Invasive Water Hyacinth Limits Globally Threatened Waterbird Abundance and Diversity at Lake Cluster of Pokhara Valley, Nepal
Abstract
:1. Introduction
2. Materials and Methods
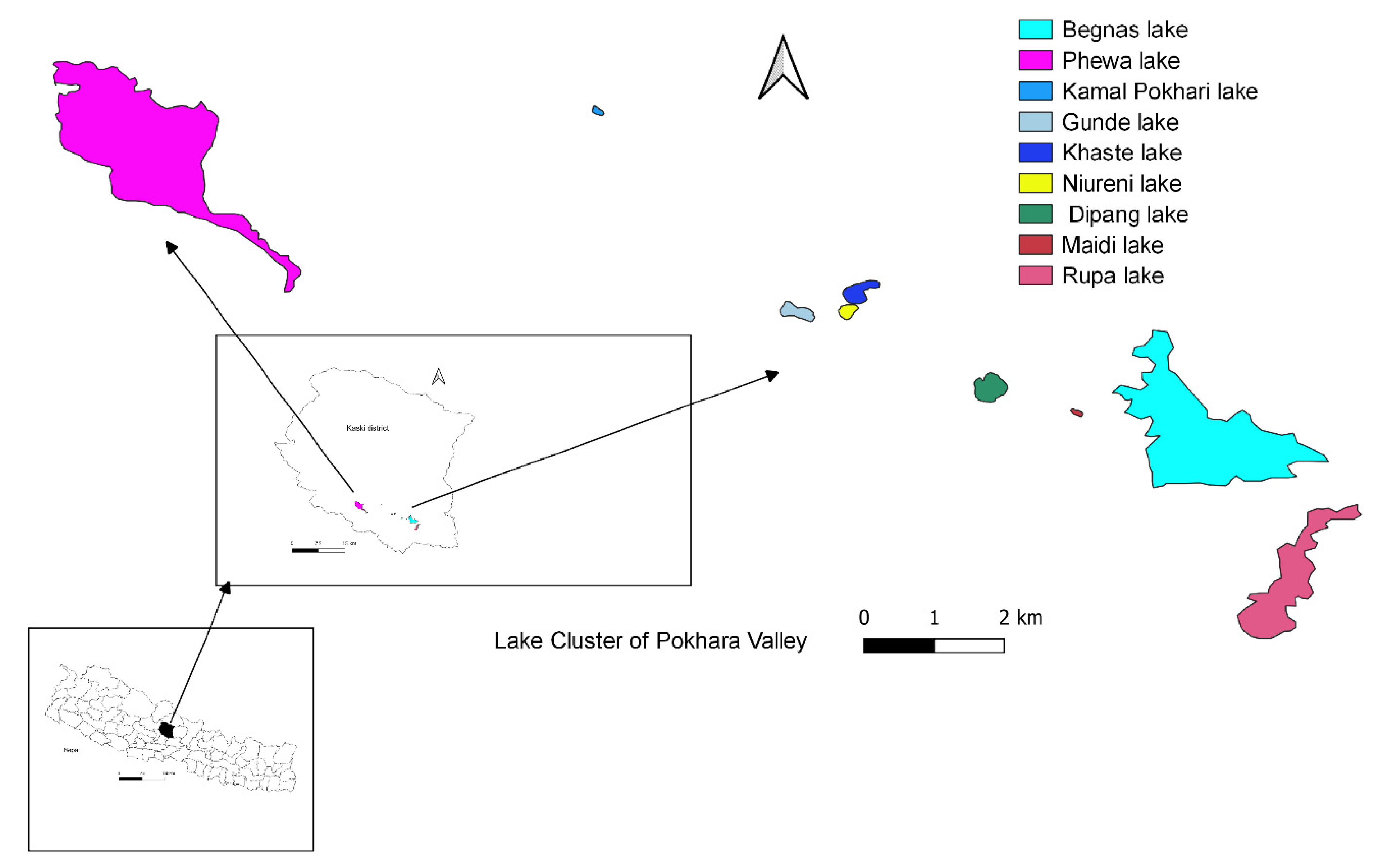
2.1. Study Area
2.2. Methods
2.3. Data Analysis
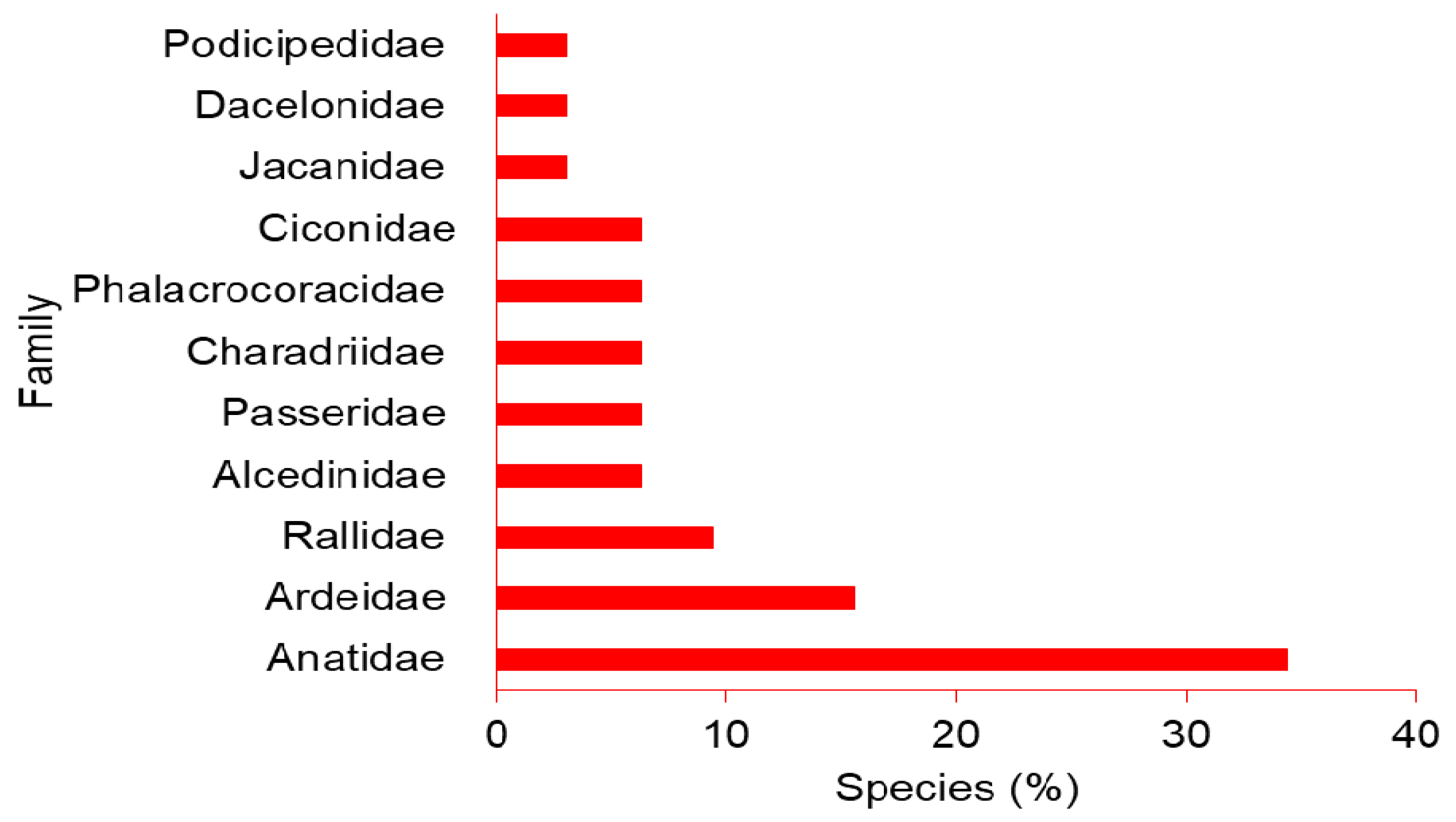
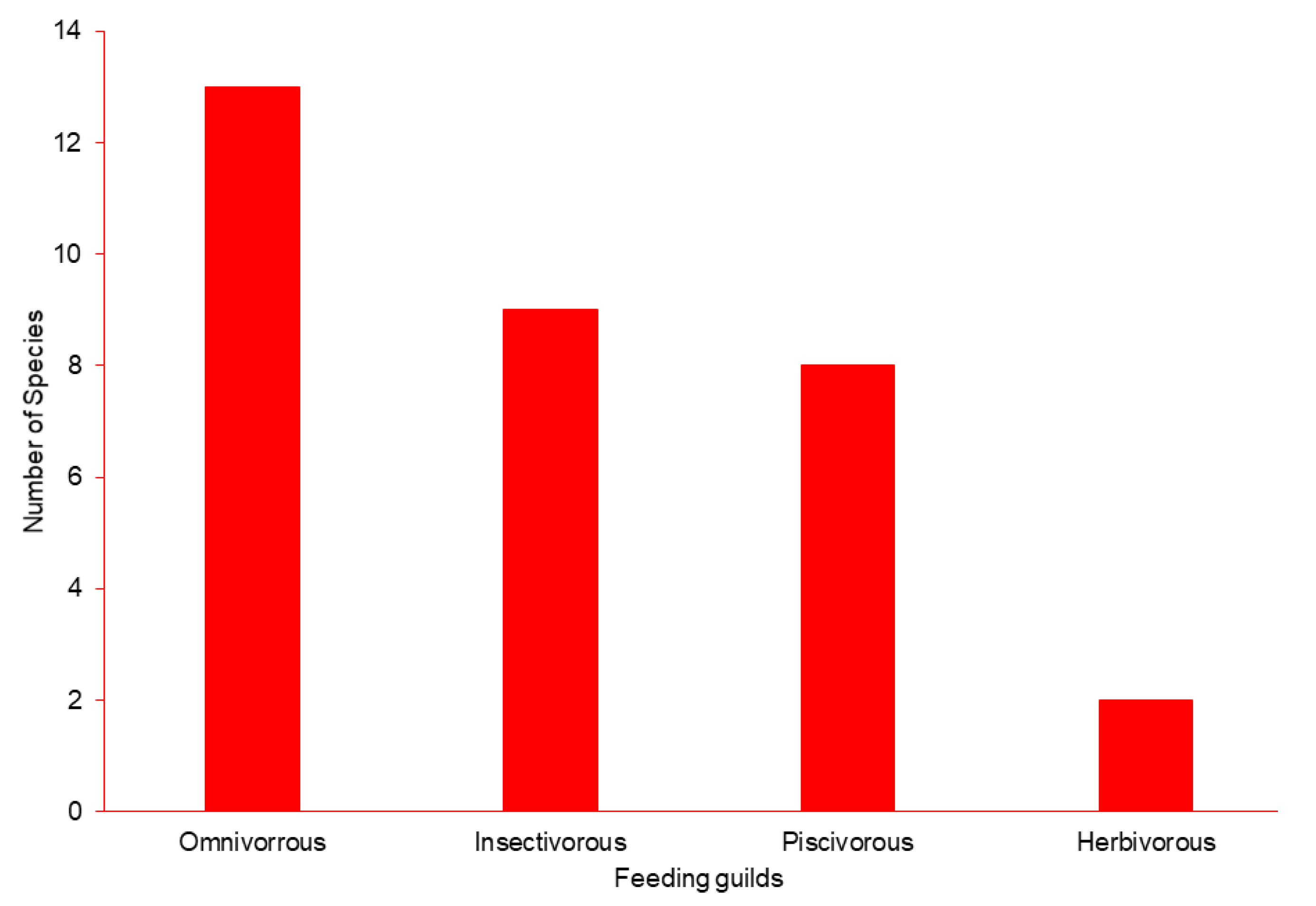
3. Results
3.1. Abundance
3.2. Physicochemical Parameters
3.3. Factors Affecting Waterbirds Abundance and Richness
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic Invasive Species: Challenges for the Future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Ongore, C.O.; Aura, C.M.; Ogari, Z.; Njiru, J.M.; Nyamweya, C.S. Spatial-Temporal Dynamics of Water Hyacinth, Eichhornia crassipes (Mart.) and Other Macrophytes and Their Impact on Fisheries in Lake Victoria, Kenya. J. Great Lakes Res. 2018, 44, 1273–1280. [Google Scholar] [CrossRef]
- Pinero-Rodríguez, M.J.; Fernández-Zamudio, R.; Arribas, R.; Gomez-Mestre, I.; Díaz-Paniagua, C. The Invasive Aquatic Fern Azolla filiculoides Negatively Impacts Water Quality, Aquatic Vegetation and Amphibian Larvae in Mediterranean Environments. Biol. Invasions 2021, 23, 755–769. [Google Scholar] [CrossRef]
- Shah, K.K.; Tiwari, I.; Tripathi, S.; Subedi, S.; Shrestha, J. Invasive Alien Plant Species: A Threat to Biodiversity and Agriculture in Nepal. Agriways 2020, 8, 62–73. [Google Scholar] [CrossRef]
- Shrestha, B.B. Invasive Alien Plant Species in Nepal. Front. Bot. 2016, 2016, 269–284. [Google Scholar]
- Charles, H.; Dukes, J.S. Impacts of invasive species on ecosystem services. In Biological Invasions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 217–237. [Google Scholar]
- Keller, R.P.; Masoodi, A.; Shackleton, R.T. The Impact of Invasive Aquatic Plants on Ecosystem Services and Human Well-Being in Wular Lake, India. Reg. Environ. Change 2018, 18, 847–857. [Google Scholar] [CrossRef]
- Pathak, H.N.; Bhuju, D.R.; Shrestha, B.B.; Ranjitkar, S. Impacts of Invasive Alien Plants on Ecosystem Services of Ramsar Lake Cluster in Middle Mountain Nepal. Glob. Ecol. Conserv. 2021, 27, e01597. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, J.S. Invasive Alien Plant Species: Their Impact on Environment, Ecosystem Services and Human Health. Ecol. Indic. 2020, 111, 106020. [Google Scholar]
- Murphy, S.T.; Subedi, N.; Jnawali, S.R.; Lamichhane, B.R.; Upadhyay, G.P.; Kock, R.; Amin, R. Invasive Mikania in Chitwan National Park, Nepal: The Threat to the Greater One-Horned Rhinoceros Rhinoceros Unicornis and Factors Driving the Invasion. Oryx 2013, 47, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.B.; Shrestha, K.K. Invasions of Alien Plant Species in Nepal: Patterns and Process. Invasive Alien Species Obs. Issues World 2021, 2, 168–183. [Google Scholar] [CrossRef]
- Rajbhandari, K.R.; Rai, S.K.; Bhatta, G.D. Flowering Plants of Nepal: An Introduction; Department of Plant Resources: Kathmandu, Nepal, 2017. [Google Scholar]
- Rana, H.K.; Sun, H.; Paudel, A.; Ghimire, S.K. Saussurea Ramchaudharyi (Asteraceae), a New Species from Nepal. Phytotaxa 2018, 340, 271–276. [Google Scholar]
- Shrestha, U.B.; Sharma, K.P.; Devkota, A.; Siwakoti, M.; Shrestha, B.B. Potential Impact of Climate Change on the Distribution of Six Invasive Alien Plants in Nepal. Ecol. Indic. 2018, 95, 99–107. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group Auckland: New Zealand, 2000; Volume 12. [Google Scholar]
- Villamagna, A.M. Ecological Effects of Water Hyacinth (Eichhornia crassipes) on Lake Chapala, Mexico. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2009; p. 194. [Google Scholar]
- Ndimele, P.E.; Kumolu-Joh, C.A.; Anetekhai, M.A. The Invasive Aquatic Macrophyte, Water Hyacinth {Eichhornia crassipes (Mart.) Solm-Laubach: Pontedericeae}: Problems and Prospects. Res. J. Environ. Sci. 2011, 5, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Khatri, U.; Thapa-Parajuli, R.B.; Paudel, U. Willingness to Pay for Water Hyacinth Control in Nepal. Am. J. Environ. Sci. 2018, 14, 226–233. [Google Scholar] [CrossRef]
- Maharjan, R.B.S.; Ming, C.L. The Potential Role of Water Hyacinth in Wastewater Treatment in Nepal. Hydro Nepal J. Water Energy Environ. 2012, 10, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, C.C.; Petersen, C.M. Water Hyacinths as a Resource in Agriculture and Energy Production: A Literature Review. Waste Manag. 2007, 27, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Hailu, D.; Negassa, A.; Kebede, B. Study on the Status of Some Physico-Chemical Parameters of Lake Koka and Its Relation with Water Hyacinth (Eichhornia crassipes) Invasion. Int. J. Fish. Aquat. Stud. 2020, 8, 405–412. [Google Scholar]
- Villamagna, A.M.; Murphy, B.R.; Karpanty, S.M. Community-Level Waterbird Responses to Water Hyacinth (Eichhornia crassipes). Invasive Plant Sci. Manag. 2012, 5, 353–362. [Google Scholar] [CrossRef]
- Mangas-Ramírez, E.; Elías-Gutiérrez, M. Effect of Mechanical Removal of Water Hyacinth (Eichhornia crassipes) on the Water Quality and Biological Communities in a Mexican Reservoir. Aquat. Ecosyst. Health Manag. 2004, 7, 161–168. [Google Scholar] [CrossRef]
- Mironga, J.M.; Mathooko, J.M.; Onywere, S.M. Effect of Water Hyacinth Infestation on the Physicochemical Characteristics of Lake Naivasha. Int. J. Humanit. Soc. Sci. 2012, 2, 103–113. [Google Scholar]
- Yongo, E.; Outa, N.O. Effects of Water Hyacinth (Eichhornia crassipes Solm) Infestation on Water Quality, Fish Species Diversity and Abundance in the Nyanza Gulf of Lake Victoria, Kenya. Lakes Reserv. Ponds 2015, 9, 67–74. [Google Scholar]
- Kumar, P.; Rai, D.; Gupta, S.K. Wetland Bird Assemblage in Rural Ponds of Kurukshetra, India. Waterbirds 2016, 39, 86–98. [Google Scholar] [CrossRef]
- Firdausy, M.S.; Mardiastuti, A.; Mulyani, Y.A. Abundance Waterbirds and the Distribution of Trees Nesting in Pulau Rambut (Rambut Island) Wildlife Sanctuary, Jakarta Bay, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 771, 012028. [Google Scholar] [CrossRef]
- Grimmett, R.; Inskipp, C.; Inskipp, T.; Baral, H.S. Birds of Nepal; Bloomsbury Publishing: London, 2016. [Google Scholar]
- Inskipp, C.; Baral, H.S.; Sunita, P.; Bhatta, T.R.; Khatiwada, M.; Inskipp, T.; Gurung, S.; Singh, P.B.; Murray, L.; Poudyal, L.; et al. The Status of Nepal ’s Birds: The National Red List Series; Zoological Society of London: London, UK, 2016; Volume 1, p. 678. ISBN 978-0-900881-60-2. [Google Scholar]
- BCN and DNPWC Birds of Nepal: An Official Checklist; Kathmandu, Nepal, 2018.
- Masifwa, W.F.; Twongo, T.; Denny, P. The Impact of Water Hyacinth, Eichhornia crassipes (Mart) Solms on the Abundance and Diversity of Aquatic Macroinvertebrates along the Shores of Northern Lake Victoria, Uganda. Hydrobiologia 2001, 452, 79–88. [Google Scholar] [CrossRef]
- Toft, J.D.; Simenstad, C.A.; Cordell, J.R.; Grimaldo, L.F. The Effects of Introduced Water Hyacinth on Habitat Structure, Invertebrate Assemblages, and Fish Diets. Estuaries 2003, 26, 746–758. [Google Scholar] [CrossRef]
- Villamagna, A.M.; Murphy, B.R.; Trauger, D.L. Behavioral Response of American Coots (Fulica Americana) to Water Hyacinth (Eichhornia crassipes) in Lake Chapala, Mexico. Waterbirds 2010, 33, 550–555. [Google Scholar] [CrossRef]
- MoFE. Integrated Lake Basin Management Plan of Lake Cluster of Pokhara Valley, Kaski, Nepal (2018–2023); Ministry of Forests and Environment: Kathmandu, Nepal, 2018. [Google Scholar]
- Gautam, R.; Kafle, G. A Preliminary Survey of Waterbirds in Phewa Lake, Kaski. Bird Conserv. Nepal Newsl. 2008, 16, 6–8. [Google Scholar]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A. Bird Census Techniques, 2nd ed.; Elsevier: Netherlands, 2000; ISBN 978-0-12-095831-3. [Google Scholar]
- APHA, WEF; AWWA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Shannon-Wiener, C.E.; Weaver, W.; Weater, W.J. The Mathematical Theory of Communication; University Illinois PressUrbana: Champaign, IL, USA.
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Bates, D.; Kliegl, R.; Vasishth, S.; Baayen, H. Parsimonious Mixed Models. arXiv Stat. 2018, arXiv:1506.04967. [Google Scholar]
- R Development Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2020. [Google Scholar]
- Adhikari, J.N.; Bhattarai, B.P.; Thapa, T.B. Factors Affecting Diversity and Distribution of Threatened Birds in Chitwan National Park, Nepal. J. Threat. Taxa 2019, 11, 13511–13522. [Google Scholar] [CrossRef]
- Adhikari, J.N.; Bhattarai, B.P.; Dhakal, D.N. Conservation Value of Beeshhazari Lake: An Insight into Diversity and Abundance of Wetland Birds. Our Nat. 2018, 16, 17–26. [Google Scholar] [CrossRef]
- Thapa, J.B.; Saund, T.B. Water Quality Parameters and Bird Diversity in Jagdishpur Reservoir, Nepal. Nepal J. Sci. Technol. 2013, 13, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Giri, B.; Chalise, M.K. Seasonal Diversity and Population Status of Waterbirds in Phewa Lake, Pokhara, Nepal. J. Wetl. Ecol. 2008, 1, 3–7. [Google Scholar] [CrossRef]
- Dhakal, H.; Ghimire, M.; Poudel, A.K. Avian Diversity of Khaste Lake Complex, Pokhara Valley, Nepal. Minivet 2020, 3, 17–25. [Google Scholar]
- Khan, T.N. Temporal Changes to the Abundance and Community Structure of Migratory Waterbirds in Santragachhi Lake, West Bengal, and Their Relationship with Water Hyacinth Cover. Curr. Sci. 2010, 99, 1570–1577. [Google Scholar]
- Kaur, S.; Kler, T.K.; Javed, M. Abundance and Diversity of Water Bird Assemblages in Relation to Village Ponds in Punjab. J. Entomol. Zool. Stud. 2018, 6, 1375–1380. [Google Scholar]
- Bartodziej, W.; Weymouth, G. Waterbird Abundance and Activity on Waterhyacinth and Egeria in the St Marks River, Florida. J. Aquat. Plant Manag. 1995, 33, 19–22. [Google Scholar]
- Martins, C.O.; Olaniyi, O.E.; Zakaria, M. Environmental Factors and Spatial Heterogeneity Affect Occupancy Estimates of Waterbirds in Peninsular Malaysia. Ornithol. Sci. 2021, 20, 39–55. [Google Scholar] [CrossRef]
- Rajpar, M.N.; Zakaria, M. Bird Abundance and Its Relationship with Microclimate and Habitat Variables in Open-Area and Shrub Habitats in Selangor, Peninsular Malaysia. J. Anim. Plant Sci. 2015, 25, 114–124. [Google Scholar]
- Doss, D.P.S.; Gopukumar, N.; Sripathi, K. Breeding Biology of the Purple Swamphen (Porphyrio porphyrio) at Tirunelveli, South India. Wilson J. Ornithol. 2009, 121, 796–800. [Google Scholar] [CrossRef]
- Mouslim, B.; Eddine, M.S.; Rassim, K.; Zihad, B.; Moussa, H. Aspects of the Breeding Ecology of the Purple Swamphen (Porphyrio porphyrio) in the Wetland Complex of Guerbes-Sanhadja, North-East Algeria. Ostrich 2014, 85, 185–191. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Wu, W.; Feng, X.; Niu, D.; Ma, Z. Birds and Their Habitat Conditions in Reed Marshes with Different Cutting Intervals at Chongming Dongtan along China’s Coasts. Glob. Ecol. Conserv. 2021, 26, e01499. [Google Scholar] [CrossRef]
- Storch, D.; Bohdalková, E.; Okie, J. The More-Individuals Hypothesis Revisited: The Role of Community Abundance in Species Richness Regulation and the Productivity-Diversity Relationship. Ecol. Lett. 2018, 21, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, L.; Xu, W. Diversity of Wintering Waterbirds Enhanced by Restoring Aquatic Vegetation at Shengjin Lake, China. Sci. Total Environ. 2020, 737, 140190. [Google Scholar] [CrossRef]
- Dahal, B.R. Effects of Invasive Weeds Particularly Water Hyacinth Eichhornia crassipes and Human Disturbances on Community Structure of Wetland Birds in Koshi Tappu Wildlife Reserve, Nepal. In International Studies in Aquatic Tropical Ecology; University of Bremen, Faculty for Biology & Chemistry: Bremen, Germany.
- Dereje, T. Preliminary Survey of Water Hyacinth in Lake Tana, Ethiopia. Glob. J. Allergy 2015, 1, 13–18. [Google Scholar]
- Rommens, W.; Maes, J.; Dekeza, N.; Inghelbrecht, P.; Nhiwatiwa, T.; Holsters, E.; Ollevier, F.; Marshall, B.; Brendonck, L. The Impact of Water Hyacinth (Eichhornia crassipes) in a Eutrophic Subtropical Impoundment (Lake Chivero, Zimbabwe). I. Water Quality. Arch. Hydrobiol. 2003, 158, 373–388. [Google Scholar] [CrossRef]
| S.N. | Scientific Name | Common Name | Family | IUCN Status | Feeding Guild |
|---|---|---|---|---|---|
| 1 | Phalacrocorax carbo Linnaeus, 1758 | Great Cormorant | Phalacrocoracidae | LC | Piscivore |
| 2 | Aythya baeri Güldenstädt, 1770 | Baer’s Pochard | Anatidae | CR | Omnivore |
| 3 | Aythya ferina Linnaeus, 1758 | Common Pochard | Anatidae | VU | Omnivore |
| 4 | Anser indicus Latham, 1990 | Bar-headed Goose | Anatidae | LC | Herbivore |
| 5 | Mareca penelope Linnaeus, 1758 | Eurasian Wigeon | Anatidae | LC | Herbivore |
| 6 | Aythya nyroca Güldenstädt, 1770 | Ferruginous Pochard | Anatidae | NT | Omnivore |
| 7 | Anas platyrhynchos Linnaeus, 1758 | Mallard | Anatidae | LC | Omnivore |
| 8 | Anas acuta Linnaeus, 1758 | Northern Pintail | Anatidae | LC | Omnivore |
| 9 | Spatulaclypeata Linnaeus, 1758 | Northern Shoveler | Anatidae | LC | Omnivore |
| 10 | Tadorna ferruginea Pallas, 1764 | Ruddy Shelduck | Anatidae | LC | Omnivore |
| 11 | Aythya fuligula Linnaeus, 1758 | Tufted Duck | Anatidae | LC | Omnivore |
| 12 | Ciconia nigra Linnaeus, 1758 | Black Stork | Ciconiidae | LC | Piscivore |
| 13 | Ciconia episcopus Boddaert, 1783 | Woolly Necked Stork | Ciconiidae | VU | Piscivore |
| 14 | Ardea cinerea Linnaeus, 1758 | Grey Heron | Ardeidae | LC | Piscivore |
| 15 | Dendrocygna javanica Horsfield, 1821 | Lesser Whistling duck | Anatidae | LC | Omnivore |
| 16 | Tachybaptus ruficollis Pallas, 1764 | Little Grebe | Podicipedidae | LC | Insectivore |
| 16 | Phalacrocorax niger Gmelin, 1789 | Little Cormorant | Phalacrocoracidae | LC | Piscivore |
| 18 | Bubulcus ibis Linnaeus, 1766 | Cattle Egret | Ardeidae | LC | Insectivore |
| 19 | Ardeola grayii Sykes, 1832 | Indian pond Heron | Ardeidae | LC | Insectivore |
| 20 | Ardeaintermedia Wagler, 1829 | Intermediate Egret | Ardeidae | LC | Insectivore |
| 21 | Egretta garzetta Linnaeus, 1766 | Little Egret | Ardeidae | LC | Insectivore |
| 22 | Fulica atra Linnaeus, 1758 | Common Coot | Ardeidae | LC | Omnivore |
| 23 | Gallinula chloropus Linnaeus, 1758 | Common Moorhen | Rallidae | LC | Omnivore |
| 24 | Porphyrio porphyrio Linnaeus, 1758 | Purple Swamphen | Rallidae | LC | Omnivore |
| 25 | Metopidius indicus Latham, 1790 | Bronze-winged Jacana | Jacanidae | LC | Omnivore |
| 26 | Alcedo atthis Linnaeus, 1758 | Common Kingfisher | Alcedinidae | LC | Piscivore |
| 27 | Alcedo meninting Horsfield, 1821 | Blue-eared Kingfisher | Alcedinidae | LC | Piscivore |
| 28 | Halcyon smyrnensis Linnaeus, 1758 | White Throated Kingfisher | Dacelonidae | LC | Piscivore |
| 29 | Motacilla maderaspatensis Gmelin, 1789 | White-browed Wagtail | Passeridae | LC | Insectivore |
| 30 | Motacilla alba Linnaeus, 1758 | White Wagtail | Passeridae | LC | Insectivore |
| 31 | Charadrius dubius Scopoli, 1786 | Little Ringed Plover | Charadriidae | LC | Insectivore |
| 32 | Vanellus indicus Boddaert, 1783 | Red Wattled Lapwing | Charadriidae | LC | Insectivore |
| Parameters | HP Habitat | HA Habitat | Statistics |
|---|---|---|---|
| Abundance | Median = 33.5(9–160) | Median = 25.5(3–205) | Mann–Whitney test, U = 805.5; p = 0.038 |
| Resident birds | Median = 33.5(9–131) | Median = 23(3–116) | Mann–Whitney test, U = 700.5; p = 0.004 |
| Threatened birds | Median = 0(0–7) | Median = 0(0–14) | Mann–Whitney test, U = 1275; p = 0.023 |
| Omnivore birds | Median = 15(0–82) | Median = 6(0–112) | Mann–Whitney test, U = 817; p = 0.046 |
| Insectivore birds | Median = 20(7–58) | Median = 16(2–58) | Mann–Whitney test, U = 772.5; p = 0.020 |
| Piscivore birds | Median = 4(1–21) | Median = 4.5(1–41) | Mann–Whitney test, U = 1064; p = 0.906 |
| Parameters | HP Habitat | HA Habitat | Statistics |
|---|---|---|---|
| Depth (m) | Median = 3 | Median = 4 | Mann–Whitney test, U = 1817; p = <0.001 |
| Temperature (℃) | Median = 25 | Median = 24.5 | Mann–Whitney test, U = 850.5; p = 0.082 |
| Transparency(m) | Median = 0.77 | Median = 1.4 | Mann–Whitney test, U = 2057; p = <0.001 |
| pH | Median = 6.6 | Median = 7.4 | Mann–Whitney test, U = 1825; p = <0.001 |
| Turbidity (NTU) | Median = 3.05 | Median = 3.05 | Mann–Whitney test, U = 949; p = 0.323 |
| TDS (mg/L) | Median = 23.5 | Median = 19 | Mann–Whitney test, U = 602; p = <0.001 |
| DO (mg/L) | Median = 4 | Median = 6.6 | Mann–Whitney test, U = 1997; p = <0.001 |
| Free CO2 (mg/L) | Median = 11.85 | Median = 6.8 | Mann–Whitney test, U = 96.5; p = <0.001 |
| Total alkalinity (mg/L) | Median = 126.5 | Median = 149.5 | Mann–Whitney test, U = 1677; p = <0.001 |
| Nitrate (mg/L) | Median = 1.25 | Median = 2.05 | Mann–Whitney test, U = 1766; p = <0.001 |
| Threatened waterbird abundance | Parameters | Estimate | Lower CL | Upper CL | z | p |
| (Intercept) | −19.600 | −5.27 × 103 | 5.23 × 103 | −0.006 | 0.995 | |
| Depth | 0.447 | 0.00346 | 0.692 | 2.610 | 0.009 | |
| Distance to settlement | 2.70 × 10−5 | −2.23 × 10−3 | 8.32 × 10−4 | 0.022 | 0.982 | |
| Bird abundance | 1.23 × 10−2 | 4.19 × 10−3 | 2.18 × 10−2 | 3.560 | 0.000 | |
| Distance to edge | −1.01 × 10−3 | −2.23 × 10−3 | 8.32 × 10−4 | −0.774 | 0.439 | |
| Winter season | 20.4000 | −5.23 × 103 | 5.27 × 103 | 0.006 | 0.995 | |
| Water hyacinth | −1.450 | −3.170 | 0.185 | −2.126 | 0.033 | |
| Transparency | −1.380 | −2.580 | 0.304 × 10−1 | −1.893 | 0.058 | |
| Temperature | −6.25 × 10−2 | −2.16 × 10−1 | 2.80 × 10−2 | −1.113 | 0.266 | |
| Threatened waterbird richness | (Intercept) | −22.200 | −8.23 × 103 | 8.19 × 103 | 0.005 | 0.996 |
| Bird abundance | 9.89 × 10−3 | 2.19 × 10−3 | 1.76 × 10−2 | 2.517 | 0.012 | |
| Depth | 3.54 × 10−1 | 9.06 × 10−2 | 6.18 × 10−1 | 2.633 | 0.008 | |
| Winter season | 20.400 | −8.20 × 103 | 8.24 × 103 | 0.005 | 0.996 | |
| Temperature | −9.80 × 10−2 | −2.67 × 10−1 | 7.11 × 10−2 | 1.136 | 0.256 | |
| Water hyacinth | −0.631 | −2.130 | 0.870 | 0.824 | 0.410 | |
| Transparency | 4.29 × 10−2 | −1.640 | 1.730 | 0.050 | 0.960 | |
| Distance to settlement | 1.96 × 10−4 | −1.67 × 10−3 | 2.06 × 10−3 | 0.205 | 0.837 | |
| Distance to edge | 2.70 × 10−4 | −1.67 × 10−3 | 2.21 × 10-3 | 0.273 | 0.785 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basaula, R.; Sharma, H.P.; Belant, J.L.; Sapkota, K. Invasive Water Hyacinth Limits Globally Threatened Waterbird Abundance and Diversity at Lake Cluster of Pokhara Valley, Nepal. Sustainability 2021, 13, 13700. https://doi.org/10.3390/su132413700
Basaula R, Sharma HP, Belant JL, Sapkota K. Invasive Water Hyacinth Limits Globally Threatened Waterbird Abundance and Diversity at Lake Cluster of Pokhara Valley, Nepal. Sustainability. 2021; 13(24):13700. https://doi.org/10.3390/su132413700
Chicago/Turabian StyleBasaula, Rajendra, Hari Prasad Sharma, Jerrold L. Belant, and Kumar Sapkota. 2021. "Invasive Water Hyacinth Limits Globally Threatened Waterbird Abundance and Diversity at Lake Cluster of Pokhara Valley, Nepal" Sustainability 13, no. 24: 13700. https://doi.org/10.3390/su132413700
APA StyleBasaula, R., Sharma, H. P., Belant, J. L., & Sapkota, K. (2021). Invasive Water Hyacinth Limits Globally Threatened Waterbird Abundance and Diversity at Lake Cluster of Pokhara Valley, Nepal. Sustainability, 13(24), 13700. https://doi.org/10.3390/su132413700






