Rice Husk Compost Production and Use in Mitigating Ammonia Volatilization from Urea
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Co-Composting and Their Characterizations
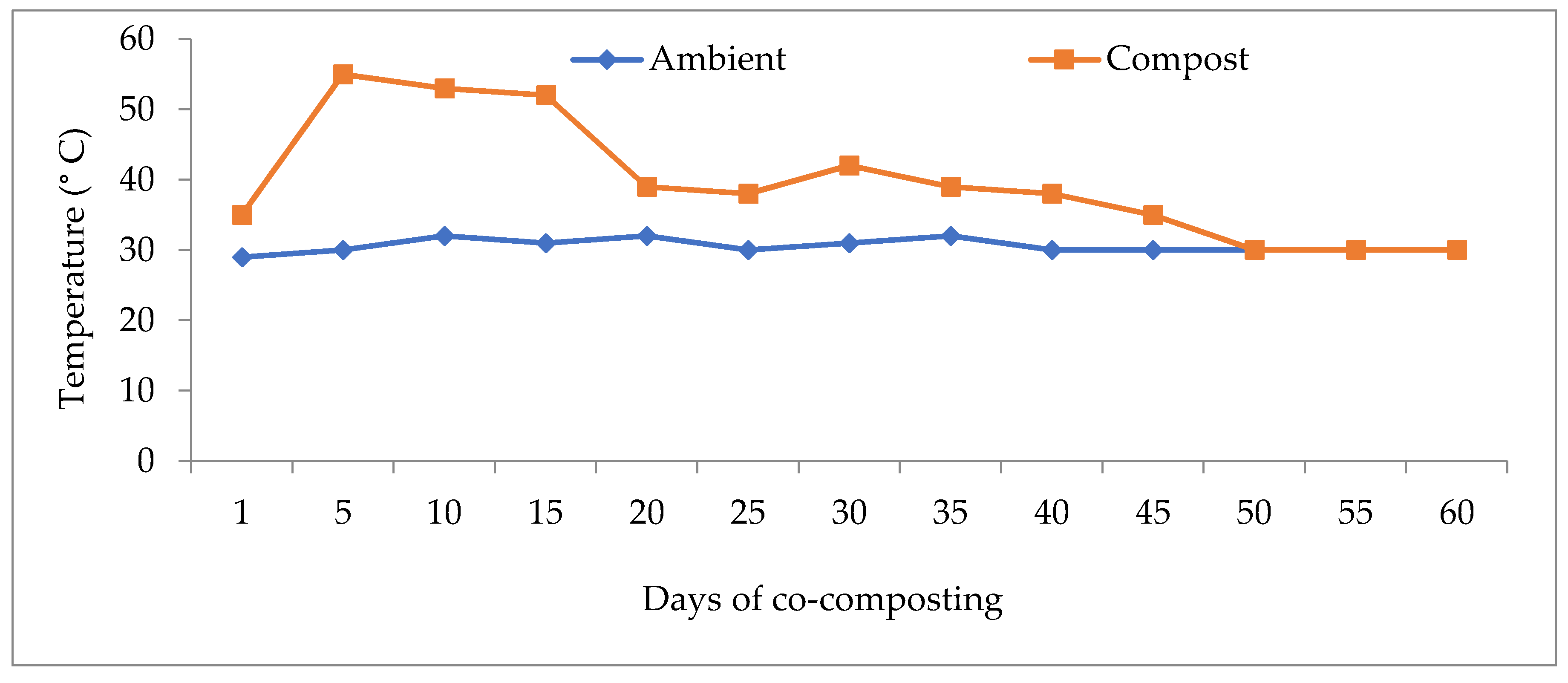
2.2. Co-Composting of Rice Husk and Chicken Dung Slurry
2.3. Characterization of Physico-Chemical Properties of Rice Husk Compost
2.4. Germination and Plant Bioassay Test of The Rice Husk Compost
- C: Control (distilled water)
- E1: Initial extract
- E2: Initial extract diluted 10×
- E3: Initial extract diluted 100×
- E4: Initial extract diluted 1000×
2.5. Soil Sampling and Characterizations
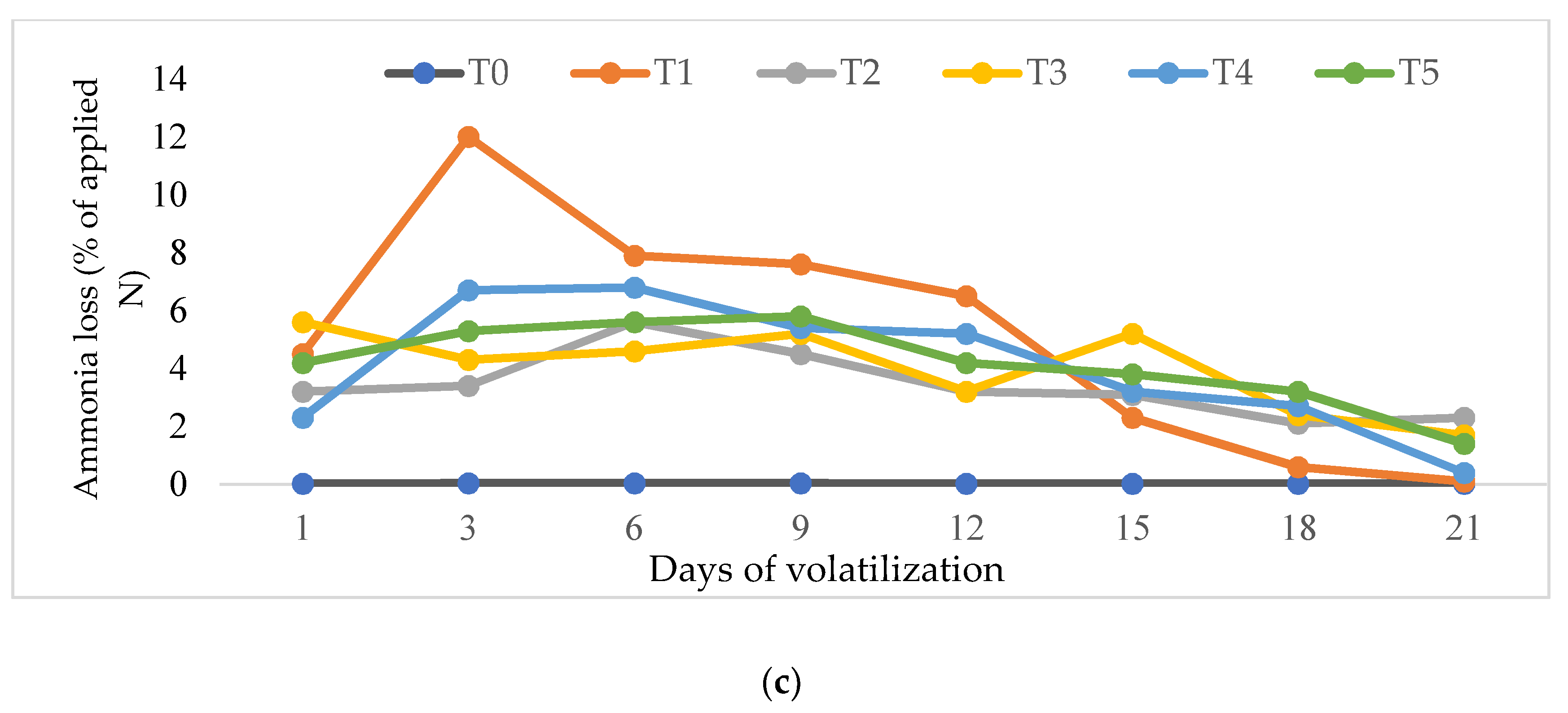
2.6. Ammonia Volatilization Study
- Soil only (T0)
- Soil + 7.3 g urea (standard recommendation at 100%) (T1)
- Soil + 7.3 g urea (standard recommendation at 100%) + 20 g compost (T2)
- Soil + 5.8 g urea (80% of standard recommendation) + 20 g compost (T3)
- Soil + 4.3 g urea (60% of standard recommendation) + 40 g compost (T4)
- Soil + 2.9 g urea (40% of standard recommendation) + 60 g compost (T5)
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Selected Physico-Chemical Characteristic of Raw Rice Husk and Chicken Slurry
3.2. Selected Physico-Chemical Characteristic of Rice Husk Compost
3.3. Phytotoxicity Test
3.4. Soil Characteristics
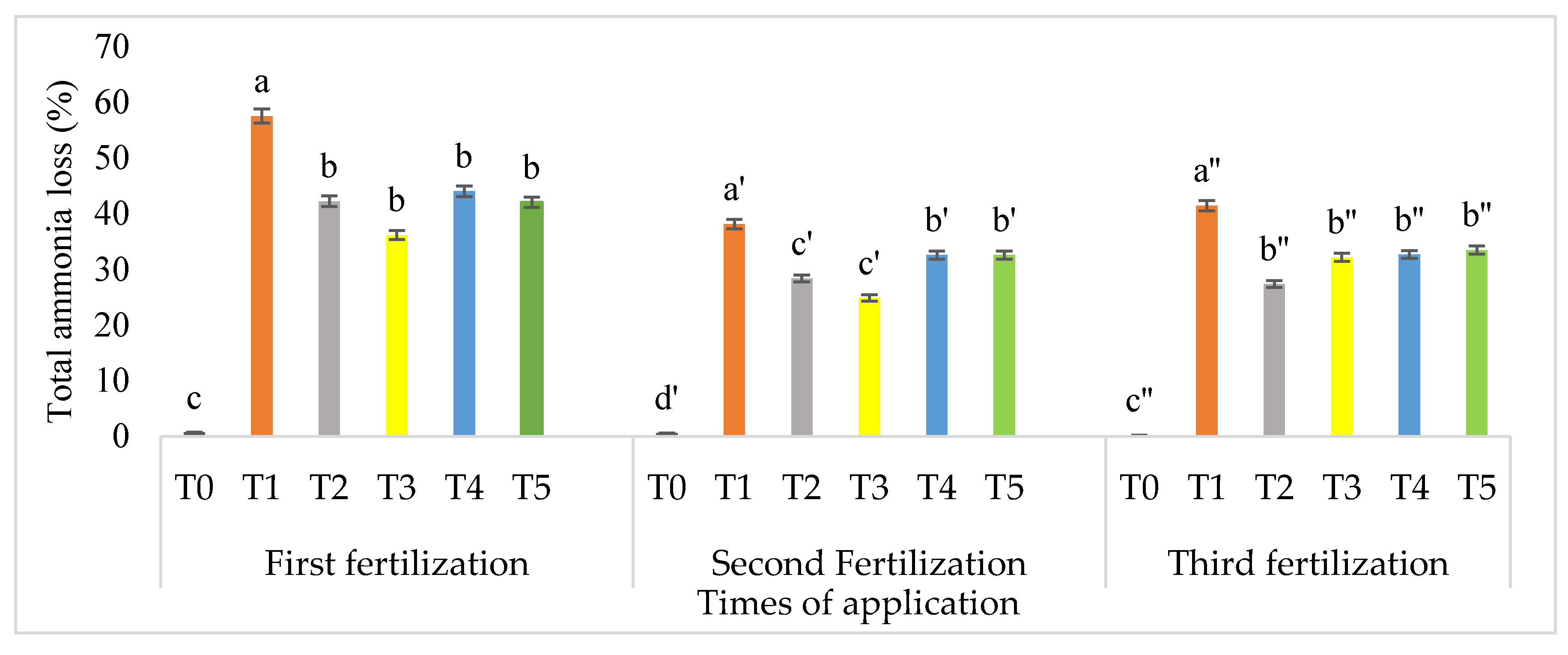
3.5. Amounts of Ammonia Volatilization at Three Different Times of Application
3.6. Temperature and pH at Three Different Timing and Rates of Urea Application
3.7. Soil Total Nitrogen, Exchangeable Ammonium, Available Nitrate, and pH
4. Discussion
4.1. Physico-Chemical Characteristics of Unprocessed Rice Husk and Chicken Slurry
4.2. Selected Physical, Chemical, and Biological Properties of the Rice Husk Compost
4.3. Selected Chemical Properties of Bekenu Series (Typic Paleudults)
4.4. Effects of Rice Husk Compost on Ammonia Volatilization
4.5. Soil Water Temperature and pH
4.6. Soil Total Nitrogen, Exchangeable Ammonium, and Available Nitrate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapanen, A.; Itävaara, M. Ecotoxicity tests for compost applications. Ecotoxicol. Environ. Saf. 2001, 49, 1–16. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, S.; Zhai, L.M.; Zhang, J.Z.; Ren, Y.Z.; Fan, B.Q.; Liu, H.B. Preparation and utilization of phosphate biofertilizers using agricultural waste. J. Integr. Agric. 2015, 14, 158–167. [Google Scholar] [CrossRef]
- Nakhshiniev, B.; Biddinika, M.K.; Gonzales, H.B.; Sumida, H.; Yoshikawa, K. Evaluation of hydrothermal treatment in enhancing rice straw compost stability and maturity. Biores. Tech. 2014, 151, 306–313. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Trade and Markets Division. 2012. Available online: http://www.fao.org/economic/est/publications/rice-publications/ricemarket-monitor-rmm/en/ (accessed on 1 May 2020).
- Obi, F.O.; Ugwuishiwu, B.O.; Nwakaire, J.N. Agricultural waste concept, generation, utilization and management. Niger. J. Tech. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Ishola, T.M.; Ishola, E.T. Composting and Sustainable Development. In Encyclopedia of Sustainability in Higher Education; Leal Filho, W., Ed.; Springer: Cham, Switzerland, 2019; pp. 21–22. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Paddy husk compost addition for improving nitrogen availability. Indian J. Agric. Res. 2019, 387, 1–7. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Minimizing phosphorus sorption and leaching of a tropical acid soil by amending Egypt rock phosphate with organic amendments. Acad. J. Agric. Res. 2015, 3, 276–288. [Google Scholar]
- Omar, L.; Ahmed, O.H.; Jalloh, M.B.; Nik Muhamad, A.M. Soil nitrogen fractions, nitrogen use efficiency and yield of Zea mays L. grown on a tropical acid soil treated with composts and clinoptilolite zeolite. Appl. Sci. 2020, 10, 4139. [Google Scholar] [CrossRef]
- Dias, J.; Gil, B.; Reidsma, P.; Giller, K.; Todman, L.; Whitmore, A.; van Ittersum, M. Sustainable development goal 2: Improved targets and indicators for agriculture and food security. Ambio 2019. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Susilawati, K.; Majid, N.M.A. Compost maturity and nitrogen availability by co-composting of paddy husk and chicken manure amended with clinoptilolite zeolite. Waste Manag. Res. 2015, 33, 322–331. [Google Scholar] [CrossRef]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C., Jr. Current approaches and future trends in compost quality criteria for agronomic, environmental, and human health benefits. Adv. Agron. 2017, 144, 143–233. [Google Scholar]
- San Martín, W. Global Nitrogen in Sustainable Development: Four Challenges at the Interface of Science and Policy. In Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Swarbreck, S.M.; Wang, M.; Wang, Y.; Kindred, D.; Sylvester- Bradley, R.; Shi, W.; Varinderpal, S.; Bentley, A.R.; Griffiths, H. A roadmap for lowering crop nitrogen requirement (report). Trends Plant Sci. 2019, 24, 892. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data. 2017, 9, 181. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Resolution Adopted by the United Nations Environment Assembly on 15 March 2019 on Sustainable Nitrogen Management; UNEP/EA.4/Res.14. 2019; United Nations Environment Programme: Nairobi, Kenya, 2019. [Google Scholar]
- Stevens, C. Nitrogen in the environment. Science 2019, 363, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Leach, A.M.; Erisman, J.W.; Bleeker, A. Nitrogen: The historical progression from ignorance to knowledge, with a view to future solutions. Soil Res. 2017, 55, 417–424. [Google Scholar] [CrossRef]
- International Nitrogen Management System (INMS). Fourth Meeting of the International Nitrogen Management System (INMS-4); Full Report of the Highlevel Segment; UN Environment HQ: Nairobi, Kenya, 2019. [Google Scholar]
- Kanter, D.R.; Bartolini, F.; Kugelberg, S.; Leip, A.; Oenema, O.; Uwizeye, A. Nitrogen pollution policy beyond the farm. Nat. Food 2020, 1, 27–32. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Almaraz, M.; Aneja, V.; Austin, A.T.; Bai, E.; Cassman, K.G.; Compton, J.E.; Davidson, E.A.; Erisman, J.W.; Galloway, J.N.; et al. A World of cobenefits: Solving the global nitrogen challenge. Earth’s Future 2019, 7, 865–872. [Google Scholar] [CrossRef]
- Sigurdarson, J.J.; Svane, S.; Karing, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Bio/Tech 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Sasaki, N.; Suehara, K.I.; Kohda, J. Effects of C/N ratio and pH of raw materials on oil degradation efficiency in a compost fermentation process. J. Biosci. Bioengin. 2003, 96, 47–52. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 672. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.F., Dinauer, R.C., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen-inorganic forms. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.G., Keeney, D.R., Baker, D.E., Miller, R.H., Rhoades, J.D., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Brewer, L.J.; Sullivan, D.M. Maturity and stability evaluation of composted yard trimmings. Comp. Sci. Util. 2003, 11, 96–112. [Google Scholar] [CrossRef]
- Palanivell, P.; Ahmed, O.H.; Susilawati, K.; Majid, N.M.A. Compost and crude humic substances produced from selected wastes and their effects on Zea mays L. nutrient uptake and growth. Sci. World J. 2013, 2013, 276235. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F.D. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Ahmed, O.H.; Aminuddin, H.; Husni, M.H.A. Ammonia volatilization and ammonium accumulation from urea mixed with zeolite and triple superphosphate. Acta Agric. Scand. Sec. B Soil Plant Sci. 2006, 58, 182–186. [Google Scholar]
- John, N.M.; Uwah, D.F.; Iren, O.B.; Akpan, J.F. Changes in maize (Zea mays L.) performance and nutrients content with the application of poultry manure, municipal solid waste and ash compost. J. Agric. Sci. 2013, 3, 270–272. [Google Scholar] [CrossRef][Green Version]
- Muda Agricultural Development Authority. Rice Check. 2014. Available online: http://www.mada.gov.my/semakan-tanaman-padi (accessed on 1 May 2020).
- Palanivell, P.; Ahmed, O.H.; Susilawati, K.; Majid, N.M.A. Improving lowland rice (O. sativa L. cv. MR219) plant growth variables, nutrients uptake, and nutrients recovery using crude humic substances. Sci. World J. 2015, 2015, 906094. [Google Scholar] [CrossRef]
- Paramananthan, S. Soil of Malaysia: Their Characteristics and Identification; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2000; Volume 1, pp. 11–125. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Tateda, M.; Trung, L.D.; Hang, H.T.M.; Ike, M.; Fujita, M. The role of compost pile turning for improving performance of composting. Jpn. J. Water Treat. Biol. 2008, 44, 21–28. [Google Scholar] [CrossRef][Green Version]
- Murimi, S.M.; Gbedemah, S.F. Suitability of Rice Husk and Chicken Droppings as Organic Fertilizer for Sustainable Agriculture in Ghana. J. Environ. Issues Agric. Dev. Ctries. 2019, 10, 63–78. [Google Scholar]
- Haug, R.T. The Practical Handbook of Compost Engineering; Routledge: Abingdon, UK, 2018; pp. 2–4. [Google Scholar]
- McCrory, D.F.; Hobbs, P.J. Additives to reduce ammonia and odour emissions from livestock wastes. J. Environ. Qual. 2001, 30, 345–355. [Google Scholar] [CrossRef]
- Ahmad, R.; Jilani, G.; Arshad, M.; Zahir, Z.A.; Khalid, A. Bioconversion of organic wastes for their recycling in agriculture: An overview of perspectives and prospects. Ann. Microbiol. 2007, 57, 471–479. [Google Scholar] [CrossRef]
- United States Department of Agriculture. National Engineering Handbook; Part 637; Composting: Washington, DC, USA, 2000; Chapter 2; pp. 2–8.
- Sullivan, D.M.; Miller, R.O. Compost quality attributes, measurements, and variability. Comp. Util. Horti. Crop. Syst. 2001, 95–120. [Google Scholar] [CrossRef]
- Tong, J.; Sun, X.; Li, S.; Qu, B.; Wan, L. Reutilization of green waste as compost for soil improvement in the afforested land of the Beijing Plain. Sustainability 2018, 10, 2376. [Google Scholar] [CrossRef]
- Wong, M.T.F.; Nortcliff, S.; Swift, R.S. Method for determining the acid ameliorating capacity of plant residue compost, urban waste compost, farmyard manure, and peat applied to tropical soils. Commun. Soil Sci. Plant Anal. 1998, 29, 2927–2937. [Google Scholar] [CrossRef]
- Kithome, M.; Paul, J.W.; Bomke, A.A. Reducing nitrogen losses during simulated composting of poultry manure using adsorbents or chemical amendments. J. Environ. Qual. 1999, 28, 194–201. [Google Scholar] [CrossRef]
- Fan, S.C.; Lin, C.S.; Hsu, P.K.; Lin, S.H.; Tsay, Y.F. The Arabidopsis nitrate Transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell. 2009, 21, 2750–2761. [Google Scholar] [CrossRef] [PubMed]
- Jauberthie, R.; Rendell, F.; Tamba, S.; Cisse, I. Origin of the pozzolanic effect of rice husks. Construc. Build. Mater. 2000, 14, 419–423. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Alva, A.K. High-water regime can reduce ammonia volatilization from soils under potato production. Commun. Soil Sci. Plant Anal. 2007, 38, 1203–1220. [Google Scholar] [CrossRef]
- Cai, G.X.; Chen, D.L.; Ding, H.; Pacholski, A.; Fan, X.H.; Zhu, Z.L. Nitrogen losses from fertilizers applied to maize, wheat, and rice in the North China Plain. Nutr. Cycl. Agron. 2002, 63, 187–195. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Reducing ammonia loss from urea and improving soil exchangeable ammonium and nitrate in non-waterlogged soils through mixing zeolite and sago (Metroxylon sagu) wastewater. Int. J. Phys. Sci. 2011, 6, 866–870. [Google Scholar]


| Parameters | Rice Husk | Chicken Slurry |
|---|---|---|
| pHwater | 7.32 ± 0.05 | 7.37 ± 0.18 |
| Total organic carbon (%) | 42.92 ± 13.36 | 47.94 ± 1.80 |
| Organic matter (%) | 74.11 ± 3.50 | 82.67 ± 1.04 |
| Total nitrogen (%) | 0.84 ± 1.41 | 1.15 ± 0.62 |
| C/N ratio | 51.09 ± 1.80 | 41.68 ± 7.88 |
| CEC (cmolc/kg) | 79.33 ± 0.42 | n.d |
| Total phosphorus (mg kg−1) | 2270.40 ± 108.28 | 1008.15 ± 105.88 |
| Ammonium (mg kg−1) | 22.15 ± 2.12 | 26.9 ± 3.67 |
| Nitrate (mg kg−1) | 18.10 ± 2.10 | n.d |
| Humic acids (%) | 3.33 ± 0.09 | n.d |
| Potassium (mg kg−1) | 1612.45 ± 0.11 | 234.14 ± 8.36 |
| Calcium (mg kg−1) | 76.67 ± 0.01 | 57.23 ± 13.88 |
| Magnesium (mg kg−1) | 561.00 ± 0.02 | 84.66 ± 16.17 |
| EC (µS cm−1) | 1528.33 ± 61.16 | 4.33 ± 0.88 |
| Parameters | Before Co-Composting (Mean ± S.E.) | After Co-Composting (Mean ± S.E.) |
|---|---|---|
| EC (µS cm−1) | 2.75 ± 0.10 | 2.79 ± 0.17 |
| pHwater | 7.15 ± 0.07 | 6.55 ± 0.05 |
| Organic material (%) | 81.33 ± 6.04 | 60.67 ± 26.99 |
| Total organic carbon (%) | 47.17 ± 3.50 | 35.19 ± 15.65 |
| Total nitrogen (%) | 0.89 ± 0.05 | 1.15 ± 0.62 |
| Carbon to nitrogen ratio | 53 ± 3.26 | 30.60 ± 2.87 |
| Humic acids (%) | 5.13 ± 0.21 | 5.87 ± 0.08 |
| CEC (cmolc kg−1) | 79.33 ± 0.42 | 100.67 ± 0.23 |
| Total phosphorus | 2639.18 ± 175.88 | 3101.18 ± 302.97 |
| Total potassium | 1859.33 ± 0.52 | 2038 ± 0.31 |
| Total magnesium (mg kg−1) | 113 ± 0.01 | 276 ± 0.04 |
| Total calcium | 662 ± 0.04 | 863 ± 0.22 |
| Ammonium | 44.5 ± 2.66 | 128.5 ± 7.87 |
| Nitrate | 34 ± 1.66 | 83.7 ± 6.34 |
| Treatment | Root Length (cm) | Shoot Length (cm) | Seed Germination (%) | Relative Seed Germination (%) | Germination Index (%) |
|---|---|---|---|---|---|
| C | 9.8 ± 0.55 | 1.2 ± 0.03 | 100 | 100 | 100 |
| E1 | 9.2 ± 0.28 | 1.3 ± 0.06 | 100 | 100 | 93.4 ± 5.21 |
| E2 | 9.2 ± 0.27 | 1.4 ± 0.10 | 100 | 100 | 94.1 ± 5.09 |
| E3 | 9.2 ± 0.26 | 1.5 ± 0.05 | 100 | 100 | 94.3 ± 5.08 |
| E4 | 9.5 ± 0.31 | 1.2 ± 0.07 | 100 | 100 | 97.3 ± 4.78 |
| Property | Value Obtained | Standard Data Range [34] |
|---|---|---|
| Mean ± S.E | ||
| pHwater | 4.67 ± 0.25 | 4.6 |
| CEC | 11.67 ± 0.21 | 8.0–24 |
| Exchangeable calcium(cmolc kg−1) | 23.10 ± 0.01 | 0.01 |
| Exchangeable magnesium | 33.33 ± 0.02 | 0.21 |
| Exchangeable potassium | 28.83 ± 0.17 | 0.19 |
| Total Nitrogen | 0.37 ± 0.42 | 0.04–0.17 |
| Organic matter (%) | 5.03 ± 1.04 | n.d |
| Total carbon | 2.91 ± 0.60 | 0.57–2.51 |
| Total phosphorus | 110.88 ± 9.07 | n.d |
| Exchangeable ammonium (mg kg−1) | 12 ± 2.49 | n.d |
| Available nitrate | 5.20 ± 1.01 | n.d |
| Treatment | NH3 Loss (% of Urea-N) | Subtraction in Total NH3 Lost as Compared with T1 (%) |
|---|---|---|
| 1st fertilization (28 days after planting) | ||
| T0 | 0.71 c | None |
| T1 | 57.60 a | None |
| T2 | 34.44 b | 40.21 |
| T3 | 32.76 b | 43.12 |
| T4 | 30.17 b | 47.63 |
| T5 | 42.10 b | 26.91 |
| 2nd fertilization (50 days after planting) | ||
| T0 | 0.62 c | None |
| T1 | 38.25 a | None |
| T2 | 28.43 b | 25.68 |
| T3 | 24.93 b | 34.83 |
| T4 | 32.17 b | 15.90 |
| T5 | 32.17 b | 15.90 |
| 3rd fertilization (70 days after planting) | ||
| T0 | 0.22 c | None |
| T1 | 41.52 a | None |
| T2 | 27.47 c | 33.84 |
| T3 | 32.22 b | 22.40 |
| T4 | 32.77 b | 21.08 |
| T5 | 33.50 b | 19.32 |
| Treatment | Total N (%) | Ammonium (mg kg−1) | Nitrate (mg kg−1) | pH |
|---|---|---|---|---|
| T0 | 0.02 d ± 0.001 | 21.12 e ± 0.05 | 10.18 b ± 0.01 | 4.18 b ± 0.01 |
| T1 | 0.14 c ± 0.01 | 66.28 d ± 9.80 | 25.19 a ± 0.05 | 6.77 a ± 0.05 |
| T2 | 0.67 b ± 0.01 | 548.14 b ± 11.54 | 24.58 a ± 4.90 | 6.16 a ± 0.02 |
| T3 | 0.64 b ± 0.05 | 386.24 b ± 6.99 | 25.24 a ± 2.40 | 6.37 a ± 0.05 |
| T4 | 0.79 a ± 0.01 | 658.34 a ± 7.05 | 24.16 a ± 5.88 | 6.22 a ± 0.01 |
| T5 | 0.76 a± 0.01 | 342.18 c ± 7.05 | 25.77 a ± 1.90 | 6.31 a ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, L.; Ahmed, O.H.; Boyie Jalloh, M.; Abdul Majid, N.M. Rice Husk Compost Production and Use in Mitigating Ammonia Volatilization from Urea. Sustainability 2021, 13, 1832. https://doi.org/10.3390/su13041832
Omar L, Ahmed OH, Boyie Jalloh M, Abdul Majid NM. Rice Husk Compost Production and Use in Mitigating Ammonia Volatilization from Urea. Sustainability. 2021; 13(4):1832. https://doi.org/10.3390/su13041832
Chicago/Turabian StyleOmar, Latifah, Osumanu Haruna Ahmed, Mohamadu Boyie Jalloh, and Nik Muhamad Abdul Majid. 2021. "Rice Husk Compost Production and Use in Mitigating Ammonia Volatilization from Urea" Sustainability 13, no. 4: 1832. https://doi.org/10.3390/su13041832
APA StyleOmar, L., Ahmed, O. H., Boyie Jalloh, M., & Abdul Majid, N. M. (2021). Rice Husk Compost Production and Use in Mitigating Ammonia Volatilization from Urea. Sustainability, 13(4), 1832. https://doi.org/10.3390/su13041832







