org/10.3390/su13041881
APA StyleXuan, M.-Y., Han, Y., & Wang, X.-Y. (2021). The Hydration, Mechanical, Autogenous Shrinkage, Durability, and Sustainability Properties of Cement–Limestone–Slag Ternary Composites. Sustainability, 13(4), 1881. https://doi.org/10.3390/su13041881







