Effects of High Voltage Electrical Discharge Plasma on Olive Mill Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Physico-Chemical Analysis
2.3. Extraction of Polyphenols
2.4. Determination of Polyphenols
3. Results and Discussion
3.1. Physico-Chemical Characteristics
3.2. COD Removal
3.3. Polyphenol Composition
3.4. Future Investigation Direction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Tufaner, F. Evaluation of COD and color removals of effluents from UASB reactor treating olive oil mill wastewater by Fenton process. Sep. Sci. Technol. 2019, 55, 3455–3466. [Google Scholar] [CrossRef]
- Zghari, B.; Doumenq, P.; Romane, A.; Boukir, A. GC-MS, FTIR and 1H, 13C NMR Structural Analysis and Identification of Phenolic Compounds in Olive Mill Wastewater Extracted from Oued Oussefrou Effluent (Beni Mellal-Morocco). J. Mater. Environ. Sci. 2017, 8, 4496–4509. [Google Scholar] [CrossRef]
- Esteves, B.M.; Rodrigues, C.S.D.; Madeira, L.M. Synthetic olive mill wastewater treatment by Fenton’s process in batch and continuous reactors operation. Environ. Sci. Pollut. Res. 2017, 25, 34826–34838. [Google Scholar] [CrossRef]
- Genethliou, G.; Kornaros, M.; Dailianis, S. Biodegradation of olive mill wastewater phenolic compounds in a thermophilic anaerobic up flow packed bed reactor and assessment of their toxicity in digester effluents. J. Environ. Manag. 2020, 255. [Google Scholar] [CrossRef]
- Landeka Dragičević, T.; Zanoški Hren, M.; Gmajnić, M.; Pelko, S.; Kungulovski, D.; Kungulovski, I.; Čvek, D.; Frece, J.; Markov, K.; Delaš, F. Biodegradation of olive mill wastewater by Trichosporon cutaneum and Geotrichum candidum. Arch. Ind. Hyg. Toxicol. 2010, 61, 399–405. [Google Scholar] [CrossRef]
- Kilic, M.; Kaya, G.; Kestioglu, K. An inventory study for treatment of olive mill wastewater by chemical, biological and advanced treatment methods. Uludağ Univ. J. Fac. Eng. 2009, 14, 183–198. [Google Scholar] [CrossRef]
- Ibrahimoglu, B.; Yilmazoglu, M.Z. Disposal of olive mill wastewater with DC arc plasma method. J. Environ. Manag. 2018, 217, 727–734. [Google Scholar] [CrossRef]
- Kuşçu, Ö.S.; Eke, E. Treatment of olive oil mill wastewater by apulsed high-voltage discharge process; process optimization and combination with Fe+2 and H2O2. J. Chem. Technol. Biotechnol. 2015, 90, 1040–1050. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qui, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Banaschik, R.; Lukes, P.; Jablonowski, H.; Hammer, M.U.; Weltmann, K.-D.; Kolb, J.F. Potential of pulsed corona discharges generated in water for the degradation of persistent pharmaceutical residues. Water Res. 2015, 84, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M. Efficient removal of phenol from aqueous solution by the pulsed high-voltage discharge process in the presence of H2O2. Chem. Int. 2015, 1, 81–86. [Google Scholar] [CrossRef]
- Kallel, M.; Belaid, C.; Boussahel, R.; Ksibi, M.; Montiel, A.; Elleuch, B. Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J. Hazard Mater. 2009, 163, 550–554. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced removal of water pollutants by dielectric barrier discharge nonthermal plasma reactor. Sep. Purif. Technol. 2019, 215, 155–162. [Google Scholar] [CrossRef]
- Sugiarto, A.T.; Sato, M. Pulsed plasma processing of organic compounds in aqueous solution. Thin Solid Films 2001, 386, 295–299. [Google Scholar] [CrossRef]
- Shen, Y.; Lei, L.; Zhang, X.; Zhou, M.; Zhang, Y. Effect of various gases and chemical catalysts on phenol degradation pathways by pulsed electrical discharges. J. Hazard Mater. 2008, 150, 713–722. [Google Scholar] [CrossRef]
- Grymonpré, D.R.; Finney, W.C.; Clark, R.J.; Locke, B.R. Hybrid gas-liquid electrical discharge reactors for organic compound degradation. Ind. Eng. Chem. Res. 2004, 43, 1975–1989. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Melliou, E.; Magiatis, P.; Gerasopoulos, D. Enhancement of bioactive phenols and quality values of olive oil by recycling olive mill waste water. J. Am. Oil Chem. Soc. 2017, 94, 1077–1085. [Google Scholar] [CrossRef]
- Sharma, G.; Rodríguez-Pardo, C.E. The dark side of CIELAB. In Color Imaging XVII: Displaying, Processing, Hardcopy, and Applications; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; p. 82920D. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Magureanu, M.; Lukes, P. Plasma Chemistry and Catalysis in Gases and Liquids; Wiley-VCH Verlag & C.o. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Hammer, T.; Kappes, T.; Baldauf, M. Plasma catalytic hybrid processes: Gas discharge initiation and plasma activation of catalytic processes. Catal. Today 2004, 89, 5–14. [Google Scholar] [CrossRef]
- Briels, T.M.P.; Van Veldhuizen, E.M.; Ebert, U. Positive streamers in air and nitrogen of varying density: Experiments on similarity laws. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Shih, K.Y.; Locke, B.R. Chemical and physical characteristics of pulsed electrical discharge within gas bubbles in aqueous solutions. Plasma Chem. Plasma Process. 2010, 30, 1–20. [Google Scholar] [CrossRef]
- Lukeš, P. Water Treatment by Pulsed Streamer Corona Discharge. Ph.D. Thesis, Institute of Chemical Technology, Prague, Czech Republic, 2001. [Google Scholar]
- Sugiarto, A.T.; Ito, S.; Ohshima, T.; Sato, M.; Skalny, J.D. Oxidative decoloration of dyes by pulsed discharge plasma in water. J. Electrost. 2003, 58, 135–145. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Zhang, X.-S.; Dai, Y.-C.; Yuan, W.-K. Pulsed high-voltage discharge plasma for degradation of phenol in aqueous solution. Sep. Purif. Technol. 2004, 34, 5–12. [Google Scholar] [CrossRef]
- Krause, H.; Schweiger, B.; Schuhmacher, J.; Scholl, S.; Steinfeld, U. Degradation of the endocrine disrupting chemicals (EDCs) carbamazepine, clofibric acid and iopromide by corona discharge over water. Chemosphere 2009, 75, 163–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Hao, X.; Lei, L. Degradation mechanisms of 4-chlorophenolin a novel gas–liquid hybrid discharge reactor by pulsed high voltage system with oxygen or nitrogen bubbling. Chemosphere 2007, 67, 702–711. [Google Scholar] [CrossRef]
- Njatawidjaja, E.; Tri Sugiarto, A.; Ohshima, T.; Sato, M. Decoloration of electrostatically atomized organic dye by the pulsed streamer corona discharge. J. Electrost. 2005, 63, 353–359. [Google Scholar] [CrossRef]
- Sun, B.; Sato, M.; Sid Clements, J. Optical study of active species produced by a pulsed streamer corona discharge in water. J. Electrost. 1997, 39, 189–202. [Google Scholar] [CrossRef]
- Bampalioutas, K.; Vlysidis, A.; Lyberatos, G.; Vlyssides, A. Detoxification and methane production kinetics from three-phase olive mill wastewater using Fenton’s reagent followed by anaerobic digestion. J. Chem. Technol. Biotechnol. 2019, 94, 265–275. [Google Scholar] [CrossRef]
- Venny; Gan, S.; Ng, H.K. Current status and prospects of Fenton oxidation for the decontamination of persistent organic pollutants (POPs) in soils. Chem. Eng. J. 2012, 213, 295–317. [Google Scholar] [CrossRef]
- Suárez, M.; Macià, A.; Romero, M.P.; Motilva, M.J. Improved liquid chromatography tandem mass spectrometry method for the determination of phenolic compounds in virgin olive oil. J. Chromatogr. A 2008, 1214, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Romani, A.; Galardi, C.; Pinelli, P.; Giaccherini, C.; Vincleri, F.F. Polyphenolic content in olive oil waste waters and related olive samples. J. Agric. Food Chem. 2001, 49, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Ordinance on limit values for wastewater emissions. Nar. Novine 2020, 26. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2020_03_26_622.html (accessed on 1 February 2021).
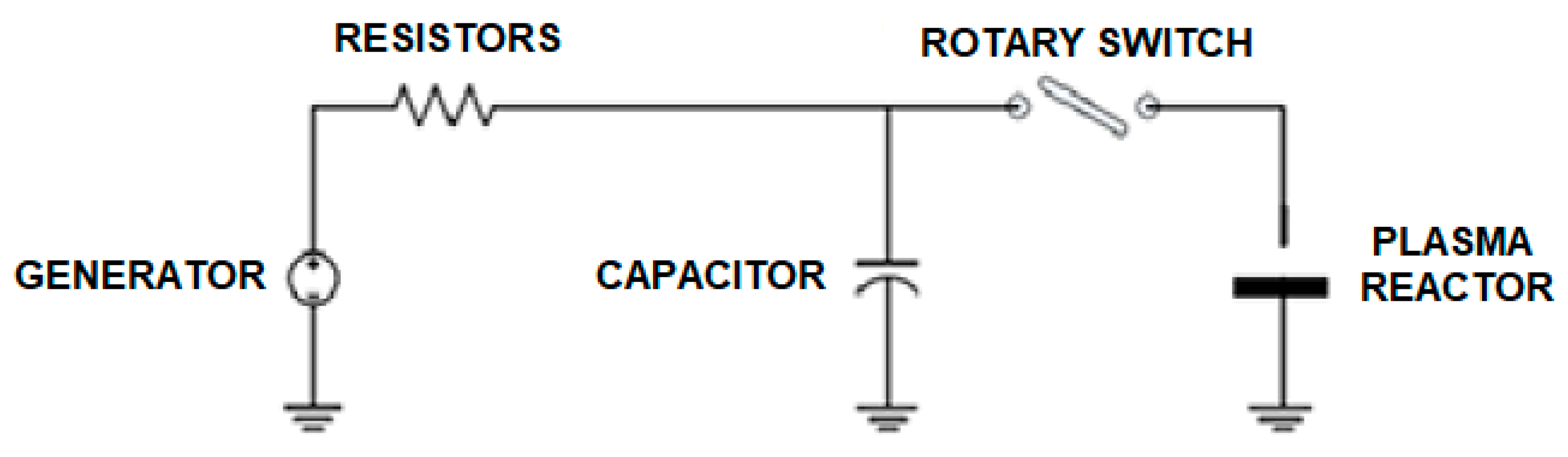
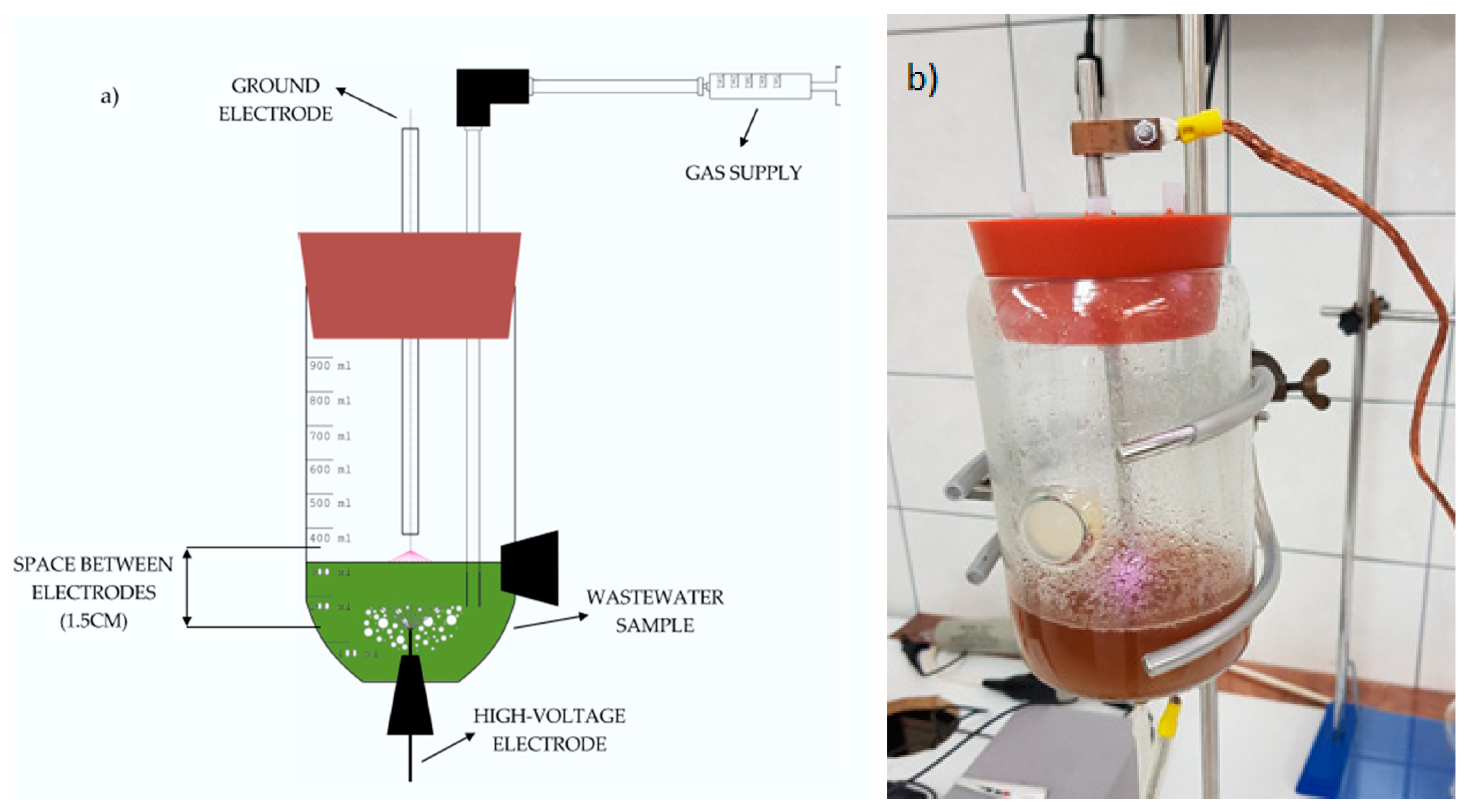
| Treatment | t (min) | pH | EC (μScm−1) | O2 (%sat) | ΔT (°C) |
|---|---|---|---|---|---|
| C | 0 | 5.04 ± 0.01 | 874.50 ± 3.54 | 29.50 ± 0.42 | / |
| A1 | 30 | 4.19 ± 0.01 | 1102.50 ± 2.12 | 10.25 ± 0.35 | 12.10 ± 0.28 |
| A2 | 30 | 4.12 ± 0.01 | 940.00 ± 2.83 | 74.70 ± 0.57 | 20.55 ± 1.34 |
| A3 | 30 | 4.61 ± 0.03 | 916.00 ± 1.42 | 280.40 ± 2.12 | 22.65 ± 0.35 |
| B1 | 30 | 4.13 ± 0.03 | 1112.00 ± 5.66 | 10.60 ± 0.85 | 14.90 ± 0.28 |
| B2 | 30 | 4.47 ± 0.04 | 944.00 ± 4.24 | 63.25 ± 0.64 | 26.95 ± 0.64 |
| B3 | 30 | 4.59 ± 0.06 | 905.00 ± 4.24 | 239.85 ± 4.31 | 25.90 ± 0.85 |
| Cad | 0 | 4.18 ± 0.01 | 949.50 ± 3.54 | 88.75 ± 0.50 | / |
| A1ad | 30 | 3.63 ± 0.02 | 1181.50 ± 0.71 | 21.95 ± 0.64 | 13.70 ± 0.28 |
| A2ad | 30 | 3.31 ± 0.04 | 1178.50 ± 0.71 | 50.00 ± 1.13 | 18.00 ± 0.14 |
| A3ad | 30 | 3.89 ± 0.09 | 1139.00 ± 2.83 | 227.40 ± 5.23 | 20.95 ± 0.64 |
| B1ad | 30 | 3.79 ± 0.01 | 1167.50 ± 2.12 | 14.60 ± 0.85 | 24.90 ± 0.57 |
| B2ad | 30 | 3.70 ± 0.01 | 1119.50 ± 0.71 | 39.20 ± 1.56 | 22.85 ± 1.06 |
| B3ad | 30 | 3.89 ± 0.01 | 1231.00 ± 5.66 | 234.05 ± 2.05 | 26.80 ± 0.21 |
| Treatment | t (min) | CODremoval (%) |
|---|---|---|
| C | 0 | - |
| A1 | 30 | 38.71 |
| A2 | 30 | 9.73 |
| A3 | 30 | 1.32 |
| B1 | 30 | 8.07 |
| B2 | 30 | 13.16 |
| B3 | 30 | 44.08 |
| Cad | 0 | - |
| A1ad | 30 | 39.80 |
| A2ad | 30 | 34.33 |
| A3ad | 30 | 37.25 |
| B1ad | 30 | 50.98 |
| B2ad | 30 | 15.69 |
| B3ad | 30 | 49.02 |
| Polyphenols (µgmL−1) | Hydroxytyrosol | Tyrosol | Oleuropein Derivatives | Vanillic acid | Secoisolariciresinoldiglocoside | Oleuropein | Pinoresinol | Total Unidentified Compounds | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||||
| C | 2.76 ± 0.09 | 8.66 ± 0.28 | 10.06 ± 0.24 | 1.06 ± 0.08 | 2.61 ± 0.34 | 0.30 ± 0.51 | 0.48 ± 0.56 | 18.87 ± 4.85 | 44.80 ± 6.96 | |
| A1 | 5.36 ± 1.20 | 8.65 ± 0.71 | 7.64 ± 0.62 | 0.83 ± 0.20 | 0.00 | 0.00 | 0.00 | 6.21 ± 2.38 | 28.69 ± 5.10 | |
| A2 | 1.91 ± 0.20 | 6.98 ± 0.53 | 7.09 ± 0.27 | 0.85 ± 0.21 | 0.00 | 0.00 | 0.62 ± 0.60 | 4.22 ± 1.03 | 21.66 ± 2.85 | |
| A3 | 0.00 | 8.09 ± 0.44 | 9.53 ± 0.33 | 1.00 ± 0.07 | 0.83 ± 0.28 | 0.00 | 0.00 | 10.50 ± 0.70 | 29.95 ± 1.83 | |
| B1 | 8.16 ± 0.44 | 10.80 ± 0.11 | 10.29 ± 0.28 | 1.26 ± 0.34 | 0.00 | 0.00 | 0.00 | 6.83 ± 0.84 | 37.34 ± 2.01 | |
| B2 | 4.62 ± 0.62 | 10.51 ± 1.30 | 9.76 ± 0.67 | 0.87 ± 0.12 | 0.00 | 0.00 | 0.00 | 6.59 ± 0.58 | 32.35 ± 3.27 | |
| B3 | 0.00 | 8.78 ± 0.84 | 9.36 ± 0.79 | 1.04 ± 0.06 | 0.88 ± 0.36 | 0.35 ± 0.61 | 0.00 | 10.01 ± 1.81 | 30.43 ± 4.47 | |
| Cad | 2.76 ± 0.09 | 8.66 ± 0.28 | 10.06 ± 0.24 | 1.06 ± 0.08 | 2.61 ± 0.34 | 0.30 ± 0.51 | 0.48 ± 0.56 | 18.87 ± 4.85 | 44.80 ± 6.96 | |
| A1ad | 2.33 ± 0.09 | 6.80 ± 0.31 | 7.34 ± 0.32 | 0.81 ± 0.03 | 0.00 | 0.00 | 0.00 | 5.23 ± 0.61 | 22.51 ± 1.36 | |
| A2ad | 0.00 | 7.45 ± 0.46 | 7.79 ± 0.17 | 0.29 ± 0.26 | 0.00 | 0.00 | 0.00 | 2.24 ± 0.19 | 17.78 ± 1.08 | |
| A3ad | 0.00 | 10.84 ± 0.50 | 9.43 ± 0.29 | 0.95 ± 0.02 | 1.43 ± 0.78 | 0.00 | 0.00 | 7.77 ± 0.70 | 30.42 ± 2.28 | |
| B1ad | 3.95 ± 0.35 | 11.08 ± 0.25 | 9.43 ± 0.46 | 0.90 ± 0.02 | 0.00 | 0.51 ± 0.44 | 0.00 | 6.18 ± 0.22 | 32.05 ± 1.75 | |
| B2ad | 1.55 ± 0.08 | 11.56 ± 0.41 | 9.50 ± 0.10 | 0.77 ± 0.08 | 0.00 | 0.00 | 0.00 | 5.99 ± 0.91 | 29.39 ± 1.57 | |
| B3ad | 0.00 | 8.59 ± 0.29 | 8.46 ± 0.22 | 0.90 ± 0.04 | 0.32 ± 0.28 | 0.00 | 0.00 | 5.80 ± 0.24 | 24.06 ± 1.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, M.; Vukušić Pavičić, T.; Kraljić, K.; Grgas, D.; Landeka Dragičević, T.; Herceg, Z. Effects of High Voltage Electrical Discharge Plasma on Olive Mill Wastewater Treatment. Sustainability 2021, 13, 1552. https://doi.org/10.3390/su13031552
Ivanov M, Vukušić Pavičić T, Kraljić K, Grgas D, Landeka Dragičević T, Herceg Z. Effects of High Voltage Electrical Discharge Plasma on Olive Mill Wastewater Treatment. Sustainability. 2021; 13(3):1552. https://doi.org/10.3390/su13031552
Chicago/Turabian StyleIvanov, Mia, Tomislava Vukušić Pavičić, Klara Kraljić, Dijana Grgas, Tibela Landeka Dragičević, and Zoran Herceg. 2021. "Effects of High Voltage Electrical Discharge Plasma on Olive Mill Wastewater Treatment" Sustainability 13, no. 3: 1552. https://doi.org/10.3390/su13031552
APA StyleIvanov, M., Vukušić Pavičić, T., Kraljić, K., Grgas, D., Landeka Dragičević, T., & Herceg, Z. (2021). Effects of High Voltage Electrical Discharge Plasma on Olive Mill Wastewater Treatment. Sustainability, 13(3), 1552. https://doi.org/10.3390/su13031552







