1. Introduction
Over the past few centuries, significant amounts of carbon in the form of CO
2, stored for millions of years in the form of deposits of coal, oil, and gas, have been returned to the Earth’s atmosphere. This has disrupted the existing balance of CO
2 and the circulation of carbon in the atmosphere, ultimately affecting the Earth’s climate and threatening the existence of life on the planet. Industrial plants and transport in the world emit about 37,900 million tons per year of products from the combustion of carbon fuels in the form of CO
2 [
1].
The growing need for energy has caused the development of new environmentally safe technologies, as well as innovative ways of obtaining energy, which would not damage the ecosystems of the Earth. It is believed that the most promising in the environmental aspect is hydrogen energy, which can be successfully used in industry, transport, and power generation.
A number of “dirty” technological solutions for obtaining hydrogen in industry are known, such as the steam conversion of methane and natural gas; the gasification of coal; the pyrolysis of liquid and combustible fossils; and the decomposition of liquid and gaseous hydrocarbons and solid domestic waste [
2].
“Green technology “refers to the obtaining of hydrogen from water; for example, it is typical to obtain hydrogen from sea water or sewage using algae [
3]. Scientists at the University of California at Berkeley found an active production of hydrogen by algae, such as
Chlamydomonas reinhardtii, in the absence of oxygen and sulfur.
The most efficient technology for producing pure hydrogen can be realized by the electrolysis of water from aqueous solutions. Under the action of direct electric current and voltage water decomposes into oxygen and hydrogen. As a result of the decomposition of water molecules, twice as much hydrogen as oxygen is obtained by volume. Water electrolysis is mainly performed in water-alkali electrolyzers and with a solid polymer electrolyte (SPE). Water-alkali electrolyzers operate at current densities of 0.2–0.3 A/cm
2 and require 4.1 to 4.5 kWh/nm
3 of energy to produce hydrogen. Electrolyzers with SPE are characterized by high current density (up to 3 A/cm
2) and low power consumption (up to 3.6–3.9 kWh/m
3 at 1 A/cm
2), while achieving the degree of hydrogen purity (over 99.99%) [
4,
5].
A significant disadvantage of obtaining hydrogen by electrolysis can be attributed to the fact that implementing of electrolysis requires electricity, which is produced by thermal power plants operating on carbon and hydrocarbon fuels. However, this problem can be partially solved by using electricity produced at nuclear power plants, which has a lesser environmental impact.
Photosynthesis is considered to be one of the most promising applications for cheap and clean energy. Photosynthesis involves the oxidation of water and the production of molecular oxygen and hydrogen as by-products. It is known that the combustion of hydrogen produces an absolutely harmless product, as water and energy are released, exceeding the energy produced by the combustion of an equivalent amount of hydrocarbons. The specific heat of the combustion of hydrogen is 120 MJ/kg, which exceeds several times the specific heat of combustion of hydrocarbon fuels; for methane, for example, which is about 50 MJ/kg [
6]. The widespread introduction of hydrogen energy will improve the planet’s ecology and solve the problem of greenhouse gas emissions into the atmosphere, thus stopping global warming.
Photosynthesis is the main process which enables the emergence of life on Earth, and it is astonishing in its scale. Plants absorb about 467 trillion kilowatt hours of solar energy in photosynthesis throughout the year [
7]. This is almost 172 times the world’s energy production in 2020. Plants bind 37.8–109 m
3 of water and recycle 79–1012 m
3 of carbon dioxide per year, releasing 47–1012 m
3 of oxygen into the atmosphere [
7]. Data on the quantity of hydrogen emitted into the atmosphere is not given due to the fact that hydrogen ions take part in the reduction processes, as well as in formation of hydrocarbons.
Currently, studies are actively carried out to reconstruct photosynthesis in laboratory conditions, in order to obtain cheap hydrogen, using only the energy of the Sun and water. It is important to note that photosynthesis proceeds with high efficiency with efficiencies close to 100%. Thus, by studying the processes of photosynthesis it will be possible to create a cost-effective production of hydrogen using photosynthetic methods.
The aim of this article is to consider the energy (light) phase of photosynthesis based on the analysis of the X-ray structure data of photosystems I and II (PS-I and PS-II), to describe the photo-electrocatalytic processes and mechanisms occurring in the chloroplasts of cyanobacteria and plants using, for this purpose, modern concepts of semiconductors and biological semiconductor structures.
In accordance with the stated aim, we highlight a number of the article’s provisions, which have an absolute novelty. The formation of liquid crystal semiconductor structure of biological membranes formed by lipids and carotenoids providing the energy and electron transfer was considered. It is shown that chlorophyll magnesium can form a chelate complex with oxygen atoms of xanthophylls, such as astaxanthin, while maintaining the system of electrically conductive conjugated bonds. It is noted in the article that biomolecules: lipids, carotenoids, coenzymes, chlorophylls, pheophytin, and heme containing metal ions, are able to form a CTC among themselves, forming a semiconductor structure. The photoelectrocatalytic mechanism of water oxidation with the formation of oxygen, protons, and electrons on the Mn4CaO5 cluster of photosystem II (PS-II), was developed based on the published X-ray structure data. It is shown that the electrons generated by PS-II via a chain of carriers are delivered into the reaction center P 700 of PS-I formed by a pair of chlorophylls, which act as electrodes (cathode and anode), forming a two-electrode electrolytic system. The general scheme of water oxidation realized in two-electrode electrolysis system P 700 PS-I is developed. A logistic chain of processes flowing in PS-I and PS-II from the viewpoint of modern semiconductor microelectronics is described.
1.1. Biological Semiconductors
Substances with a specific electrical conductivity of 10
−4 to 10
10 ohm·cm are referred to semiconductors. Low (a million times lower) concentration of electrons in semiconductors compared to metals determines the peculiarities of their electro photophysical behavior. Semiconductor properties of biological compounds are caused by conjugated bonds in their structure. Examples of semiconductor structure are chlorophyll and carotene molecules. In these compounds, the electron does not belong to any single atom but is delocalized in the whole structure of the molecule. Electron delocalization results in the splitting of the upper and lower energy levels of the monomeric link and the formation of bands whose width increases with the number of links in the conjugated segment. As a rule, the length of delocalization π- electrons corresponds to 5–7 monomeric links [
8,
9].
Electron transfer in biological semiconductor structures can be described within the framework of the theory of band conduction. According to this theory, when exposed to light photons, electrons from the lowest unoccupied molecular orbital (LUMO) move to the highest occupied molecular orbital (HOMO) with a higher energy level. The energy difference between LUMO and HOMO is called the optical gap or forbidden zone. As a rule, the band gap in semiconductor structures with conjugated bonding system is 1.3–2.5 eV [
10].
It is important to note that increasing the number of links in the hydrocarbon chain does not lead to the disappearance of the forbidden zone and conversion of biomolecules to metal, since the optical gap in biomolecules with a conjugated bonding system is narrower and their absorption maximum is not in the UV, but in the visible spectrum, as it happens in nature.
We conducted studies on the formation of CTC in β-carotene and lipid-cardiolipin systems. The formation of CTCs between β-carotene and lipid-cardiolipin was studied. No absorption bands in the visible region of the spectrum were detected for cardiolipin, whereas β -carotene has characteristic bands at 425, 450, and 475 nm. A significant broadening (up to 100 nm) of the β-carotene absorption band into the long-wavelength region is observed in the formation of the cardiolipin-β-carotene complex. A slight shift of the absorption bands to the longer wavelength region up to 480 nm versus the initial length of 450 nm is observed for the β-carotene-lecithin complex.
According to infrared spectra data, the indications of intermolecular interaction and CTC formation are as follows: the shift of absorption bands, change of their shape, appearance of new bands, and a redistribution of their intensities from spectra of individual compounds to mixtures. On the basis of the obtained spectra conclusions were made about the interaction of β-carotene with lecithin.
1.2. Natural Compounds with Semiconducting Properties
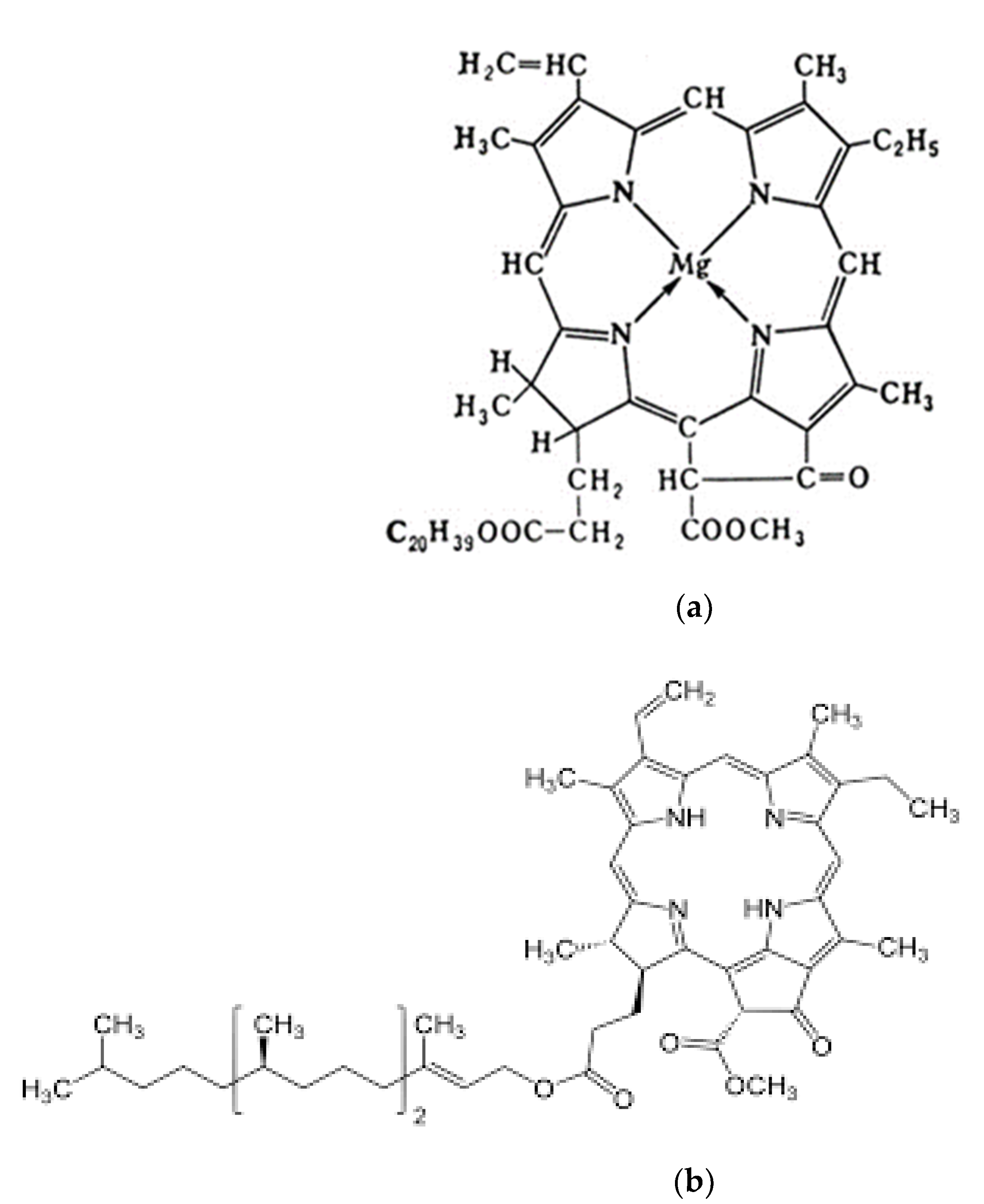
The semiconducting molecular structure of chlorophyll has a flat porphyrin ring, in the center of which there is a Mg
2+ atom ion, which has a coordination number of 6. The flat porphyrin ring structure is formed by a system of conjugated bonds formed by carbon and nitrogen atoms (
Figure 1a). Chlorophyll electrons are uniformly distributed along the “periphery” of the porphyrin ring to form an overall closed 18 π-electron cloud with an excess of π-electrons [
11].
Chlorophyll electrons are “delocalized” and migrate within the ring structure. By absorbing photons, chlorophyll electrons become excited and the dipole electric moment of the molecule is in the plane of the porphyrin ring. Chlorophyll molecule has a hydrophobic phyton chain of 20 hydrocarbon atoms, through which it is fixed in the liquid crystal structure of the biological membrane. The maxima of chlorophyll in absorption bands lie in the 440 and 700 nm wavelength regions, and chlorophyll b in the 460 and 660 nm regions [
11].
In the 450–550 nm range, when the maximum intensity of sunlight is reached, the minimum absorption of light by chlorophyll molecules is observed. Chlorophylls act as light-collecting molecules for electron and energy transfer in cyanobacteria and plant cells. In addition, they form two-electrode electrolysis systems in PS-I and PS-II involved in water oxidation.
The semiconductor pheophytin a is similar to the molecular structure of chlorophyll a, but lacking the Mg
2+ ion (
Figure 1b). Pheophytin is the initial electron carrier in PS II from P680 to plastoquinone. All properties of chlorophyll a are characteristic of pheophytin a. In photosynthesis, pheophytin functions in a complex with chlorophyll and forms a single electron cloud of π-electrons during the interaction of two flat porphyrin rings. It is considered that chlorophyll and pheophytin molecules can exchange magnesium ion, providing efficient electron transfer from the PS-II cluster (electron source) to the PS-I and PS-II electrolysis systems, acting as a semiconductor current amplifier.
Carotenoids. Natural semiconductors include a large class of carotene compounds (over 600). Two groups of carotenoids (
Figure 1c) form carotenoids: β-carotenes and oxygen-containing carotenes-xanthophylls. Carotenes have long chains of conjugated bonds. There are two cyclohexane rings at the two opposite ends of the β-carotene molecule. Carotenes devoid of cyclohexane rings are commonly referred to as aliphatic or acyclic. Carotenoids generally contain 40 carbon atoms. Carotenoids are obligatory structural components of photosynthetic and mitochondrial membranes. Having a conjugated bonding system, carotenoids exhibit semiconductor properties, forming a common π-electron cloud along the entire hydrocarbon chain, providing delocalization of π electrons throughout the conjugated chain of the molecule.
Carotenoids differ in the configuration of their molecular structures as electron carriers. Carotenes that have one or two cyclohexane rings are capable of forming CTCs in the molecular structures of cells. Carotenes, without cyclohexane rings, stabilize specialized cell bio-membranes by pulling together their two monolayers through hydrophobic binding forces. Thus, an electrically conductive semiconducting liquid-crystalline lipid-carotenoid structure of biological membranes is formed. This design of biological membrane enables energy and charge migration to lipids with ethylene bonding system. The interaction of carotenes and lipids is accompanied by the formation of CRP and the transport of energy and charges along the biological membrane to the biocatalyst active center. In addition, carotenoids can form complex compounds with metals such as Ca
2+, Cu
2+, Pb
2+, Zn
2+, Cd
2+, and Hg
2+ [
12].
Plastoquinone (ubiquinone). Natural semiconductors also include bioorganic compounds that have ethylene (isoprenyl) groups in their structure (
Figure 1d). Electron transfer in such compounds occurs due to the helical conjugation of ethylene groups. As a result of the twisting of ethylene groups, the double bonds arranged opposite each other form π-π bonds, forming a single electron cloud with delocalized π-electrons. The existence of helical conjugation was established by spectrophotometric studies. Virtanen J.A. and Kinnunen P.K.J. showed that the absorption shift towards long wavelengths occurs with increasing number of double bonds, and the stronger the greater the conjugation of polyenes [
13]. Some lipids containing in their structure alternating ethylene fragments have vertical electronic conductivity, they include lipid-dolichol, which has spiral conjugation.
Thus, plastoquinones involved in photosynthesis, as well as its analogue ubiquinone localized in mitochondria, belong to semiconductors. Plastoquinone is 2,3-dimethyl-1,4-benzoquinone, the molecule includes nine isoprene fragments. Plastoquinone participates in the electron transfer chain in the energetic phase of photosynthesis. In photosynthesis, plastoquinone accepts two protons from the chloroplast stroma and two electrons from PS-II. The protons are then transferred to the inner thylakoid cavity, and the electrons are transferred to a protein complex formed by cytochromes, cytochrome b6f. The plastoquinone molecule is capable of regeneration, alternating oxidation and reduction. Considering the functional role of plastoquinone in the cell from the perspective of modern microelectronics, we can call it a photodiode involved in the process of photosynthesis.
Phylloquinone, menaquinone (vitamin K2), a similar function of the photodiode in PS-I is, performed by phylloquinone, which occurs in nature in the form of a number of semiconductor structures (
Figure 1e). Nine variants of vitamin K2 are known, differing in the number of isoprenyl groups in their side chains. Long-chain menaquinones have up to 13 ethylene groups. Phylloquinone is one of the key cofactors in the electron transfer chain in PS-I. Through helical conjugation, phylloquinones transport electrons along the hydrocarbon chain. Phylloquinones are present in almost all complexes that perform light-dependent charge separation [
14].
Lipids. Molecular structures capable of energy and charge transfer in complex with aliphatic carotenoids can also include a number of lipids with fragments of ethylene groups in their hydrocarbon chain. Moreover, the number of double bonds in the lipid structure can vary from 1 to 6 depending on the fatty acids forming the lipid molecules.
1.3. Energy and Electron Migration in Natural Semiconductors
When natural semiconductors are excited by light with wavelengths in the visible range of the spectrum, molecular excitons are formed, which are a bound state of an electron (on LUMO) and a hole (on HOMO). The exciton at the beginning of its formation has a delocalization length of about 14 carbon atoms [
15]. Then its length decreases to 5–7 units in time on the order of 0.1 ps [
16]. Excitons in natural semiconductors can move (migrate) along the chain over a distance exceeding their delocalization length [
16,
17,
18,
19] or migrate from one chain to another over a distance of up to 10–20 nm [
20,
21]. During migration, when reaching the interface between the donor and acceptor (donor-acceptor interface), the charge transfer from the donor to the acceptor occurs due to the difference in the energies of the LUMOs. Thus, the separation (dissociation) of the exciton into an electron and a hole takes place. As a result, the electron in the acceptor phase and the hole in the donor phase leave the interface and participate in redox enzymatic reactions implemented in biological membranes.
The formation of the complex with charge transfer (CTC) of donor and acceptor:
donor D
− acquires positive charge D
+ while acceptor molecule A
+ receives electron from donor and becomes anion A
− and turns into potential electron donor etc.
CTC is an intermolecular donor-acceptor complex, in the ground state of which a part of the electron density of the donor molecule is transferred to the acceptor molecule. CTC is usually formed due to the interaction between the donor HOMO and the acceptor HOMO [
22]. In this case, the donor HOMO makes the main contribution to HOMO CTC, and the acceptor HOMO makes the main contribution to HOMO CTC. Part of the electron density transfer from the donor to the acceptor turns out to be energetically advantageous and is accompanied by the stabilization of the complex.
According to the Mulliken theory, the degree of charge transfer is largely determined by the ionization potential of the donor D, and the electronic affinity of the acceptor A, or more precisely, their difference:
The smaller this difference, the more stable is the state of the ion-radical pair, and hence, the greater is the charge transfer in the ground state. Charge transfer from the donor to the acceptor occurs during CTC excitation, the frequency of the ν transition with charge transfer can be represented by Equation (3) [
23].
where C is a constant determined by the degree of charge transfer (depends on the overlap of the donor and acceptor wave functions) of the Coulomb interaction of positive and negative charges in the CTC, as well as the reorganization energies; h is Planck’s constant.
Thus, molecular CTCs with semiconducting properties include a combination of electron donor D and electron acceptor A molecules:
where δ is the degree of charge transfer (0 < δ < 1).
Full charge transfer from a donor to an acceptor molecule produces ion-radical salts A
+D
−, which can possess semiconductor properties. Studies conducted over the last 10 years have shown that in some mixtures between the donor and acceptor, CTC can be formed in the ground electron state without the need for photoinduction [
23,
24,
25,
26,
27].
A group of bioorganic compounds that do not exhibit the properties of semiconductors, being in a contact with other biomolecules, self-organizes, forming semiconductors. For example, lipids containing 1 to 6 double bonds in hydrocarbon chains can form CTCs with carotenoids.
Metal-organic complexes with a metal ion in the center exhibit semiconducting properties. They form intermolecular bonds by overlapping d-d or d-π-electrons. Examples of such complexes are semiconductor structures formed by chlorophyll and carotene. The magnesium atom ion Mg
2+ located in the center of the porphyrin structure of chlorophyll with coordination number 6 forms a chelate complex with xanthophylls such as astaxanthin, lutein, neoxanthin and violaxanthin. It was found that carotenoids in cell structures are in dynamic contact with other biomolecules, forming complexes with energy and charge transfer, the consequence of which is considerable expansion of the carotenoid absorption band. Carotenoid complexes in BM show high stability. Spectrophotometric studies [
28,
29] showed the possibility of formation of CTC for a mixture of β-carotene with quinone (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) and the appearance of an intense absorption band of β-carotene in the 1030 nm region. Thus, carotenoids are capable of forming CTC. That is, the transfer of energy during photosynthesis is carried out from the chlorophyll to the carotinoids, and then through the lipid-carotinoid semiconductor structure to the biocatalyst active centre via the carotene-coenzyme complex. This does not violate Förster’s rule of energy transfer from the pigment with the shorter wavelength to the pigment with the longer wavelength at all.
Energy and charge transfer is possible in macromolecular biological units containing a complex of electrophotoactive molecules, whose intermolecular interaction forms a semiconducting structure.
Thus, in [
30,
31] the structure of the internal antenna of PS-Ι of the thermophilic cyanobacterium Synechococcus elongatus is given, showing the localization of electrophotoactive molecules in the absence of a protein component. The spatial orientation of chlorophylls (blue) and carotenoids (orange) were determined from X-ray studies performed at 2.5 Å resolution [
30,
31]. The crystal structure of PS-Ι has been shown to consist of 12 protein subunits of 127 cofactors, including: 96 chlorophyll molecules, 22 carotenoids, 2 phylloquinones, 4 lipids, 3 iron-sulfur clusters Fe
4S
4, Ca
2+ ion, in addition the structure contains 201 water molecules.
It should be noted that in living objects, in contrast to nonliving ones, there is a spatial structural organization of water, accompanied by the formation of fractal crystals, which are formed on hydrophilic segments of biopolymers and bioorganic compounds which exhibit affinity to water. According to [
32] to maintain the existence of water fractal crystals the formed excitons do not give all the energy to water medium, but only a small part of it. In this case most part of energy by means of fractal crystals-antennas is re-radiated to neighbour molecules and biopolymers. The distance of radiation-free energy transfer in aqueous solutions exceeds considerably the energy migration in non-aqueous media. It is established that the energy transferred to other molecules changes their energy balance and causes resonance excitation of functional groups of biomolecules, which causes their conformational changes [
32].
It should be taken into consideration that electro-photoactive molecules surround amino acid residues of protein subunits, with their own system of π-electron bonds. Interacting with each other, the molecules form a single semiconductor structure of PS-Ι, providing high efficiency of solar energy capture and electron transfer in the biocatalyst structure.
3. Conclusions
Disruption of the existing carbon cycle as well as the balance of CO2 in the atmosphere can lead to significant climate change and threaten the existence of life on Earth. To prevent the development of an irreversible process associated with the greenhouse effect and the consequent climate warming, new environmentally friendly technologies in the energy production sector are being actively developed. The production of hydrogen by photosynthesis is being considered as an alternative to chemical and electrochemical technologies for obtaining energy. Obtaining fuel-hydrogen using renewable energy from the sun and water-is a clean technology. In addition, the combustion of hydrogen produces an environmentally friendly product-water. In order to create an industrial production of hydrogen from water, functioning on the principle of photosynthesis, it is necessary to know the mechanisms and processes taking place in chloroplasts of cyanobacteria and plants.
As is known, bioenergetic processes of cells proceed in chloroplasts biological membranes, which are two hydrophilic surfaces separated by a hydrophobic liquid-crystalline zone. In addition to lipids, pigments, cofactors, enzymes and a number of other bioorganic compounds are embedded in the BM structure. BMs containing carotenoids are a semiconductor, highly ordered environment where biophysical, biochemical, and photosynthetic processes take place.
Charge transfer in BM containing carotenoids occurs by the donor-acceptor mechanism with the formation of CTC. Energy and electron transfer in the semiconductor structure of chloroplast membranes can be described within the framework of the generally accepted theory of band conduction. CTCs are formed due to the interaction of the upper filled molecular orbital of the donor with the lower vacant molecular orbital of the acceptor. The lipid-carotenoid structure of the BM provides spatial separation of the oxidant pasone (hole) (e+) and reductant electron (e−) charges, which is possible during light excitation of the semiconductor structure. The charge transfer is accompanied by exciton migration of energy through the semiconductor structure of the BM. It is shown that excitons can traverse distances from 10 to 20 nm. in time of 10−14 s. The high rate of exciton migration allows energy losses to be minimized.
An important role in redox reactions of the cell is played by ions Mg2+, Ca2+, Fe2+, Zn2+, Mn2+, Co2+, Ni2+, Cu2+, which form CTC with xanthophylls, such as astaxanthin and lutein. In the porphyrin system, the Mg2+ ion of chlorophyll forms a chelate complex with the oxygen atoms of astaxanthin and lutein, while maintaining a conjugated bonding system, providing efficient energy and electron migration along the semiconductor structure of BM.
Experimental studies and literature data showed that a large group of biomolecules exhibiting semiconducting properties is capable of forming BMPs between themselves: lipids, carotenoids, coenzymes, chlorophylls, pheophytin, heme containing metal ions, plastoquinone and some amino acid residues of proteins and enzymes. This found its manifestation in photosynthetic processes related to the generation of H+, O2↑, e− ions from water. Initially, the photoenzymatic reaction produces hydrogen peroxide from water, which is oxidized in the active center of PS-II on the Mn4CaO5 cluster to form O2↑, H+, e−. Mn4+ is reduced to Mn2+ and then oxidized to Mn4+ with the transfer of reducing equivalents of PS-I. The electrons generated by PS II are delivered to PS-I P 700 via the carrier chain, where the electrolysis of water proceeds with the formation of gaseous oxygen and hydrogen.
Using the literature data on the three-dimensional structure of the thermophilic cyanobacterium Synechococcus elongatus and the analysis of its photosynthetic apparatus, we can conclude that the reaction center of PS-I is formed from a pair of Chl1, which represent a two-electrode electrolytic system. In a biological electrolyzer, the role of electrodes-cathode and anode-is performed by pairs of Chl1, Chl2, and Chl3 molecules, which directly take part in the electrolysis of water. When two chlorophyll molecules come in contact, the Chl1 pair in the electrolytic system forms a common electron cloud, i.e., overlapping of the double electric layers of the two electrodes is observed, which leads to a significant decrease in resistance between the two electrodes and the appearance of the strongest electric field that improves water electrolysis. In nanoelectrode systems, when the distance between the two electrodes is less than 220 nm, electrolyte-free pure water electrolysis is possible. The effect of water electrolysis increases significantly with increasing temperature and exposure to solar energy. To efficiently harvest solar energy, chloroplast thylakoids are arranged in granules, which are linked by lamellae into a single energy network that provides energy to PS I and PS II. The above-described processes implemented in PS-I and PS-II can be explained on the basis of basic concepts accepted in modern microelectronics.