Development of a Self-Sustaining Wastewater Treatment with Phosphorus Recovery for Small Rural Settlements
Abstract
1. Introduction
2. Materials and Methods
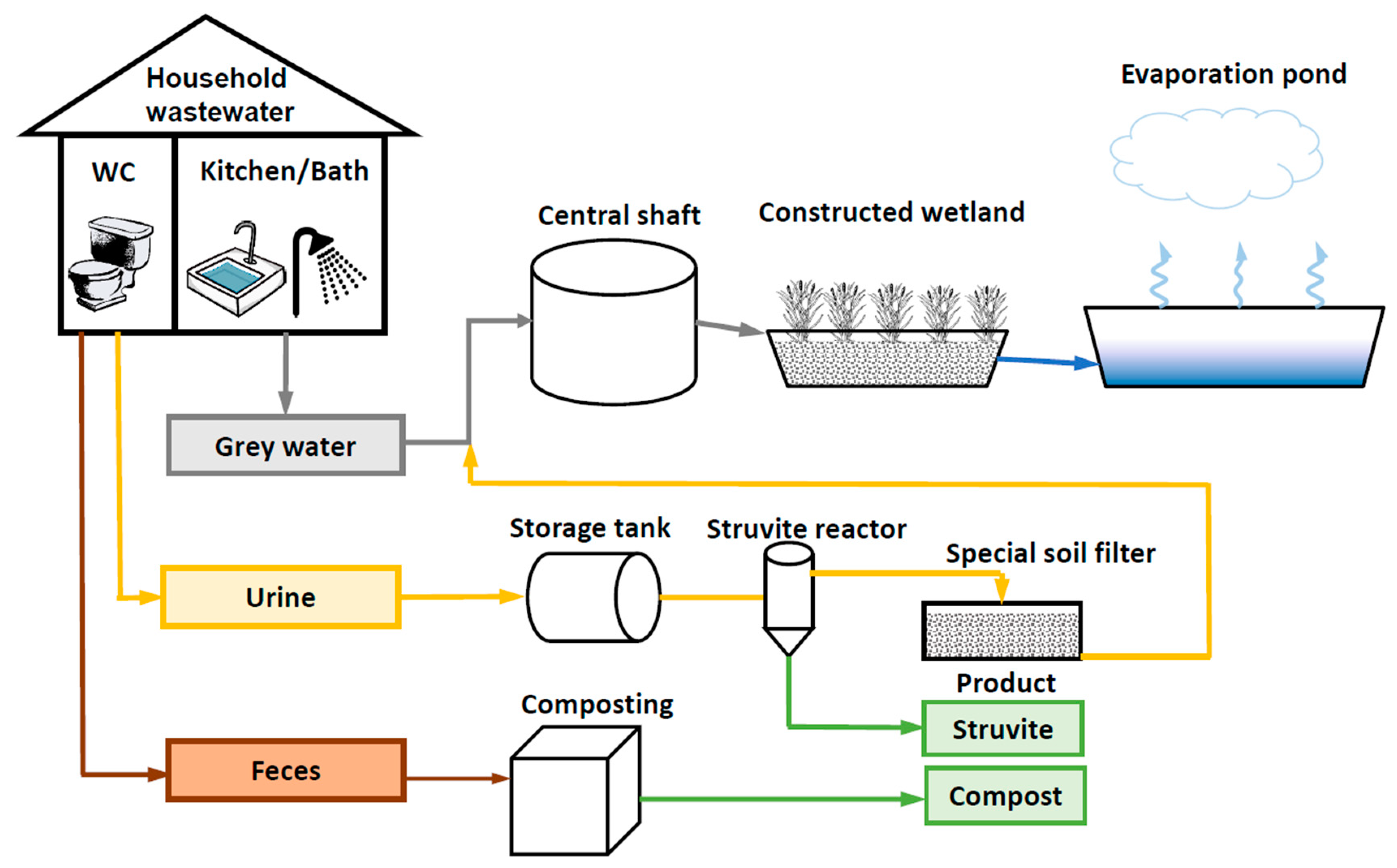
2.1. Development and Implementation of the Decentralized Wastewater Treatment and Resource Recovery System
2.2. P-Recovery by Struvite Precipitation from Stored Urine
2.3. Urine Supernatant Treatment
2.4. Analytical Methods
3. Results and Discussion
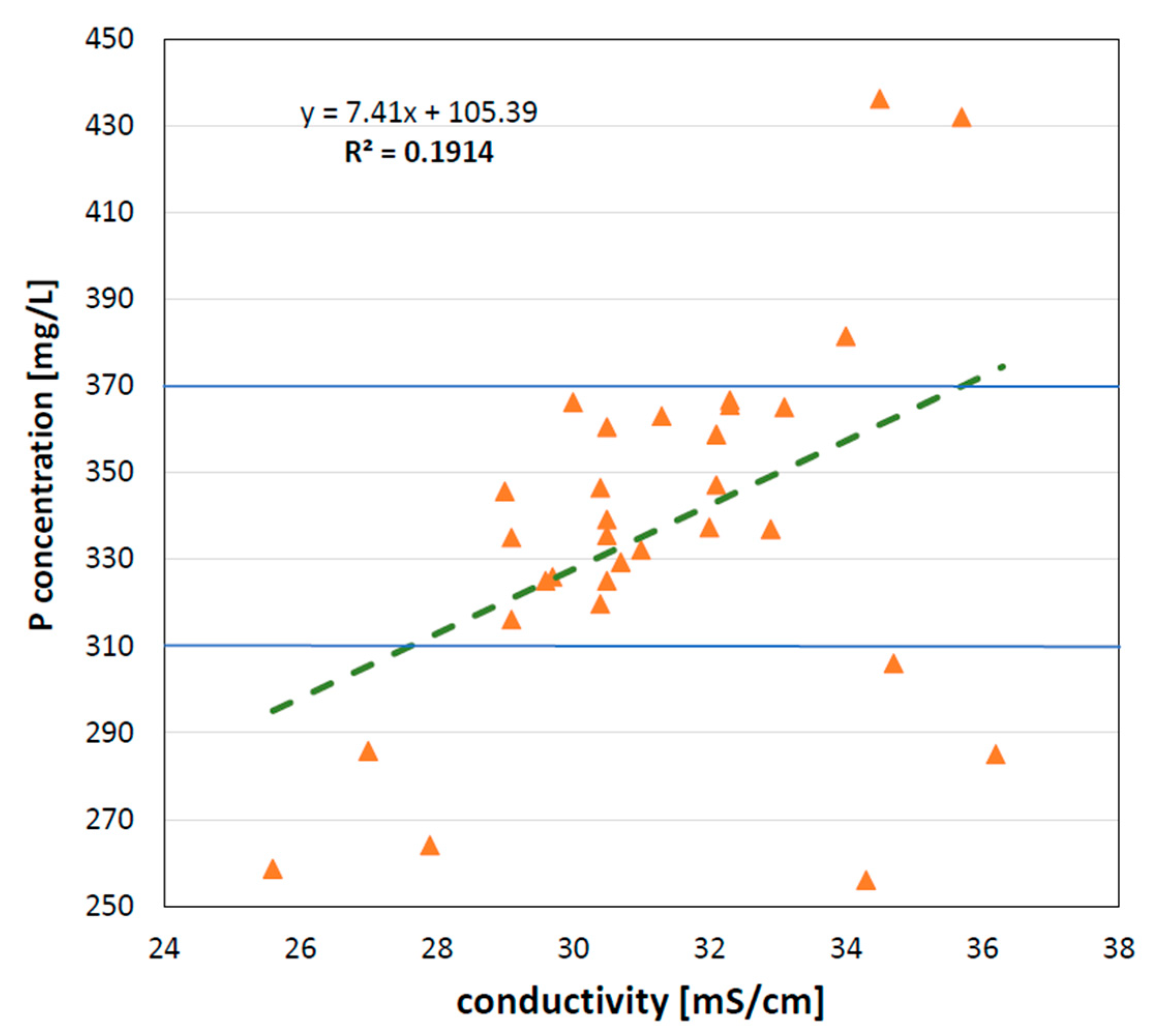
3.1. Composition of Stored Urine
3.2. Phosphorus-Recovery via Struvite Precipitation
- Selection of a light MgO, which needs to be pre-tested
- Fast addition in form of suspension
- Overdosage of MgO in the range of β = 1.3–1.5
- Stirring speed 78 rpm and stirring time 1.5 h
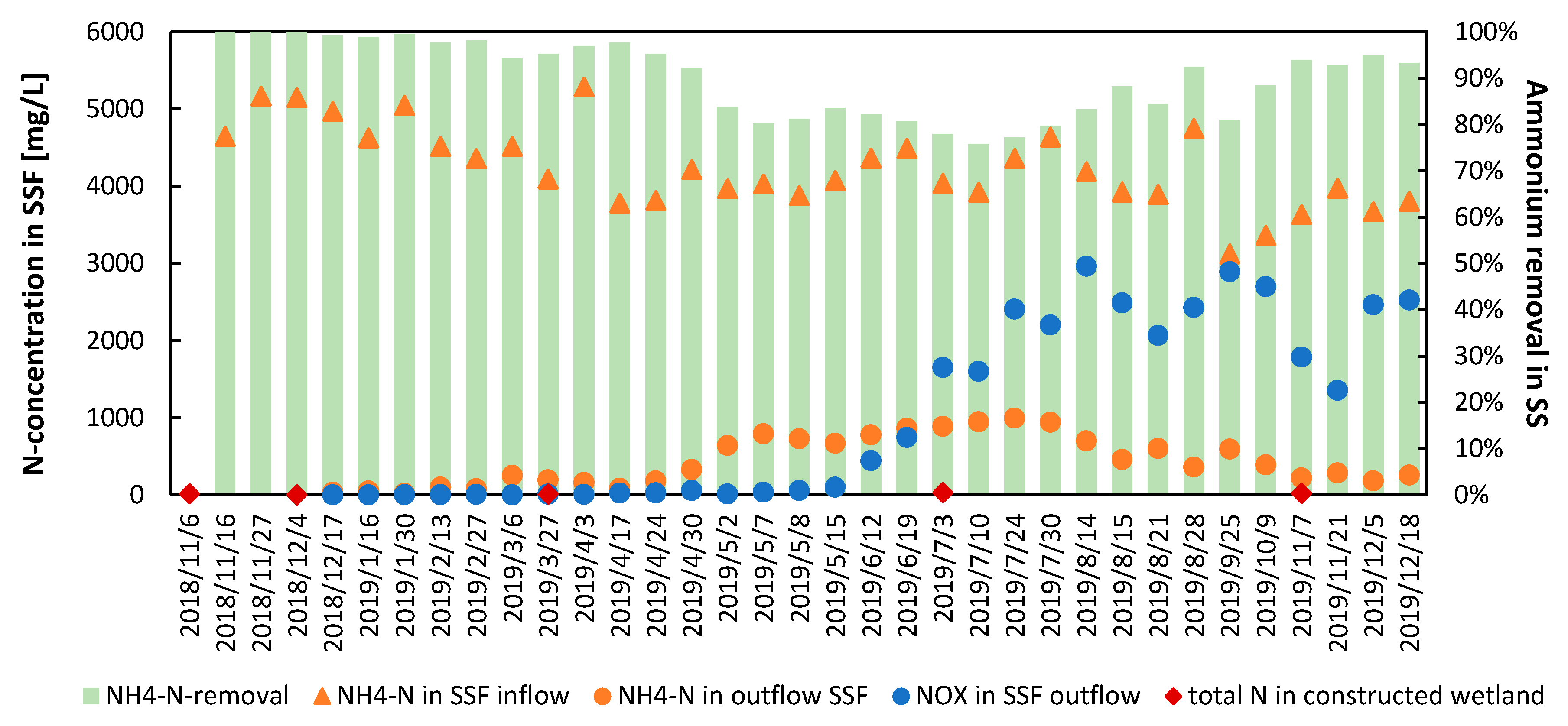
3.3. Ammonium Removal in the Special Soil Filter
3.4. Effluent of the Constructed Wetland
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller specific inner surface area (m2/g) |
| β | molar ratio Mg:P |
| Cond. | Conductivity (mS/cm) |
| Ca | calcium, concentration (mg/L) |
| K | potassium, concentration (mg/L) |
| Mg | Magnesium, concentration (mg/L) |
| MgO | Magnesium oxide |
| Na | sodium, concentration (mg/L) |
| NH4 | Ammonium |
| NH4-N-removal | Ammonium removal (%) |
| NH4-N | Ammonium-nitrogen, concentration (mg/L) |
| NO3-N | nitrate-nitrogen, concentration (mg/L) |
| NO2-N | nitrite-nitrogen, concentration (mg/L) |
| Ntot | total nitrogen, concentration (mg/L) |
| P | phosphorus |
| PO4-P | phosphate-phosphorus, concentration (mg/L) |
| Ptot | total phosphorus, concentration (mg/L) |
| ROS | resource-oriented sanitation |
| struvite | Magnesium-ammonium-phosphate, MgNH4PO4∗6H2O |
| SSF | special soil filter |
| Temp. | Temperature [°C] |
References
- Dalecha, T.; Assefa, E.; Krasteva, K.; Meinhold, K. Struvite production from source separated urine: Application and economic feasibilityin Arba Minch, Ethiopia. Sustain. Sanit. Pract. 2014, 19, 16–22. [Google Scholar]
- Morales, N.; Boehler, M.; Buettner, S.; Liebi, C.; Siegrist, H. Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale. Water 2013, 5, 1262–1278. [Google Scholar] [CrossRef]
- Etter, B.; Tilley, E.; Khadka, R.; Udert, K.M. Low-cost struvite production using source-separated urine in Nepal. Water Res. 2011, 45, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.L.; Boisen Staal, L.; Macintosh, K.A.; McGrath, J.W.; Bailey, J.; Black, L.; Gro Nielsen, U.; Reitzel, K.; Williams, P.N. Phosphorus speciation and fertiliser performance characteristics: A comparison of waste recovered struvites from global sources. Geoderma 2020, 362, 114096. [Google Scholar] [CrossRef]
- Wei, S.P.; van Rossum, F.; van de Pol, G.J.; Winkler, M.-K.H. Recovery of phosphorus and nitrogen from human urine by struvite precipitation, air stripping and acid scrubbing: A pilot study. Chemosphere 2018, 212, 1030–1037. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Gujer, W. The behaviour of pharmaceuticals and heavy metals during struvite precipitation in urine. Water Res. 2007, 41, 1859–1868. [Google Scholar] [CrossRef]
- Liu, B.; Giannis, A.; Zhang, J.; Chang, V.W.-C.; Wang, J.-Y. Characterization of induced struvite formation from source-separated urine using seawater and brine as magnesium sources. Chemosphere 2013, 93, 2738–2747. [Google Scholar] [CrossRef]
- Ray, H.; Saetta, D.; Boyer, T.H. Characterization of urea hydrolysis in fresh human urine and inhibition by chemical addition. Environ. Sci. Water Res. Technol. 2018, 4, 87–98. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Ishii, S.K.L.; Boyer, T.H. Life cycle comparison of centralized wastewater treatment and urine source separation with struvite precipitation: Focus on urine nutrient management. Water Res. 2015, 79, 88–103. [Google Scholar] [CrossRef]
- Sakthivel, S.R.; Tilley, E.; Udert, K.M. Wood ash as a magnesium source for phosphorus recovery from source-separated urine. Sci. Total Environ. 2012, 419, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M.-L. Optimization of struvite precipitation in synthetic biologically treated swine wastewater--determination of the optimal process parameters. J. Hazard. Mater. 2013, 244–245, 357–369. [Google Scholar] [CrossRef]
- Krähenbühl, M.; Etter, B.; Udert, K.M. Pretreated magnesite as a source of low-cost magnesium for producing struvite from urine in Nepal. Sci. Total Environ. 2016, 542, 1155–1161. [Google Scholar] [CrossRef]
- Winker, M.; Rieck, C. Abschlussbericht-Projekt Saniresch; Bundesministerium fur Bildung und Forschung: Bonn, Germany, 2013. [Google Scholar]
- Antonini, S.; Nguyen, P.T.; Arnold, U.; Eichert, T.; Clemens, J. Solar thermal evaporation of human urine for nitrogen and phosphorus recovery in Vietnam. Sci. Total Environ. 2012, 414, 592–599. [Google Scholar] [CrossRef]
- Antonini, S.; Paris, S.; Eichert, T.; Clemens, J. Nitrogen and Phosphorus Recovery from Human Urine by Struvite Precipitation and Air Stripping in Vietnam. Clean Soil Air Water 2011, 39, 1099–1104. [Google Scholar] [CrossRef]
- Etter, B.; Udert, K.M.; Gounden, T. Valorisation of Urine Nutrient—Promoting Sanitation & Nutrient Recovery through Urine Seperation: Final Project Report; Eawag: Dübendorf, Switzland, 2015. [Google Scholar]
- Celen, I.; Türker, M. Recovery of ammonia as struvite from anaerobic digester effluents. Environ. Technol. 2001, 22, 1263–1272. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Astals, S.; Mata-Alvarez, J.; Chimenos, J.M. Feasibility of coupling anaerobic digestion and struvite precipitation in the same reactor: Evaluation of different magnesium sources. Chem. Eng. J. 2015, 270, 542–548. [Google Scholar] [CrossRef]
- Tao, W.; Bayrakdar, A.; Wang, Y.; Agyeman, F. Three-stage treatment for nitrogen and phosphorus recovery from human urine: Hydrolysis, precipitation and vacuum stripping. J. Environ. Manag. 2019, 249, 109435. [Google Scholar] [CrossRef]
- Bruch, I.; Fritsche, J.; Bänninger, D.; Alewell, U.; Sendelov, M.; Hürlimann, H.; Hasselbach, R.; Alewell, C. Improving the treatment efficiency of constructed wetlands with zeolite-containing filter sands. Bioresour. Technol. 2011, 102, 937–941. [Google Scholar] [CrossRef]
- Bruch, I.; Alewell, U.; Hahn, A.; Hasselbach, R.; Alewell, C. Influence of soil physical parameters on removal efficiency and hydraulic conductivity of vertical flow constructed wetlands. Ecol. Eng. 2014, 68, 124–132. [Google Scholar] [CrossRef]
- Garcia, J.; Rousseau, D.P.L.; Morato, J.; Lesage, E.; Matamoros, V.; Bayona, J.M. Contaminant Removal Processes in Subsurface-Flow Constructed Wetlands: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 561–661. [Google Scholar] [CrossRef]
- De Gisi, S.; Casella, P.; Notarnicola, M.; Farina, R. Grey water in buildings: A mini-review of guidelines, technologies and case studies. Civ. Eng. Environ. Syst. 2016, 33, 35–54. [Google Scholar] [CrossRef]
- Arden, S.; Ma, X. Constructed wetlands for greywater recycle and reuse: A review. Sci Total Environ. 2018, 630, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Grundsätze für Bemessung, Bau und Betrieb von Kläranlagen mit Bepflanzten und unbepflanzten Filtern zur Reinigung Häuslichen und Kommunalen Abwassers, 2017th ed.; Deutsche Vereinigung für Wasserwirtschaft Abwasser und Abfall: Hennef, Germany, 2017.
- Niwagaba, C.; Nalubega, M.; Vinnerås, B.; Sundberg, C.; Jönsson, H. Bench-scale composting of source-separated human faeces for sanitation. Waste Manag. 2009, 29, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Simha, P.; Ganesapillai, M. Ecological Sanitation and nutrient recovery from human urine: How far have we come? A review. Sustain. Environ. Res. 2017, 27, 107–116. [Google Scholar] [CrossRef]
- Hashemi, S.; Boudaghpour, S.; Han, M. Evaluation of different natural additives effects on the composting process of source separated feces in resource-oriented sanitation systems. Ecotoxicol. Environ. Saf. 2019, 185, 109667. [Google Scholar] [CrossRef]
- Freguia, S.; Logrieco, M.; Monetti, J.; Ledezma, P.; Virdis, B.; Tsujimura, S. Self-Powered Bioelectrochemical Nutrient Recovery for Fertilizer Generation from Human Urine. Sustainability 2019, 11, 5490. [Google Scholar] [CrossRef]
- Zamora, P.; Georgieva, T.; Salcedo, I.; Elzinga, N.; Kuntke, P.; Buisman, C.J.N. Long-term operation of a pilot-scale reactor for phosphorus recovery as struvite from source-separated urine. J. Chem. Technol. Biotechnol. 2017, 92, 1035–1045. [Google Scholar] [CrossRef]
- Hug, A.; Udert, K.M. Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 2013, 47, 289–299. [Google Scholar] [CrossRef]
- Stolzenburg, P.; Capdevielle, A.; Teychené, S.; Biscans, B. Struvite precipitation with MgO as a precursor: Application to wastewater treatment. Chem. Eng. Sci. 2015, 133, 9–15. [Google Scholar] [CrossRef]
- Barbosa, S.G.; Peixoto, L.; Meulman, B.; Alves, M.M.; Pereira, M.A. A design of experiments to assess phosphorous removal and crystal properties in struvite precipitation of source separated urine using different Mg sources. Chem. Eng. J. 2016, 298, 146–153. [Google Scholar] [CrossRef]

| ExperimentSet | MgO Type | MgO Adding Strategy | Molar Ratio of Mg:P (β) | Stirring Speed rpm | Repeat |
|---|---|---|---|---|---|
| 1 | G. MgO | poud. sl. | 2.0 | 78 | 2 |
| 2 | G. MgO | susp. sl. | 2.0 | 78 | 3 |
| 3 | G. MgO | susp. fa. | 2.0 | 78 | 3 |
| 4 | G. MgO | susp. fa. | 1.5 | 78 | 4 |
| 5 | G. MgO | susp. fa. | 1.3 | 78 | 3 |
| 6 | G. MgO | susp. fa. | 1.3 | 120 | 2 |
| 7 | G. MgO | susp. fa. | 1.3 | 56 | 2 |
| 8 | R. MgO | susp. fa. | 2.0 | 78 | 2 |
| 9 | W. MgO | susp. fa. | 2.0 | 78 | 2 |
| 10 | W. MgO | susp. fa. | 1.3 | 78 | 2 |
| 11 | W. MgO | susp. fa. | 1.0 | 78 | 2 |
| Temp. | Cond. | pH | PO4-P | Ptot | NH4-N | Ntot | Mg2+ | Ca2+ | K+ | Na+ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | mS/cm | - | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | Mg/L | ||
| In this study | average | 13.2 | 31.3 | 9.2 | 335 | 382 | 4337 | 4833 | 1.1 | 8.5 | 1576 | 1764 |
| min. | 6.8 | 25.6 | 9.0 | 264 | 266 | 3433 | 3525 | 0.0 | 4.9 | 1367 | 1457 | |
| max. | 19.0 | 36.3 | 9.4 | 457 | 484 | 5365 | 5855 | 2.8 | 18.8 | 1856 | 2026 | |
| median | 12.0 | 30.7 | 9.2 | 340 | 379 | 4243 | 4798 | 0.0 | 8.1 | 1561 | 1757 | |
| Dalecha et al. [1] a | 28.8 | 8.6 | 293 | |||||||||
| Etter et al. [3] (n = 14) | 25.9 | 9.0 | 195 | |||||||||
| Wei et al. [5] a | 8.9 | 384 | 5615 | <50 | 1166 | 2300 | ||||||
| Sakthivel et al. [11] b | 25.0 | 8.8 | 187 | 2720 | 1.8 | 17.7 | 1330 | 1670 | ||||
| Freguia et al. [30] a | 45.0 | 8.8 | 330 | 7800 | 10.0 | 2400 | 2900 | |||||
| Zamora et al. [31] a | 9.3 | 223 | 3700 | 0.9 | 17.0 | 1900 | 2400 | |||||
| Hug und Udert [32] (n = 9) | 25.0 | 8.9 | 197 | 2540 | 1.6 | 16.5 | 1980 | |||||
| Concentration of PO4-P in Stored Urine (Mg/L) in 28 Samples (See Table 1) | Mg:P Ratio in Dependence of the Mass of MgO Per Filling with 160 L Urine | |||||
|---|---|---|---|---|---|---|
| 80 g | 90 g | 100 g | 110 g | 120 g | ||
| min. | 256 | 1.5 | 1.7 | 1.9 | 2.1 | 2.3 |
| 70%-lower | 310 | 1.3 | 1.4 | 1.6 | 1.7 | 2.0 |
| average | 335 | 1.2 | 1.3 | 1.4 | 1.6 | 1.7 |
| median | 340 | 1.2 | 1.3 | 1.4 | 1.6 | 1.7 |
| 70%-upper | 370 | 1.0 | 1.2 | 1.3 | 1.4 | 1,6 |
| max. | 436 | 0.9 | 1.1 | 1.2 | 1.2 | 1.3 |
| Date | Ntot | NH4-N | NO3-N | Ptot |
|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | |
| 2018-11-06 * | 15.1 | 3.10 | <0.06 | 0.70 |
| 2018-12-04 | 3.1 | 0.10 | <0.06 | 0.44 |
| 2019-03-27 | 13.6 | 0.77 | <0.06 | 0.10 |
| 2019-07-03 | 34.0 | 0.74 | 31.76 | 0.08 |
| 2019-11-07 | 20.9 | 0.08 | 20.20 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Alewell, U.; Bruch, I.; Steinmetz, H. Development of a Self-Sustaining Wastewater Treatment with Phosphorus Recovery for Small Rural Settlements. Sustainability 2021, 13, 1363. https://doi.org/10.3390/su13031363
Xiao J, Alewell U, Bruch I, Steinmetz H. Development of a Self-Sustaining Wastewater Treatment with Phosphorus Recovery for Small Rural Settlements. Sustainability. 2021; 13(3):1363. https://doi.org/10.3390/su13031363
Chicago/Turabian StyleXiao, Jingsi, Ulrike Alewell, Ingo Bruch, and Heidrun Steinmetz. 2021. "Development of a Self-Sustaining Wastewater Treatment with Phosphorus Recovery for Small Rural Settlements" Sustainability 13, no. 3: 1363. https://doi.org/10.3390/su13031363
APA StyleXiao, J., Alewell, U., Bruch, I., & Steinmetz, H. (2021). Development of a Self-Sustaining Wastewater Treatment with Phosphorus Recovery for Small Rural Settlements. Sustainability, 13(3), 1363. https://doi.org/10.3390/su13031363








