Abstract
Rare soil organisms are normally considered of less importance for ecosystem functioning. We present results that oppose this view. In otherwise well-aerated soils, anaerobic/microaerophilic production or consumption of the trace gas N2O occurs in small soil volumes, when intense decomposition activity at the site leads to local oxygen depletion. At such patch scales, the control of microbial growth and oxygen consumption may depend on the specific organisms present. We assessed N2O turnover in an experiment, where soil dilution from 10−2 over 10−4 to 10−6 followed by microbial regrowth resulted in similar microbial biomass and respiration but reduced diversity. We found an increasing number of very high N2O turnover rates when soil dilution increased from 10−2 over 10−4 to 10−6, as revealed from a significantly increased skewness of the frequency distribution of N2O turnover levels. N2O turnover also tended to increase (p = 0.08) by 20–30% when soil was diluted from 10−2 to 10−6. This suggests that rare soil organisms regulate the local activity of fast-growing microorganisms and thus reduce the probability that anoxic/microaerophilic soil volumes develop. Future studies may reveal which less abundant organisms prevent development of anoxic/microaerophilic conditions in well-aerated soils.
1. Introduction
Soils are inhabited by myriads of organisms, among which a huge number of rare species contribute most to phylogenetic diversity [1]. Normally these rare organisms are regarded as of less importance based on the Mass Ratio Hypothesis developed for plants, i.e., the effect of a species on an ecosystem process is proportional to its relative abundance [2], but more recent work on soil organisms suggests that this may not be true [3,4].
We studied the importance of rare organisms on the dynamics of processes that take place in small volumes of soil. Statistically, all rare organisms will not be present in any smaller soil volume, unlike the more abundant species. We, therefore, believe that the influence of rare organisms on soil functioning will be more obvious for processes developing in discrete small soil volumes compared to processes that occur more evenly distributed in the soil volume.
In well-aerated and homogenous soils, microbial turnover (production/consumption) of the greenhouse gas N2O occurs in small soil volumes of low/no oxygen [5]. Denitrifying microorganisms produce and consume N2O under anoxic conditions [6], whereas nitrifying bacteria form N2O under microaerophilic conditions [7,8]. Generally, N2O exchange exhibits tremendous spatiotemporal variation, reflecting the transient nature of anoxic small-volume hotspots [9]. In well-aerated, structurally homogenous soils, such low/no oxygen conditions depend on the spatial distribution of microbial activity and, hence, oxygen consumption [10]. Even though the genetic potential for N2O turnover prevails throughout a soil volume, hot spots of high N2O turnover only exist in a fraction of this volume [11]. These low-oxygen hotspots may exist for weeks during decomposition of an organic substance [12].
At the centimeter scale, we envisage that microbial activity and, thus, oxygen consumption depend on the specific organisms present and the nature of the interactions between species coexisting within the soil volume. Therefore, the extent to which oxygen consumption exceeds oxygen supply via diffusion may depend on the species present locally. With reduced microbial diversity, we anticipate that the level of interspecific antagonistic interactions declines [13,14]. The level of antagonistic interactions between bacterial populations is a crucial parameter for the control (and, hence, oxygen consumption) of fast-growing microbial populations. For instance, growth control of fast-growing microorganisms was reduced at experimentally reduced microbial diversity [15,16]. With reduced growth control in spatially confined microsites, oxygen consumption will increase locally in the microsites. We hypothesize that more low-oxygen microsites will develop and, thus, N2O turnover will increase when control of local respiration activity is reduced in soils of low microbial diversity. The ability to produce N2O is phylogenetically very widespread in the microbial community, and the community of potentially N2O producing bacteria is redundant and highly resistant to changes, as reviewed by [17]. It is, therefore, unlikely that the N2O production potential will be affected when microbial diversity is experimentally reduced.
In the present study, we used soil dilution to extinction [18] to examine the role of rare microbial species in controlling fast-growing microorganisms in soils from three land management types: intensive arable, rotation arable, and permanent pasture. Dilution series of organisms re-inoculated into sterilized soil can give soils with comparable microbial biomass that differ in microbial species composition [19]. Such experimental treatments enable testing the effect of varying microbial community composition on soil processes.
2. Materials and Methods
2.1. Soil Preparation
This study is part of an experiment described in detail elsewhere [19,20]: Soils were collected from fields with different land managements, meaning different cultivation intensities (physical soil disturbances). The land managements were intensive cereal rotation (high cultivation intensity: annual crops, including winter wheat and spring barley, annual tillage), crop rotation (medium cultivation intensity: five-year rotation scheme including winter wheat + catch crop (grass), ley for hay, grass seed production, spring barley, potato, no tillage during the year of ley, sampled during winter wheat + catch crop), and grassland (low cultivation intensity: permanent grassland, no tillage for at least 10 years) at two farms in southern Sweden [21]. Twelve soil samples were obtained (two farms, three cultivation intensities, two field replicates). Soil samples (36 kg) were sieved, the main part was γ-sterilized (>25 kGray at ISOTRON, Ede, The Netherlands), and the rest stored at 4 °C as inoculum. For each of the 12 soil samples, we prepared three inoculum dilutions. We blended 25 g with 200 mL sterilized demineralized water, centrifuged the suspension for 10 min at 1000× g and 4 °C, and filtered the supernatant through a 45-µm sieve to remove soil fauna. For the highest diversity (0.5 × 10−2 dilution), 5 kg sterilized soil was inoculated with the 25 g fresh soil–water suspension (mixed to ensure homogenization). Prior to this inoculation, 2 mL of the 200 mL suspension above were transferred to 198 mL sterile water. This 100-times diluted suspension was added to 5 kg sterilized soil to obtain the 0.5 × 10−4 dilution and the procedure was repeated to get the 0.5 × 10−6 dilution. We incubated the 36 soil samples obtained from the 12 individual soil samples in sealed plastic bags in the dark for eight months at 15% moisture (gravimetric) and room temperature to establish recolonization [19].
2.2. Experimental Setup
Portions of 200 g (dry weight) from each of the 36 samples were distributed in 24 pots (864 pots in total) and planted with winter wheat Triticum aestivum cv. Carenius for eight weeks [19]. Plants were watered regularly, approximately 25 mL three times a week to keep soil moisture at 15% (w/w). Following this eight-week period, microbial biomass was similar at the three dilution treatments from the two field replicates (Table 1 in [19]), whereas dilution reduced the number of bacterial operational taxonomic units (OTUs) (p < 0.05). In the permanent grassland soil, number of OTUs were 10% lower at 10−4 and 25% lower at 10−6 than at 10−2, and in arable soils (wheat and crop rotation), OTU numbers were reduced with 5% and 27% at 10−4 and 10−6 compared to 10−2 (Table 2 in [19]). A second growing phase was established to further investigate the consequences of this microbial species loss over longer time. Here, 50 g (fresh weight) of soil from each pot from phase 1 was retained and stored at 4 °C. The remaining soil from all pots and all treatments from phase 1 was mixed and γ-sterilized (>25 kGray). The aim was to create a homogenous background to limit abiotic differences and instead highlight the differences in microbial community composition. Portions of 200 g of the sterilized soil were re-inoculated with the 50 g living soil that had been retained. The bacterial OTU richness in this inoculum was reduced (p < 0.05) by 8–13% in the 10−4 and by 24–27% in the 10−6 dilutions compared to the 10−2 dilution for the intensive arable as well as the pasture soils (Table S1b in [19]). Thus, the experimental design is the same as in phase 1, with 24 pots for each soil/inoculum treatment. Pots were sown with one seed of T. aestivum cv. Carenius. Pots were placed in a fully randomized block design with 24 blocks in a greenhouse under 60% relative humidity, 16 h L, 8 h D, 21 °C/16 °C, and additional illumination by 400-W growing bulbs (Philips SONT-T Agro, Philips, Eindhoven, The Netherlands). Light intensity at plant level was 225 μmol photosynthetically active radiation (PAR). Each of the 24 blocks contained one replicate of each soil type/inoculation treatment. Pots were watered regularly. The experimental setup is outlined graphically in Figure S1.
2.3. CO2 and N2O Measurements
Six weeks after germination of seedlings, all pots were adjusted to 70% water-holding capacity (46% air-filled porosity) and placed in a 68-cm-tall cylinder of 7.4-cm diameter in order to collect N2O and CO2. CO2 was measured to assess if N2O exchange rates were related to the overall soil respiratory activity. To facilitate gas collection the cylinder was placed inside an autoclavable bag. The bag was taped closely around the cylinder to achieve an air volume of 2.92 L and closed with a tie-rip. Sealed autoclave bags with added N2O were used to test that the sealing procedure was sufficient for the incubation period (results not shown). Gas production was measured on whole pots including dark respiration from plants: An 8-mL gas sample was taken before and after 1.5 h of incubation in a dark room and stored in airtight 5-mL Exetainer vials. The change in CO2 and N2O concentrations over this period together with the volume of air in the bag gave the absolute production of CO2 and N2O in the soil. The concentration change was small over 1.5 h so we can be sure that accumulation was linear. We measured CO2 and N2O concentration in the gas samples on gas chromatographs equipped with a thermal conductivity (Mikrolaboratoriet, Aarhus, Denmark) and electron capture (Shimadzu GC8A) detector, respectively. Gases were separated before detection on 1.8-m Haysep Q columns operated at 45 °C.
2.4. Statistics
We tested the effects of farm, cultivation intensity, and dilution on the N2O exchange and CO2 emission with a full factorial three-way ANOVA (farm × cultivation intensity × dilution (quantitative)), block as main factor in SAS Enterprise Guide 7.1. By using two farms we tried to avoid generalizing results that only apply to a single farm location. We also tested to what extent the frequency distribution of N2O exchange rates at the three dilutions were symmetric. This was done by the coefficient of skewness that showed the extent to which the frequency distribution deviated from the normal distribution. The skewness coefficient also depicted whether the distribution was asymmetric toward high or low values. To evaluate if the skewness differed between dilution treatments, we calculated the confidence intervals for the skewness coefficients based on the standard error for skewness coefficient (SES), where SES = √(6n(n − 1)/((n − 2)(n + 1)(n + 3)).
3. Results
N2O exchange was positive as well as negative, which means that we detected N2O release and N2O absorption by the soil, respectively. Since N2O is an intermediate product in the denitrification process that occurs under anoxic conditions, results are shown as N2O exchange adding consumption and production of the gas, both as positive values. The frequency distribution of N2O exchange shifted toward higher values when dilution increased above 10−2 (Figure 1). Skewness increased significantly toward the right (i.e., the frequency distribution was more asymmetric towards high values) when dilution increased (Table 1).
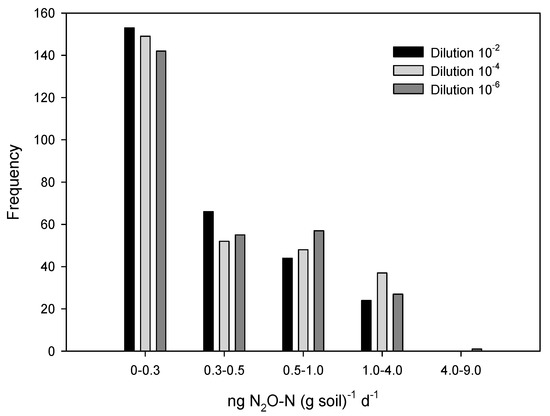
Figure 1.
Frequency distribution of N2O exchange rates (the sum of N2O production and consumption) at dilution 10−2, 10−4, and 10−6.

Table 1.
Mean N2O exchange (ng N2O–N (g soil) −1 d−1) and skewness of frequency of N2O exchange in the three dilution treatments.
The ANOVA also showed that N2O exchange tended to increase with reduced diversity (p = 0.08, Table 2), in particular from the 10−2 to the 10−4 dilution (Figure 2a).

Table 2.
Result of ANOVA on N2O exchange.
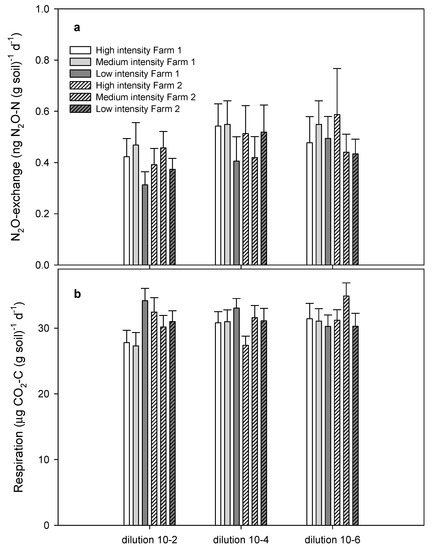
Figure 2.
(a) N2O exchange (the sum of N2O production and consumption); (b) respiration activity. Soil originated from agricultural fields of high, medium, and low cultivation intensities at two different farms. Sterilized soils were re-inoculated 5 g fresh soil kg−1 sterile soil to obtain the highest diversity (0.5 × 10−2 dilution). Similarly, re-inoculation of sterilized soil with 50 or 0.5 mg fresh soil kg−1 sterile soil gave the medium (0.5 × 10−4 dilution) and low diversity (0.5 × 10−6 dilution). Error bars represent standard errors.
The average numeric N2O exchange was 22% higher at the 10−4 and 10−6 dilutions compared to the 10−2 dilution (Table 1, Figure 2a). Neither farm origin nor cultivation intensity affected N2O exchange (Table 2). The increased N2O exchange with dilution was not due to an overall increased oxygen demand since CO2 formation did not vary with reduced diversity (F = 0.94, p = 0.33) and was quite similar at the 10−2 and 10−6 dilution, spanning from a 7% reduction at low intensity to a 4% and 15% increase at medium and high intensity (Figure 2b). The mean N2O exchange was not significantly different among dilutions, but the larger skewness of the frequency distribution of N2O exchange at high dilution means that significantly more pots had high exchange rates when dilution increased.
4. Discussion
The main aim of our study was to assess the impact of reduced microbial diversity on the exchange of N2O, which, in well-aerated soil, depends on the generation of oxygen-depleted patches in the soil matrix. Local high microbial activity at hotspots where oxygen consumption exceeds oxygen supply via diffusion generates such oxygen-depleted patches. Even though N2O production potential by denitrifying bacteria was previously found to decrease by 50–60% following regrowth when soil was diluted 105 times [22], this apparently did not affect our actual N2O exchange that tended to increase with dilution.
The microbial diversity was reduced by dilution and, after subsequent regrowth in the soil, microbial biomass and respiration activity were unaffected, which means that the increase in N2O turnover was not due to a general increase in oxygen consumption via enhanced respiration activity. Plant performance (biomass and nitrogen content) did not vary between treatments in this experiment [20], and we, therefore, assumed that the root-derived contribution to soil respiration was similar across treatments. The reduction in microbial diversity with dilution [19], therefore, implies that conclusions on effects of diversity reduction on system performance can be drawn independently of microbial biomass or overall respiration activity. We believe that the increase in respiration driving the increased N2O turnover was very local and insignificant when considering the whole soil core. Therefore, no increase in overall CO2 production is to be expected when N2O turnover gets more intense. The increased skewness of the frequency distribution of N2O exchange shows that the likelihood of large N2O exchange increased at higher dilutions. This means that the frequency of local hotspots of low/no oxygen increased when microbial diversity was reduced.
We believe that loss of rare species promoted the occurrence of anoxic patches due to reduced competition and/or decreased number of inhibitory interactions within confined soil volumes. With species loss, reduced levels as well as complexity of interactions among organisms should increase the likelihood for dominance of a few species via reduced growth control [23]. For instance, with reduced diversity, antagonism, which is common among rhizosphere bacteria [24], may be reduced. Further, reduced interspecific competition at reduced diversity may stimulate decomposition [25] and, thus, oxygen consumption. It has also been shown that at low diversity, reduced competition, antagonisms, or predation exerted by concurrent organisms increase the chance for establishment and proliferation of aggressive fast-growing microorganisms [16]. Taken together, evidence suggests that local, intense microbial activity will occur more frequently at reduced diversity, thereby increasing the likelihood of anoxic microsites in the soil matrix. It remains to be shown which less abundant organisms control the development of anoxic/microaerophilic spots in well-aerated soils. In our laboratory setup, inoculation with microorganisms from different arable management schemes in a uniform sterilized soil background did not impact N2O exchange. As we used the same background soil for all microbial treatments, this result does not necessarily imply that management or cultivation practices will not affect N2O exchange in the field. Certainly, agricultural management practices such as tillage and the derived effects on soil structure and nitrogen fertilization are known to affect N2O dynamics [26,27].
We have shown that at least processes occurring very locally in the soil can depend on the presence of rare organisms. Such a dependency on diversity could be because the dynamics that create anoxic volumes occur locally in a small sub-volume of the soil matrix where number of species will be low. In contrast, processes depending on conditions in a larger volume of soil may not be as dependent on a reduction in diversity as we found here. The long tail of rare organisms that has received less attention in relation to soil functioning and biogeochemical processes may, therefore, at least indirectly have a role in the regulation of specific processes.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/13/4/1685/s1, Figure S1: Experimental outline.
Author Contributions
Conceptualization, S.C., W.H.G.H., and M.V.; methodology, S.C. and W.H.G.H.; validation, S.C. and M.V.; formal analysis, S.C.; investigation, W.H.G.H. and V.K.; resources, S.C.; data curation, S.C. and M.V.; writing—original draft preparation, S.C.; writing—review and editing, S.C., M.V., W.H.G.H., and V.K.; visualization, S.C. and M.V.; project administration, S.C. and W.H.G.H.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union Seventh Framework project SOILSERVICE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We thank Annette Spangenberg and Sara Bentzon-Tilia for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mi, L.; Wang, G.; Jin, J.; Sui, Y.; Liu, J.; Liu, X. Comparison of microbial community structures in four Black soils along a climatic gradient in northeast China. Can. J. Soil Sci. 2012, 92, 543–549. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Hol, W.H.G.; de Boer, W.; Termorshuizen, A.J.; Meyer, K.M.; Schneider, J.H.M.; van Dam, N.M.; van Veen, J.A.; van der Putten, W.H. Reduction of rare soil microbes modifies plant-herbivore interactions. Ecol. Lett. 2010, 13, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Kusel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Tiedje, J.M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In Biology of Anaerobic Microorganisms; Zehnder, A.J.B., Ed.; John Wiley and Sons: New York, NY, USA, 1988; pp. 179–244. [Google Scholar]
- Smith, M.S.; Tiedje, J.M. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 1979, 11, 261–267. [Google Scholar] [CrossRef]
- Aboobakar, A.; Cartmell, E.; Stephenson, T.; Jones, M.; Vale, P.; Dotro, G. Nitrous oxide emissions and dissolved oxygen profiling in a full-scale nitrifying activated sludge treatment plant. Water Res. 2013, 47, 524–534. [Google Scholar] [CrossRef]
- Goreau, T.J.; Kaplan, W.A.; Wofsy, S.C.; McElroy, M.B.; Valois, F.W.; Watson, S.W. Production of NO2- and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 1980, 40, 526–532. [Google Scholar] [CrossRef]
- Groffman, P.M.; Butterbach-Bahl, K.; Fulweiler, R.W.; Gold, A.J.; Morse, J.L.; Stander, E.K.; Tague, C.; Tonitto, C.; Vidon, P. Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 2009, 93, 49–77. [Google Scholar] [CrossRef]
- Sexstone, A.J.; Revsbech, N.P.; Parkin, T.B.; Tiedje, J.M. Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci. Soc. Am. J. 1985, 49, 645–651. [Google Scholar] [CrossRef]
- Graf, D.R.H.; Zhao, M.; Jones, C.M.; Hallin, S. Soil type overrides plant effect on genetic and enzymatic N2O production potential in arable soils. Soil Biol. Biochem. 2016, 100, 125–128. [Google Scholar] [CrossRef]
- Christensen, S.; Simkins, S.; Tiedje, J.M. Temporal patterns of soil denitrification: Their stability and causes. Soil Sci. Soc. Am. J. 1990, 54, 1614–1618. [Google Scholar] [CrossRef]
- Becker, J.; Eisenhauer, N.; Scheu, S.; Jousset, A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 2012, 15, 468–474. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Bakker, M.G.; Bradeen, J.M.; Kinkel, L.L. Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 2015, 96, 134–142. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, J.D.; Hill, P.; Chroňáková, A.; Grekova, M.; Topalova, Y.; Elhottová, D.; Krištůfek, V. Survival of genetically marked Escherichia coli O157: H7 in soil as affected by soil microbial community shifts. ISME J. 2007, 1, 204–214. [Google Scholar] [CrossRef]
- Liu, M.; Bjørnlund, L.; Rønn, R.; Christensen, S.; Ekelund, F. Disturbance promotes non-indigenous bacterial invasion in soil microcosms: Analysis of the roles of resource availability and community structure. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Salonius, P.O. Metabolic capabilities of forest soil microbial-populations with reduced species-diversity. Soil Biol. Biochem. 1981, 13, 1–10. [Google Scholar] [CrossRef]
- Hol, W.H.G.; de Boer, W.; de Hollander, M.; Kuramae, E.E.; Meisner, A.; van der Putten, W.H. Context dependency and saturating effects of loss of rare soil microbes on plant productivity. Front. Plant Sci. 2015, 6, 485. [Google Scholar] [CrossRef]
- Carvalho, S.; van der Putten, W.H.; Hol, W.H.G. The potential of hyperspectral patterns of winter wheat to detect changes in soil microbial community composition. Front. Plant Sci. 2016, 7, 759. [Google Scholar] [CrossRef]
- de Vries, F.T.; Thebault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; de Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef]
- Philippot, L.; Spor, A.; Henault, C.; Bru, D.; Bizouard, F.; Jones, C.M.; Sarr, A.; Maron, P.A. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Kelsic, E.D.; Zhao, J.; Vetsigian, K.; Kishony, R. Counteraction of antibiotic production and degradation stabilizes microbial communities. Nature 2015, 521, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition interrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbies, K.E.; Massheder, A.R. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2018, 69, 10–20. [Google Scholar] [CrossRef]
- van Groeningen, J.W.; Velthof, G.L.; Oenema, O.; van Groeningen, K.J.; van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).