Safety Disposal of Rice Straw by Biodegradation Using Streptomyces Tendae
Abstract
1. Introduction
2. Materials and Methods
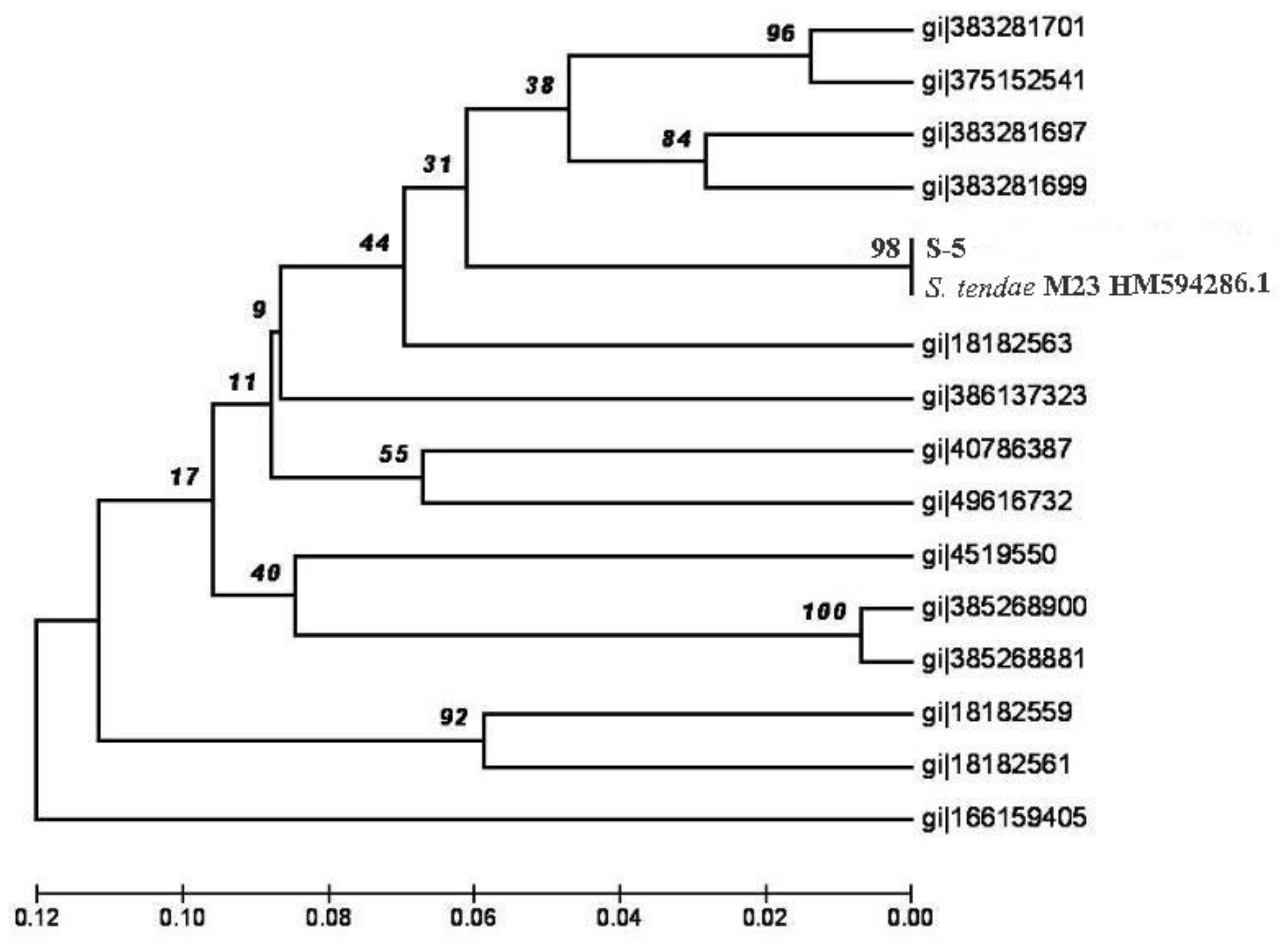
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, H.S.; Soni, B.; Shrivastava, A.R.; Shrivastava, S. Diversity and versatility of actinomycetes and its role in antibiotic production. J. Appl. Pharm. Sci. 2013, 3, S83–S94. [Google Scholar]
- Jeffrey, L.S.H. Isolation, characterization and identification of actinomycetes from agriculture soils at Semongok, Sarawak. Afr. J. Biotechnol. 2008, 7, 3700–3705. [Google Scholar]
- Das, A.; Hamedani, K.; Soudbakhsh, M.; Prashanthi, K.; Bhattacharya, S.; Suryan, S. Enzymatic screening, antibacterial potential and molecular characterization of Streptomyces isolated from Wayanad District in Kerala, India. Int. J. Pharm. Bio Sci. 2012, 2, 201–210. [Google Scholar]
- Das, P.; Solanki, R.; Khanna, M. Isolation and screening of cellulolytic actinomycetes from diverse habitats. Int. J. Adv. Biotechnol. Res. 2014, 5, 438–451. [Google Scholar]
- Prakash, D.; Nawani, N.; Prakashetal, M. Actinomycetes: A repertory of green catalysts with a potential revenue resource. BioMed Res. Int. 2013, 2013, 264020. [Google Scholar] [CrossRef] [PubMed]
- Mtui, G.Y.S. Lignocellulolytic enzymes from tropical fungi: Types, substrates and applications. J. Sci. Res. Essays 2012, 7, 1544–1555. [Google Scholar]
- Isikgorand, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. J. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Deswal, D.; Sharma, A.; Gupta, R.; Kuhad, R.C. Application of lignocellulolytic enzymes produced under solid state cultivation conditions. Bioresour. Technol. 2012, 115, 249–254. [Google Scholar] [CrossRef]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef]
- Sukumaran, R.K.; Singhania, R.R.; Pandey, A. Microbial cellulases production, applications and challenges. J. Sci. Ind. Res. 2005, 64, 832–844. [Google Scholar]
- Soni, H.; Kango, N. Hemicellulases in lignocellulose biotechnology: Recent patents. Recent Pat. Biotechnol. 2013, 7, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into lignin degradation and its potential industrial applications. J. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar]
- de-Souza, W.R. Microbial degradation of lignocellulosic biomass. In Sustainable Degradation of Lignocellulosic Biomass-Techniques, Applications and Commercialization, 1st ed.; Chandel, E.D., Ed.; InTech E-Publishing Inc.: Rijeka, Croatia, 2013; Volume 3, pp. 120–142. [Google Scholar]
- Kumar, R.; Biswas, K.; Soalnki, V.; Kumar, P.; Tarafdar, A. Actinomycetes: Potential bioresource for human welfare: A review. Res. J. Chem. Environ. Sci. 2014, 2, 5–16. [Google Scholar]
- Kadam, K.L.; Forrest, L.H.; Jacobson, W.A. Rice straw as a lignocellulosic resource: Collection, processing, transportation, and environmental aspects. J. Biomass Bioenergy 2000, 18, 369–389. [Google Scholar] [CrossRef]
- Kumar, A.; Gaind, S.; Nain, L. Evaluation of thermophilic fungal consortium for paddy straw composting. J. Biodegrad. 2008, 19, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Hatamoto, M.; Tanahashi, T.; Murase, J.; Matsuya, K.; Hayashi, M.; Kimura, M.; Asakawa, S. Eukaryotic communities associated with the decomposition of rice straw compost in a Japanese rice paddy field estimated by DGGE analysis. J. Biol. Fertil. Soils 2008, 44, 527–532. [Google Scholar] [CrossRef]
- Tang, J.C.; Shibata, A.; Zhou, Q.; Katayama, A. Effect of temperature on reaction rate and microbial community in composting of cattle manure with rice straw. J. Biosci. Bioenergy 2007, 104, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.S.; Godden, B.; Helvenstein, P.; Penninckx, M.J.; McCarthy, A.J. Lignocarbohydrate solubilization from straw by actinomycetes. Appl. J. Environ. Microbiol. 1990, 56, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Allgaier, M.; Reddy, A.; Park, J.I.; Ivanova, N.; D’haeseleer, P.; Lowry, S.; Sapra, R.; Hazen, T.C.; Simmons, B.A.; Vander-Gheynst, J.S.; et al. Targeted discovery of glycoside hydrolases from a switchgrass-adapted compost community. PLoS ONE 2010, 5, e8812. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.L. Notes on the biology of Azotobacter. J. Appl. Microbiol. 2008, 14, 89–94. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. J. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef]
- Archibald, F.S. A new assay for lignin-type peroxidases employing the dye azure B. Appl. J. Environ. Microbiol. 1992, 58, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Prapagdee, B.; Kuekulvong, C.; Mongkolsuk, S. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int. Boil Sci. J. 2008, 4, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Garcia, M.C.; Suarez-Estrella, F.; Lopez, M.J.; Moreno, J. In vitro studies on lignocellulose degradation by microbial strains isolated from composting processes. Int. Biodeterior. Biodegrad. 2007, 59, 322–328. [Google Scholar] [CrossRef]
- Chefetz, B.; Hatcher, P.G.; Hader, Y.; Chen, Y. Chemical and biological characterization of organic matter during composting of municipal solid waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Storer, D.A. A simple high sample volume aching procedure for determination of soil organic matter. J. Soil Sci. Plant Anal. 1984, 15, 759–772. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Stozhko, N.Y.; Belysheva, G.M.; Inzhevatova, O.V.; Kolyadina, L.I.; Cremisini, C.; Galletti, M. Determination of heavy metals in wines by anodic stripping voltammetry with thick-film modified electrode. Anal. Chem. Acta 2004, 514, 227–234. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods of dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Nigam, P.; Singh, D. Solid-state (substrate) fermentation systems and their applications in biotechnology. J. Basic Microbiol. 1994, 34, 405–423. [Google Scholar] [CrossRef]
- Abdulkhair, W.M. Chitinolytic activity of highly halotolerant Streptomyces tendae against Fusarium oxysporum PTK2. Afr. J. Biotechnol. 2012, 11, 15523–15532. [Google Scholar] [CrossRef]
- McCarthy, A.J. Lignocellulose-degrading actinomycetes. FEMS Microbiol. Lett. 1987, 3, 145–163. [Google Scholar] [CrossRef]
- Abdulla, H.M.; El-Shatoury, S.A. Actinomycetes in rice straw decomposition. J. Waste Manage 2007, 27, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.H.; Fakhrul-Razi, A.; Abd-Aziz, S.; Hanafi, M.M.; Roychoudhury, P.K.; Alam, M.Z. A potential resource for bioconversion of domestic wastewater sludge. Bioresour. Technol. 2002, 85, 263–272. [Google Scholar] [CrossRef]
- Castillo, M.P.; Ander, P.; Stenstrom, J. Lignin and manganese peroxidase activity in extracts from straw solid substrate fermentations. J. Biotechnol. Technol. 1997, 11, 701–706. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulose activities. J. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Tan, Y.H.; Wahab, M.N. Extracellular enzyme production during anamorphic growth in the edible mushroom, Pleurotus Sajor-Caju. World J. Microbiol. Biotechnol. 1997, 13, 613–617. [Google Scholar] [CrossRef]
- Veeken, A.H.M.; Adani, F.; Nierop, K.G.J.; de Jager, P.A.; Hamelers, H.V.M. Degradation of bio-macromolecules during high rate composting of wheat straw-amended feces. J. Environ. Qual. 2001, 30, 1675–1684. [Google Scholar] [CrossRef]
- Lee, I.B.; Kim, P.J.; Chang, K.W. Evaluation of stability of compost prepared with Korean food wastes. J. Soil Sci. Plant. Nutr. 2002, 48, 1–8. [Google Scholar] [CrossRef]
- Wenzel, M.; Schonig, I.; Berchtold, M.; Kampfer, P.; Konig, H. Aerobic and facultative anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. J. Appl. Microbiol. 2002, 92, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Thawai, C.; Tanasupawat, S.; Itoh, T.; Suwanborirux, K.; Suzuki, K.; Kudo, T. Micromonospora cburnea sp nov., isolated from a Thai peat swamp forest. Int. J. Syst. Evol. Microbiol. 2005, 55, 417–422. [Google Scholar] [CrossRef]

| Actinobacterial Isolates | Mean of Hydrolytic Activity (mm) | ||
|---|---|---|---|
| Starch | Cellulose | Lignin | |
| S-1 to S-4 | No activity | No activity | No activity |
| S-5 | 30 | 25 | 22 |
| S-6 to S-11 | No activity | No activity | No activity |
| S-12 | 25 | 20 | 20 |
| S-13 to S-19 | No activity | No activity | No activity |
| S-20 | 27 | 25 | 25 |
| S-21 to S-30 | No activity | No activity | No activity |
| Medium | Growth | Color | ||
|---|---|---|---|---|
| Aerial Mycelia | Substrate Mycelia | Diffusible Pigment | ||
| TYEB | P | 264 L. Gray | 79 l.gy. YBr | None |
| YMEA | M | 10 Pk Gray | 70 l. oy | None |
| OMEA | G | 263 L. Gray | 76 l. yBr | 79 l.gy. YBr |
| ISSA | M | 10 Pk Gray | 79 l.gy. YBr | None |
| GAA | G | 264 L. Gray | 79 l.gy. YBr | None |
| PYEIA | P | 10 Pk Gray | 79 l.gy. YBr | None |
| TA | P | 10 Pk Gray | 79 l.gy. YBr | None |
| Parameter | Test | Result |
|---|---|---|
| Morphology | Shape of spore chain | Spiral |
| Color of spore mass | Gray | |
| Motility | NM | |
| Cell wall hydrolysis | Diaminopimelic acid (DAP) | LL_DAP |
| Sugar pattern | ND | |
| Enzymatic activity | Amylase, pectinase, cellulase, protease, catalase and nitrate reductase | Positive |
| Lipase and lecithinase | Negative | |
| Physiology | Melanoid production and NaCl tolerance up to 5% | Positive |
| H2S production and streptomycin resistance | Negative | |
| Degradation of xanthine and esculin | Positive | |
| Utilization of sugars | D-glucose, D-galactose, D-fructose, mannitol, sucrose, raffinose and rhamnose | Positive |
| L-arabinose and xylose | Negative | |
| Utilization of amino acids | L-cysteine, L-valine, L-histidine, L-alanine, L-lysine, L-leucine, L-tyrosine, L-phenylalanine and L-proline | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dhabaan, F. Safety Disposal of Rice Straw by Biodegradation Using Streptomyces Tendae. Sustainability 2021, 13, 13640. https://doi.org/10.3390/su132413640
Al-Dhabaan F. Safety Disposal of Rice Straw by Biodegradation Using Streptomyces Tendae. Sustainability. 2021; 13(24):13640. https://doi.org/10.3390/su132413640
Chicago/Turabian StyleAl-Dhabaan, Fahad. 2021. "Safety Disposal of Rice Straw by Biodegradation Using Streptomyces Tendae" Sustainability 13, no. 24: 13640. https://doi.org/10.3390/su132413640
APA StyleAl-Dhabaan, F. (2021). Safety Disposal of Rice Straw by Biodegradation Using Streptomyces Tendae. Sustainability, 13(24), 13640. https://doi.org/10.3390/su132413640





