1. Introduction
Most wine consumers have distinct quality expectations when they are making a purchasing decision. Among many individual ideas of what wine quality means, a visually clear product that is free of haze and any flavor defects independent of the price point is usually the common denominator [
1]. Polymeric compounds like proteins, polyphenols, and polysaccharides can interact with one another [
2,
3] or other smaller molecules in the wine like heavy metals [
4] and sulfate [
5], causing a haze and in severe cases a precipitation in the bottle. Some influencing factors have been identified that increase the potential for haze formation, such as temperature fluctuation and agitation [
6,
7], ethanol concentration and pH [
8], or the concentration of pathogenesis-related proteins like chitinases, which by itself is influenced by the grape growing conditions [
9], disease pressure in the vineyard [
5], and the grape cultivar [
5,
10]. Managing these factors in the winemaking process, especially in the case of protein concentration, has been a major focus point for quality management in the past decades. The main solution currently is the use of bentonite, an aluminum phyllosilicate, which removes proteins based on charge interactions and physical absorption [
7]. The cationic part of this argillaceous earth can vary but the most common examples are sodium, potassium, calcium, and sodium–calcium bentonite [
11].
While the use of bentonite in wine is fairly well understood, the application has disadvantages, especially if it is used on a larger scale. The main concerns are handling of dust before the application due to the health hazard associated with it [
12,
13] and the disposal of used bentonite [
14]. While bentonite is a natural clay material, it has to be mined from special deposits which limits the sustainability of the application and professional disposal is required. The practice to mix used bentonite with vineyard soil can lead to severe oxygen deficiency in the soil as well as destruction of the soil structure and drainage. In addition to that, heavy metals can leach from the bentonite into the soil and accumulate [
15]. Due to the current lack of recycling options, the sustainability of bentonite use is questionable. The direct absorption of aroma compounds on the bentonite clay could severely affect the sensory profile of the wine [
16]. Volatiles can also be indirectly removed by bentonite as they can bind to macromolecules like proteins through hydrophobic interactions [
17]. Efforts to find a safer, less expensive, or more sustainable alternative have been ongoing for several years [
18]. A promising and renewable approach is
Saccharomyces paradoxus, a yeast strain that has high concentrations of chitin in its cell wall and was shown to absorb protein onto the cell surface [
19]. In contrast to bentonite where a lower wine pH correlates with better protein removal efficiency, the mechanism appears to be independent from pH with
S. paradoxus and solely based on the direct interaction between chitin and protein [
18,
20] as previously demonstrated by a binding assay in a model system [
19].
While the Commission Regulation (EU) No. 53/2011 does not allow the use of chitin in wine directly [
21], a purified alternative material derived from chitin for selective protein removal is chitosan, a linear polysaccharide composed of D-glucosamine and
N-acetyl-D-glucosamine. It is widely used as a preservative and stabilizer in food and beverages; its use in wine is mostly focused on the control of spoilage microorganisms like
Brettanomyces [
22]. Chitosan was shown to remove chitinases, which can, among others, lead to haze formation [
23], and also reduce the mineral content in wine [
24]. It was shown that a low wine pH increases electrostatic interactions and solubility of chitosan [
22].
Polystyrene surfaces have been studied as a matrix to adsorb proteins for medicinal purposes and to study binding mechanisms [
25]. Although other synthetic polymers are also known to bind proteins [
26], the use in wine as a bentonite alternative has not been reported yet.
The objective of this study was to evaluate a selection of different more sustainable protein fining agents in comparison to bentonite in a broad range of wine styles in order to identify an alternative solution for achieving protein stability. While the main experiments were done in real wine, concentration effects and removal rates were verified in model systems as well.
2. Materials and Methods
2.1. Winemaking
Grape cultivars for this experiment were selected based on their expected tendency to develop a higher level of protein instability, with focus on cultivars that usually exhibit low polyphenolic content and high pH values. All grapes were sourced from the California State University Fresno Farm in 2019. Harvesting decisions were made based on maturity and stylistic goal, following the schedule of the commercial University Winery. Aiming also for a wider range of ethanol concentrations, Muscat Canelli was included at a late harvest sugar level.
Muscat Canelli, Cabernet Sauvignon, and Zinfandel grapes were destemmed and pressed right after hand-harvesting, thus minimizing skin contact and phenolic extraction. The three resulting wines were white or slightly pinkish. A Barbera rose and a Touriga Nacional red wine were partially or fully fermented on the skins to extract phenolic material. All wines were fermented with a
Saccharomyces cerevisiae yeast (Ionys WF, Lallemand Inc., Montreal, QC, Canada) at 20 °C, clarified and stabilized with the addition of 100 ppm of sulfur dioxide (SO
2). The Muscat Canelli and the Barbera received two doses of SO
2 to reach the 20–30 ppm target for free sulfur dioxide. The analytical data of all wines are shown in
Table 1. All wines were racked twice and were visually clear before the fining trials.
2.2. Wine Fining Trials
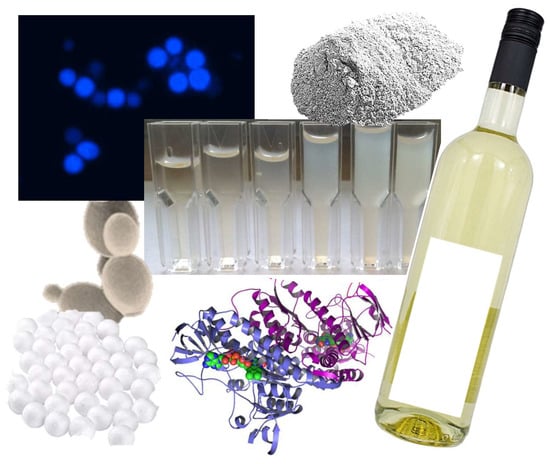
Saccharomyces paradoxus yeast cultures were ordered from the UC Davis Pfaff Yeast culture collection and propagated in YPD broth growth medium (MilliporeSigma, Burlington, MA, USA) until sufficient cell density was achieved (yeast deposit at the bottom of the flask). Pre-trials showed no difference in protein binding activity between living and dead cells, so all experiments were performed with dead S. paradoxus yeast. Cells were harvested by centrifugation, washed twice in sterile phosphate buffered saline (PBS) at pH 7.2 (VWR International, Radnor, PA, USA), and autoclaved at 121 °C for 20 min. The dead yeast cells were then washed twice in PBS again and stored at −20 °C until use. Chitin levels of the cell wall were checked under a fluorescence microscope (Model CX41F, Olympus Corporation Tokyo, Japan), after staining with Calcofluor White (Sigma-Aldrich, St. Louis, MO, USA). A commercial chitosan preparation for wine was sourced from AEB (Chitocel©, AEB biochemical USA, Lodi, CA, USA). Bentonite was purchased from Erbslöh Geisenheim GmbH (NaCalit® PORE-TEC, Erbslöh Geisenheim GmbH, Geisenheim, Germany). The polystyrene foam beads were sourced from Juvo Plus Inc. (Monrovia, CA, USA).
Since all wines were finished products where very little variability during the fining trial can be expected, the fining trials were only done in duplicates of the setup shown in
Table 2. Pure fining agents and additives were added directly or, in the case of bentonite, after swelling in water for 12 h. Fining trials were performed in 375 mL wine bottles. The bottles were placed on a shaker table and agitated for 10 h overnight. Two sets of samples (15 mL each) were taken out of each bottle, centrifuged at 6000 RPM (3750 RCF) for 10 min, filtered through a 0.45 µm syringe filter, and analyzed for protein stability with two different methods where appropriate.
White and light rosé wines (Muscat Canelli and Cabernet Sauvignon Blanc de Noir) were tested with Bentotest
® solution (Erbslöh Geisenheim AG, Geisenheim, Germany) according to the manufacturer’s instructions. All wines were also tested with a heat-test method at 80 °C for 6 h as recommended by Iland (2004) [
27]. Turbidity in each sample was analyzed by spectrophotometry (Lambda 25 spectrophotometer, PerkinElmer Inc., Waltham, MA, USA) at 860 nm [
3]. The influence of fining agents on color characteristics of all wines was checked at 420 nm and 520 nm using the same UV-Vis spectrophotometer.
Finished wine analyses (ethanol, titratable acidity, pH, free SO2, and total SO2) were performed using FT-MIR spectroscopy (FT2, FOSS, Hillerød, Denmark).
2.3. Model Wine Fining Trials
In order to evaluate the concentration effect of selected fining methods, additional trials were performed in duplicate in 50 mL centrifuge tubes. Instead of using wine with an unknown protein concentration, a model system containing egg-white protein (dried powder, Barry Farm, Wapakoneta, OH, USA) was chosen. This variation in methodology allows for a more accurate calculation of removal rates and concentration dependent efficiencies. The model wine (103 g/L ethanol, 2.7 g/L tartaric acid, 2.0 g/L malic acid, 0.15 g/L potassium metabisulfite, 1.0 g/L egg-white protein, pH adjusted to 3.5 with 10 M sodium hydroxide) was prepared and stored at 4 °C for seven days to equilibrate. It was then filtered through a 0.45 µm membrane and divided among 50 mL tubes. A control with no addition, Na-Ca bentonite (0.5 g/L, 1.0 g/L, 1.5 g/L), the
S. paradoxus strain P01-167 (0.3 g/L, 0.6 g/L, 1.0 g/L), Chitosan (0.5 g/L, 1.0 g/L, 1.5 g/L), Polystyrene (0.1 g/L, 0.3 g/L, 0.5 g/L), and carboxymethyl cellulose (CMC) (0.05 g/L, 0.10 g/L, 0.15 g/L) were prepared and placed on a shaker table for agitation for 10 h overnight. The CMC used was a food grade sodium salt powder from Modernist Pantry LLC (Portsmouth, NH). Fining efficiency was evaluated using a photometric Bradford protein assay [
28] compared to the control.
2.4. Statistical Analyses
Data handling and statistical analysis via one-way Analysis of Variance and paired t-test were performed using SigmaPlot 14 (Systat Software Inc., San Jose, CA, USA) and correlations were calculated with XLstat 2018.3 (Addinsoft, New York, NY, USA).
3. Results and Discussion
The fining trials reveal large differences in efficiency between the additives but also among the range of wine styles that were tested. Since protein stability can be assessed by heat test in all styles of wine but Bentotest
® is limited to white wines according to the manufacturer’s instructions,
Table 3 shows different sets of data for the various wines that were tested. It was reported before that Bentotest
®-treatment leads to higher turbidity readings than a heat-test [
9,
29], most likely because the acids in the testing solution denature most of the proteins, not just the heat labile fraction. The same can be seen for the two white wines that were tested with both methods. Wines that were shown to be stable with the heat test after fining still showed a significant haze after Bentotest
® (0.018 AU at 860 nm is equivalent to 65 NTU).
The turbidity difference in these experiments was 84% on average between the two methods as shown in
Table 3. The practical use of the two test results for the winemaker depends on the goal of protein stabilization. If wines are sold locally and are only stored at variable temperatures temporarily, the heat test provides information about the conditions in the hot trunk of a vehicle. The Bentotest
® treatment on the other hand takes into account that other protein fractions besides the heat labile portion could cause problems during overseas shipping or extended holding periods in customs for example. White wines that are produced for export purposes require a thorough stabilization procedure that prevents any macromolecule from precipitating, hence a more thorough testing for instabilities. Judging by Bentotest
® treatment in the present experiments, only the Muscat Canelli wine could be sufficiently stabilized by 1 g/L bentonite, while the Cabernet Blanc de Noir would require a higher dosage rate. None of the other stabilization methods tested here could provide sufficient protein removal to pass the Bentotest
® check. However, in order to compare all wines across all experiments, only the heat test data will be used for further evaluation.
None of the fining methods used in this study had a significant effect on color in any of the wines (data not shown). Although the bentonite and chitosan sediment showed a slight pink color after the application to red and rosé wines, the wines did not show a significant color reduction at 520 nm compared to the control. The same is true for the white wines where the yellow/brown color was observed at 420 nm. There was no significant color reduction or increase in browning, an effect that could be expected after the wines were agitated on a shaker table overnight. Agitation in the presence of oxygen might lead to non-enzymatic browning effects, however this effect was not statistically significant in these experiments. A previous study done in Pinot noir showed a color reduction with the use of bentonite [
30] and related that observation to positively charged pigments that could be removed by bentonite; in our experiments, however, this could not be confirmed.
In order to evaluate the differences in chitin levels between the
Saccharomyces cerevisiae strain that was used for all wine fermentations and
Saccharomyces paradoxus used as a fining agent, the two species were grown on the same YPD medium, harvested, washed, and stained. A fluorescent stain that selectively binds to chitin in the cell wall helps to visualize the overall differences in chitin (
Figure 1). While
S. cerevisiae shows a strong fluorescence around the bud scars of the cell and only a slight glow of the cell wall,
S. paradoxus emits a bright fluorescence over the whole surface of the cell. Cells that do not bind to the stain as well as others, show media residue on the cell surface that cannot be washed off with PBS. That illustrates the challenge of using yeast as a fining agent. If the chitin in the cell wall is not accessible to bind a florescent stain, it is most likely also inaccessible to protein, resulting is substantial inconsistencies regarding fining efficiency.
This effect is also visible when
S. paradoxus is used in real wine.
Figure 2 shows the average reduction in haze for every fining agent as a comparison between white and red wines. The trend that the average efficiency is better in white wines (40.5% more efficient on average) could be attributed to phenolic material that competes with proteins for potential binding sites on the surface of the additive. If the data for the two
S. paradoxus strains are combined into one dataset, the difference between fining efficiency in white and red wines actually becomes significant (
p = 0.022). The lack of statistically significant data with P01-161 and P01-167 separately in
Figure 2 can be attributed to the size of the sample set and the relatively large standard deviation due to the efficiency variability among wine styles.
Although the binding mechanism between chitin and haze-forming proteins like chitinases is documented [
23] and is not expected to be disrupted by phenolics, yeast cells have a wide variety of surface properties and charges that can bind pigments, tannins, and polysaccharides [
31]. This surface cover might essentially disrupt the efficient fining performance in red wines more than in whites. Chitosan showed a slightly higher efficiency than the chitin in white wines which might be attributed to the purified form that was added. In red wines, however, the protein removal rate was the lowest of all fining agents tested in this study. The reasons for this can be speculated to be due to stronger interactions and higher affinities between tannins, polysaccharides, and the chitosan, making the binding sites unavailable for proteins.
The difference between white and red wine efficiency is less pronounced with bentonite and polystyrene, which can be attributed to the mechanism with which these fining agents bind to protein. Bentonite uses mostly charge–charge interactions to bind molecules with a positive net charge, although there are other minor adsorption effects due to the porous nature of the clay material [
11]. Polystyrene foam beads on the other hand were shown to possess a high degree of surface hydrophobicity [
32], which implies the attraction of a non-polar fraction of proteins from the wine. These mechanisms are distinctly different and suggest that the removal efficiency shown in these experiments does not include the same portion of proteins. It is more likely that a changing degree of polarity, hydrophobicity, and surface charges remove different fractions of proteins from wine. A combination of both fining agents could potentially improve the efficiency by lowering the required addition, and as a result, making the use more sustainable.
In order to show this behavior under more controlled and reproducible conditions and to evaluate different concentrations of the most promising fining agents, trials in model wine were conducted and the protein concentration was analyzed with a colorimetric assay instead of the turbidity measurement. The goal was to see the actual protein removal rate rather than the presence or absence of haze forming protein material. The results of these trials are summarized in
Figure 3. Carboxymethyl cellulose (CMC) was added to these experiments because it was previously shown to selectively bind to protein [
3,
33] and remove it through precipitation. The application as a fining agent in wine on a larger scale is currently cost-prohibitive and therefore economically not feasible, so CMC was only investigated in the model system.
The model wine used in these experiments had 1 g/L egg-white protein added to it since the complex mixture of proteins mimics wine conditions better than a single pure protein alone [
3]. In addition to that, the International Organisation of Vine and Wine (OIV) is recommending egg-white protein for a bentonite protein adsorption trial (International Oenological Codex, Oeno 11/2003 modified Oeno 441-2011) [
34]. Interestingly, the analytical response of egg-white protein expressed as bovine serum albumin (BSA) equivalents is very close to the initial protein addition to the model as can be seen with the control in
Figure 3.
The addition of bentonite reduces the protein concentration stepwise by about 60% of the initial concentration in a clear linear fashion (R2 = 0.998). A similar behavior can be observed with Saccharomyces paradoxus; however, the reduction rate is lower with around 40% at the highest addition and confirms the range that was observed in real wine. It is plausible that higher additions to wine would achieve a higher protein removal rate; however, growing and purifying large quantities of cells would not be economically viable above the tested range and limit the application for larger wineries. The use of this renewable fining agent might therefore be limited to wines that have a lower level of protein instability.
The chitosan application in the model system did not produce the same results as in real wine. There are no statistically significant differences between any of the treatments and the control. While this result shows the limited use of chitosan as a fining agent, it also proves the selective nature of the fining mechanism. In wine, where pathogenesis related proteins like chitinases are naturally present, the chitosan can remove these haze proteins. The model with egg-white does not have the right affinity and the proteins remain in the system.
Polystyrene also displays a fining efficiency that is independent from the concentration that was added to the model wine. While 0.1 g/L and 0.5 g/L have the same effect as 1.0 g/L of bentonite and seem to confirm the results in real wine, the 0.3 g/L treatment is not statistically significantly different from the control. This inconsistency could be due to problems with the mixing behavior of polystyrene in wine. The small foam spheres are very light and hydrophobic, which can result in insufficient contact between the liquid and the additive on the shaker table. If the contact is optimized, however, the use of polystyrene for protein removal in wine is plausible. Even though polystyrene is an artificial polymer, it can potentially be recovered and recycled. For that reason, it is still considered a more sustainable alternative for protein fining than bentonite in this study.
As far as the efficiency of CMC is concerned, the results in
Figure 3 suggest that the removal is very efficient but does not follow the same concentration effect as bentonite for example. It seems like the lowest addition of CMC leads to the highest removal rate while an addition above the legal limit of 100 mg/L has a much lower effect. There are two factors that could explain this observation: contact time and the three-dimensional structure of the macromolecule CMC. Both attributes were shown to influence the reaction kinetics of CMC in wine [
3,
33], suggesting that contact time and the ability to unfold the CMC structure in order to expose binding sites was not sufficient in the present experiments. However, there is currently no explanation for the apparent inverse concentration effect.