Figure 1.
Schematic diagram of the polymer membrane separation system. (A) N2 or CO2, (B) floating flow controller, (C) pressure gauge, (D) precision regulator, (E) humidifier, (F) temperature and humidity meter, (G) membrane separation system, and (H) mass flow meter.
Figure 1.
Schematic diagram of the polymer membrane separation system. (A) N2 or CO2, (B) floating flow controller, (C) pressure gauge, (D) precision regulator, (E) humidifier, (F) temperature and humidity meter, (G) membrane separation system, and (H) mass flow meter.
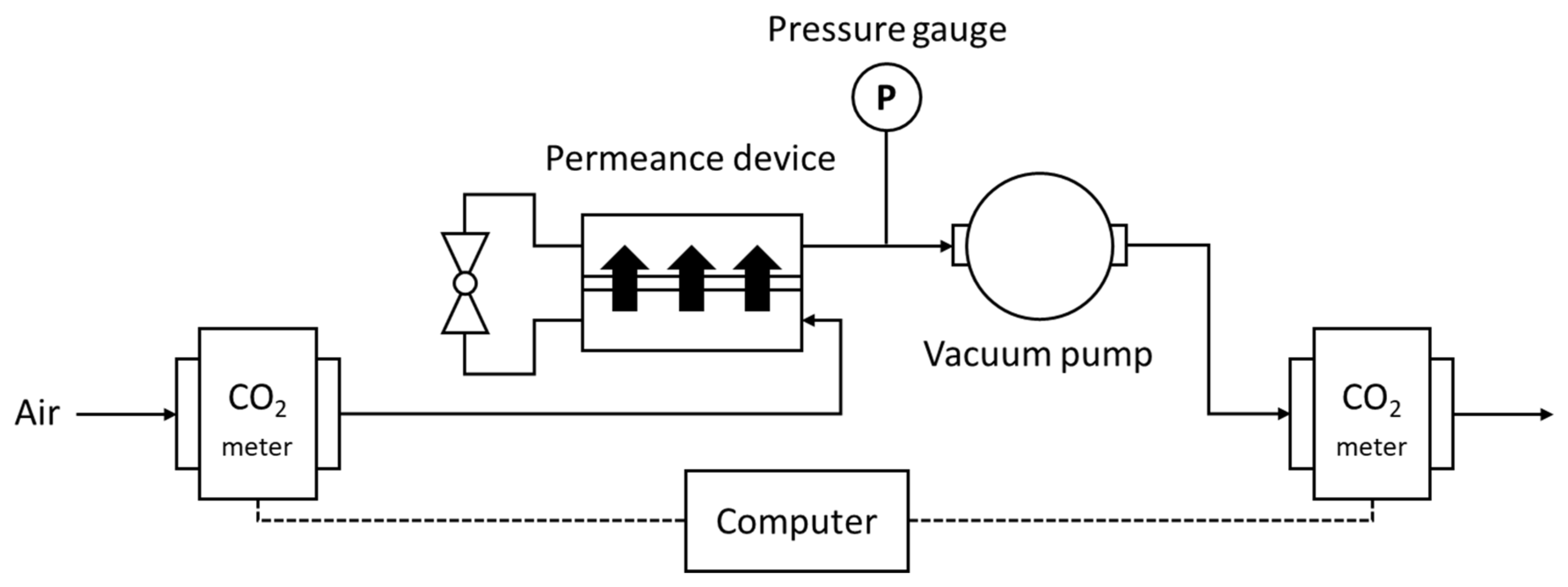
Figure 2.
CO2 membrane separation energy measurement system.
Figure 2.
CO2 membrane separation energy measurement system.
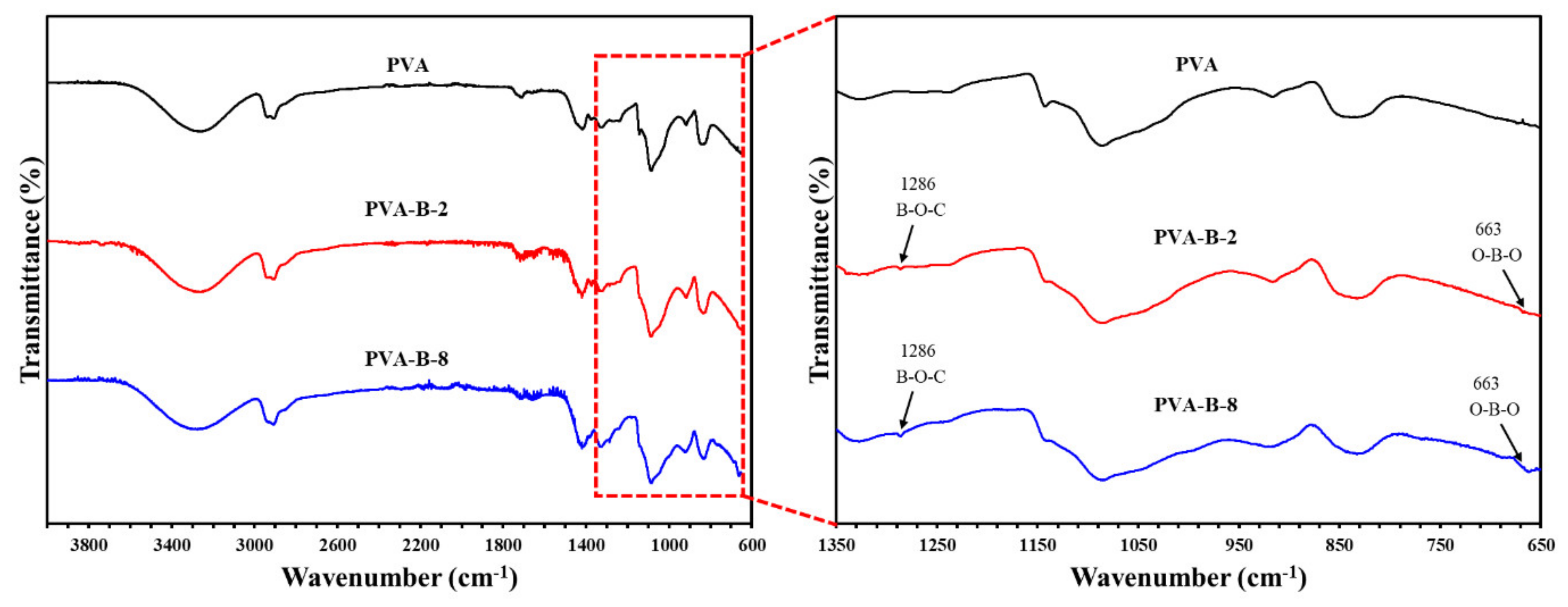
Figure 3.
Infrared spectrum of PVA, PVA-B-2, and PVA-B-8 samples.
Figure 3.
Infrared spectrum of PVA, PVA-B-2, and PVA-B-8 samples.
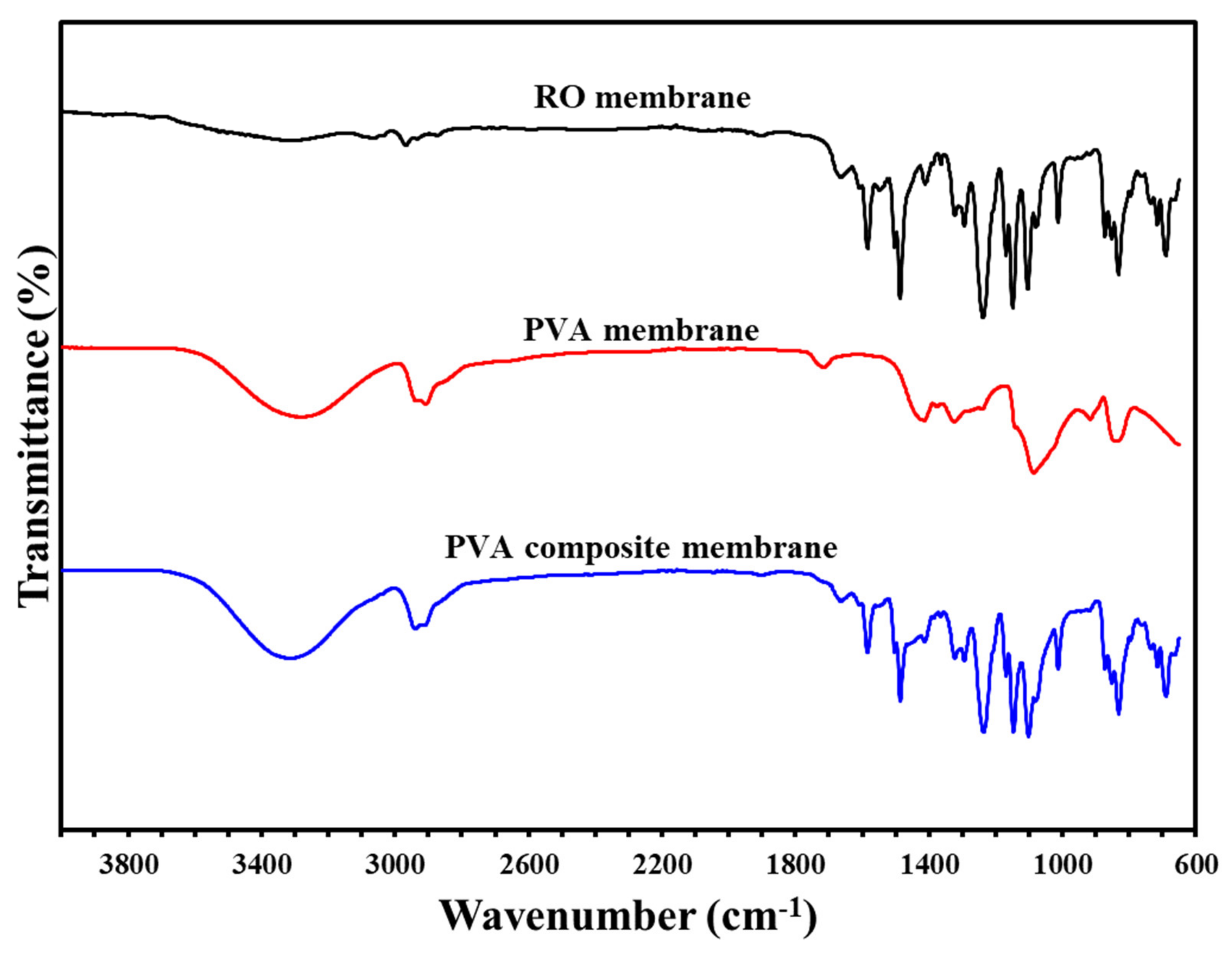
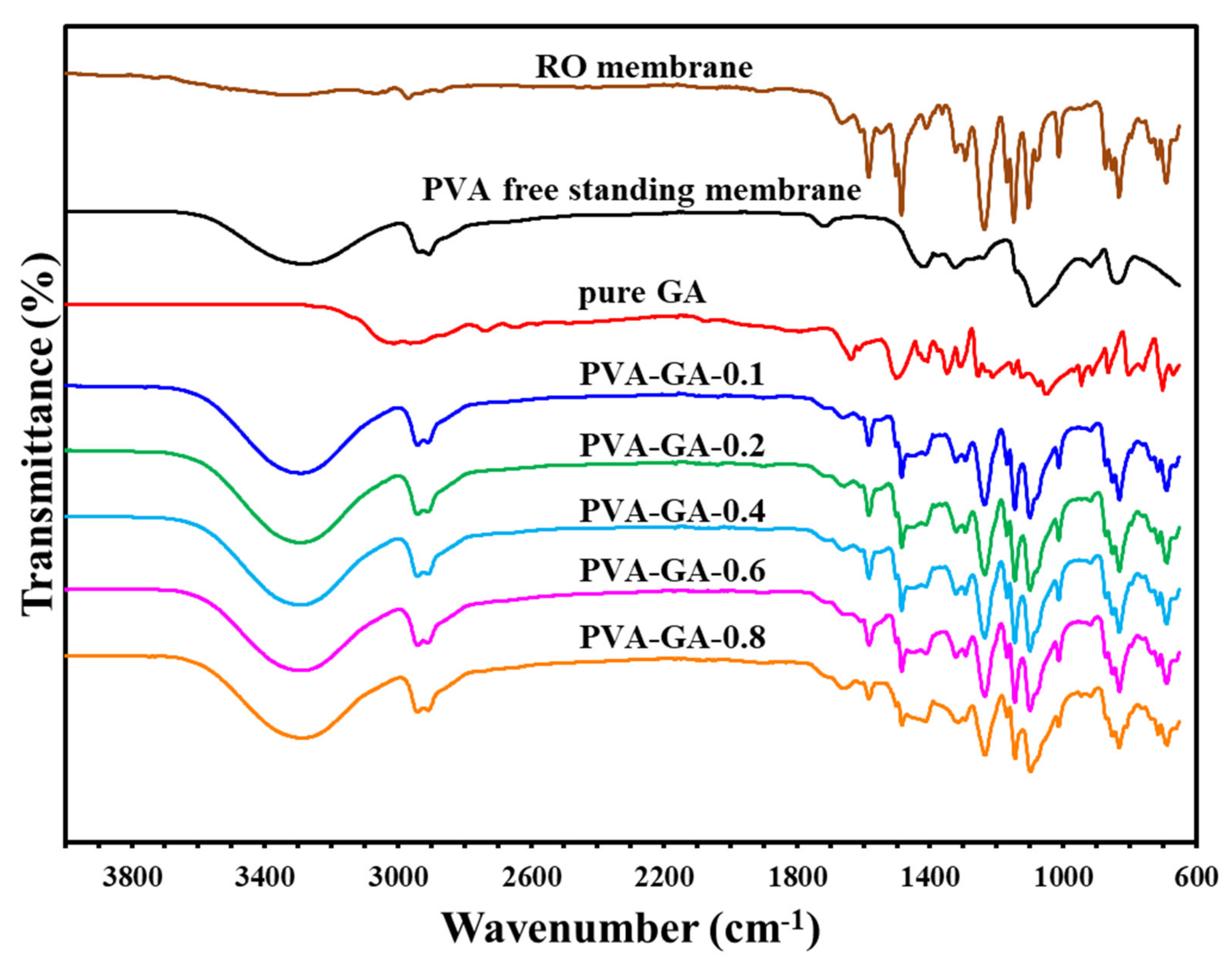
Figure 4.
The infrared spectrum of the components of the composite membranes and each layer.
Figure 4.
The infrared spectrum of the components of the composite membranes and each layer.
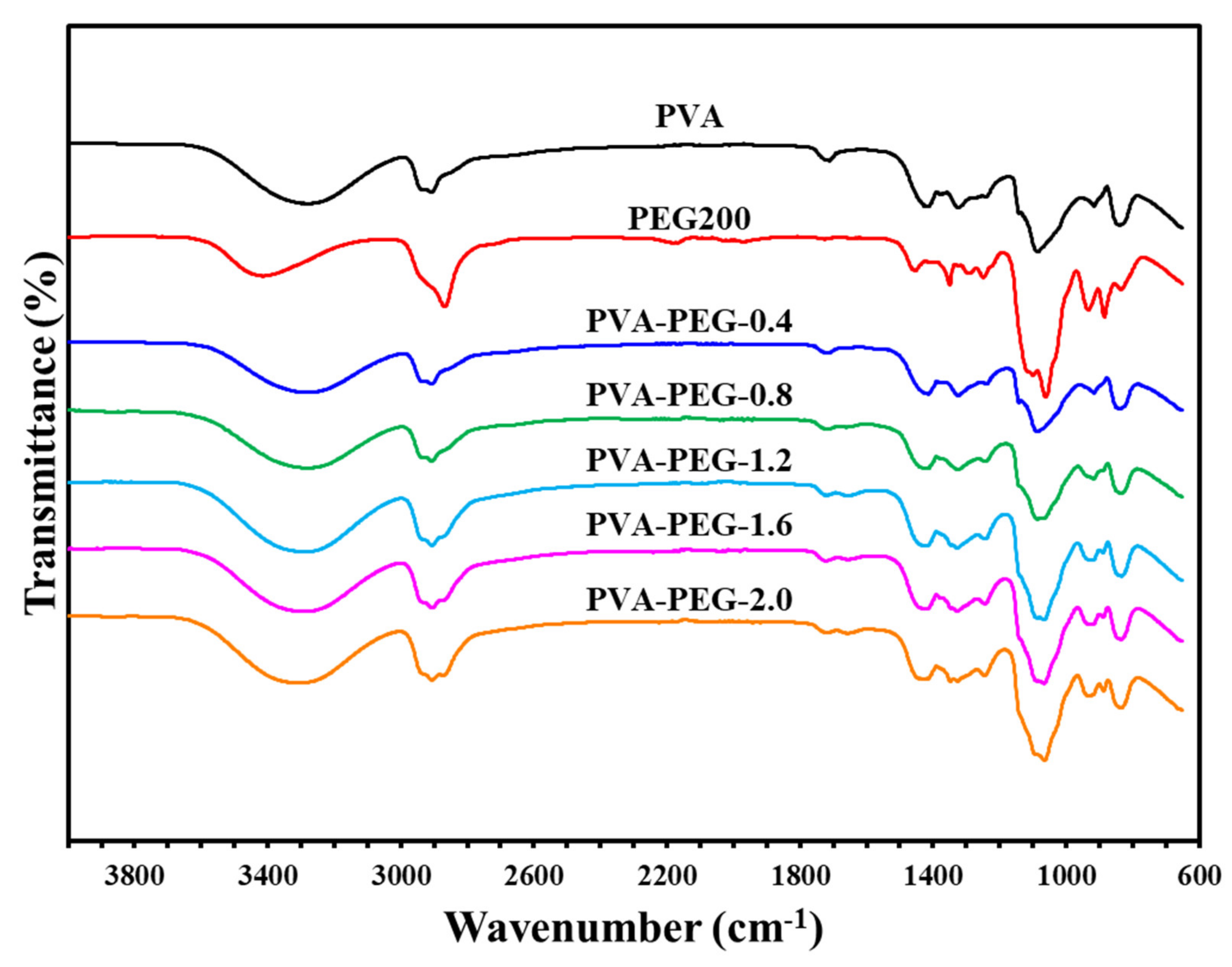
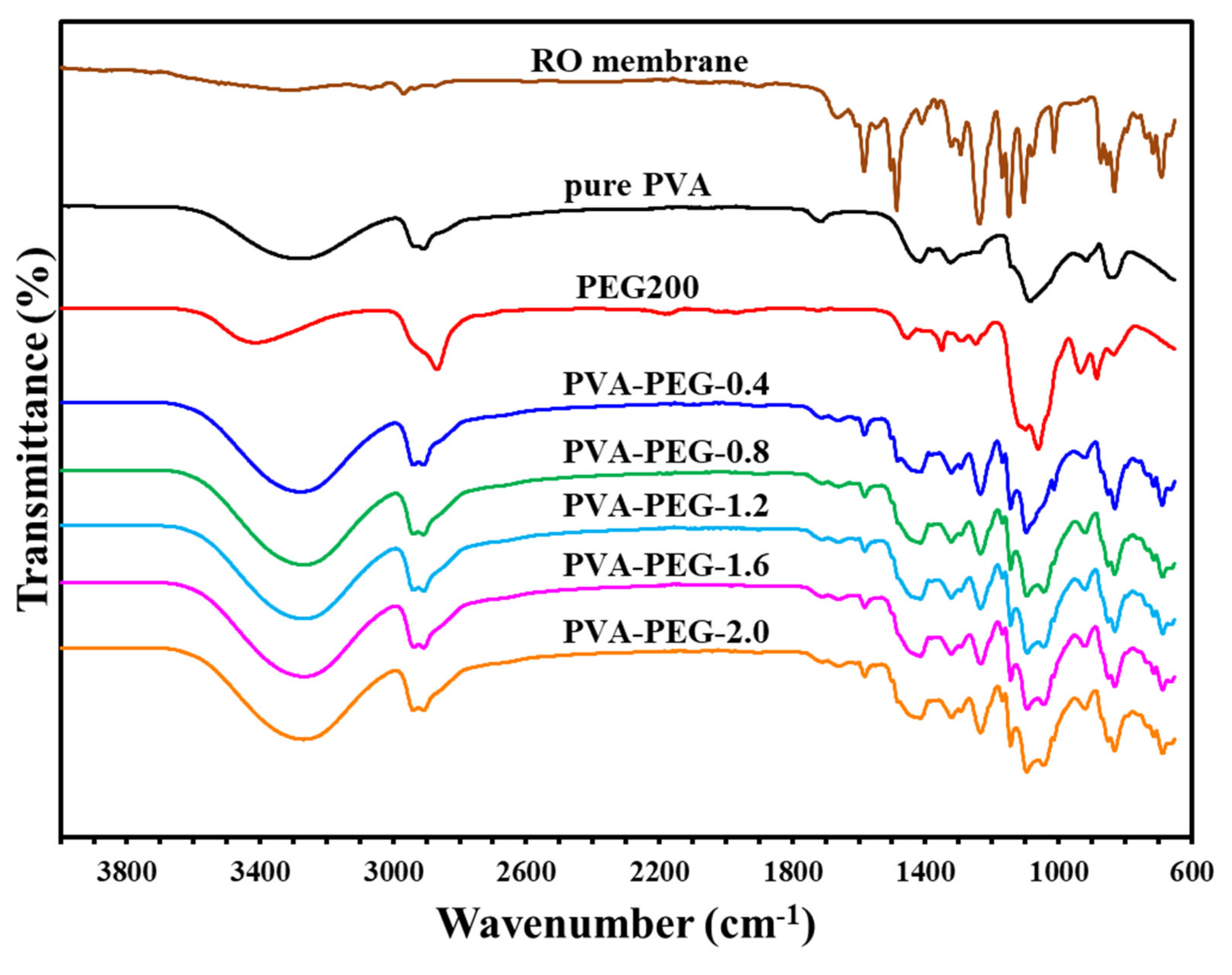
Figure 5.
Infrared spectrum of polyvinyl alcohol-polyethylene glycol self-supporting membranes.
Figure 5.
Infrared spectrum of polyvinyl alcohol-polyethylene glycol self-supporting membranes.
Figure 6.
Infrared spectrum of polyvinyl alcohol–polyethylene glycol composite membranes.
Figure 6.
Infrared spectrum of polyvinyl alcohol–polyethylene glycol composite membranes.
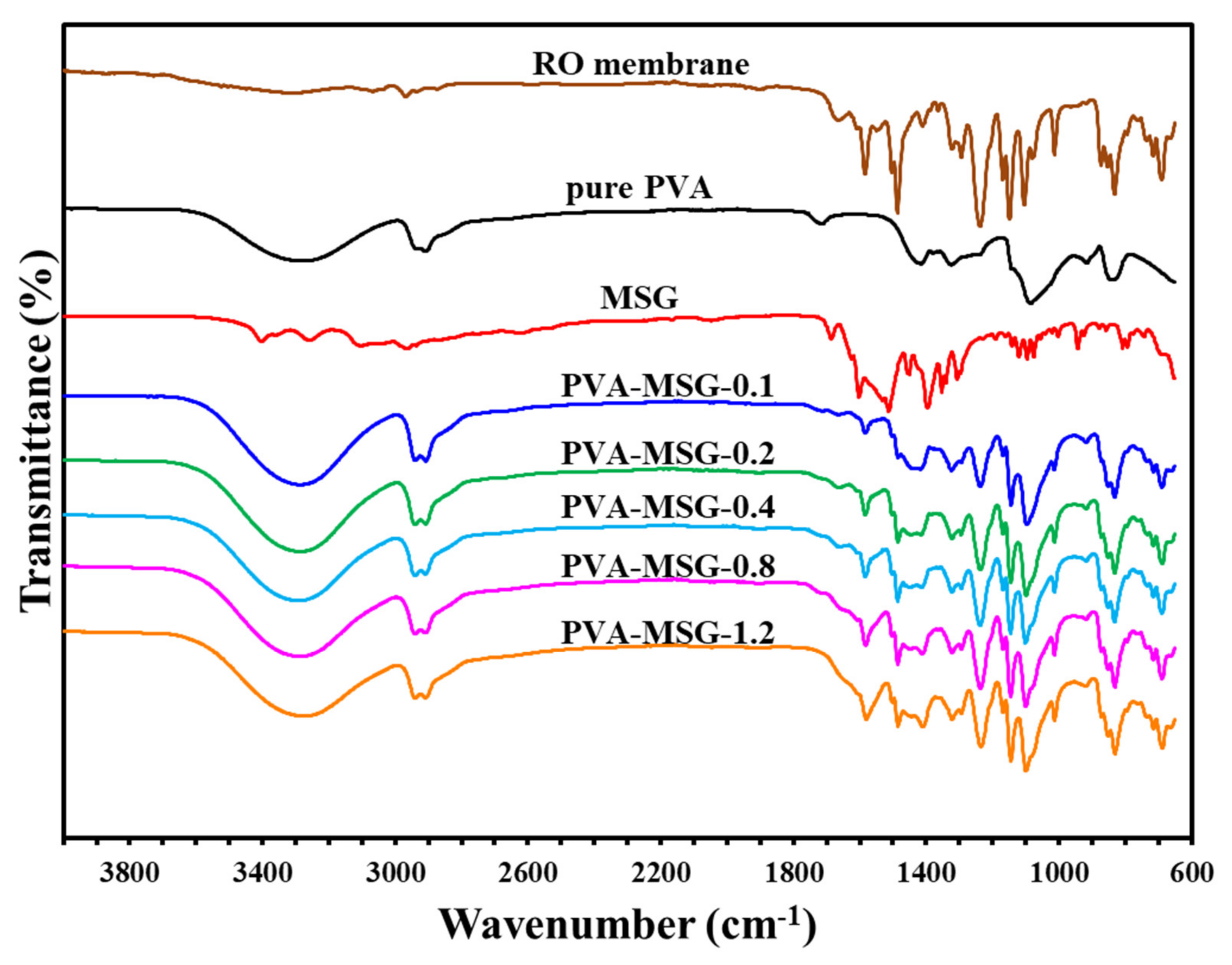
Figure 7.
Infrared spectrum of polyvinyl alcohol–sodium glutamate self-supporting membranes.
Figure 7.
Infrared spectrum of polyvinyl alcohol–sodium glutamate self-supporting membranes.
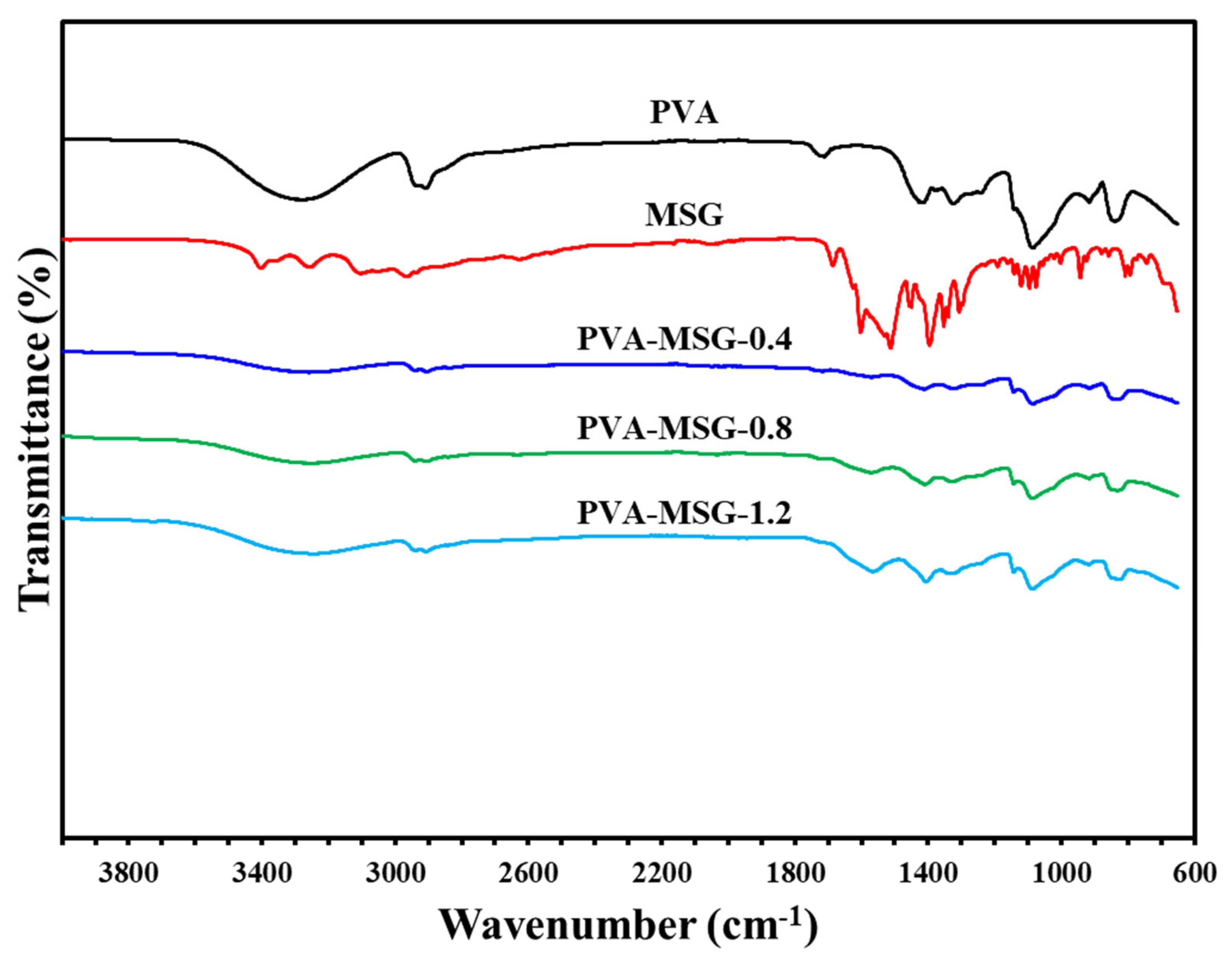
Figure 8.
Infrared spectrum of polyvinyl alcohol-sodium glutamate composite membranes.
Figure 8.
Infrared spectrum of polyvinyl alcohol-sodium glutamate composite membranes.
Figure 9.
TGA diagram of polyvinyl alcohol–glutamic acid composite membranes.
Figure 9.
TGA diagram of polyvinyl alcohol–glutamic acid composite membranes.
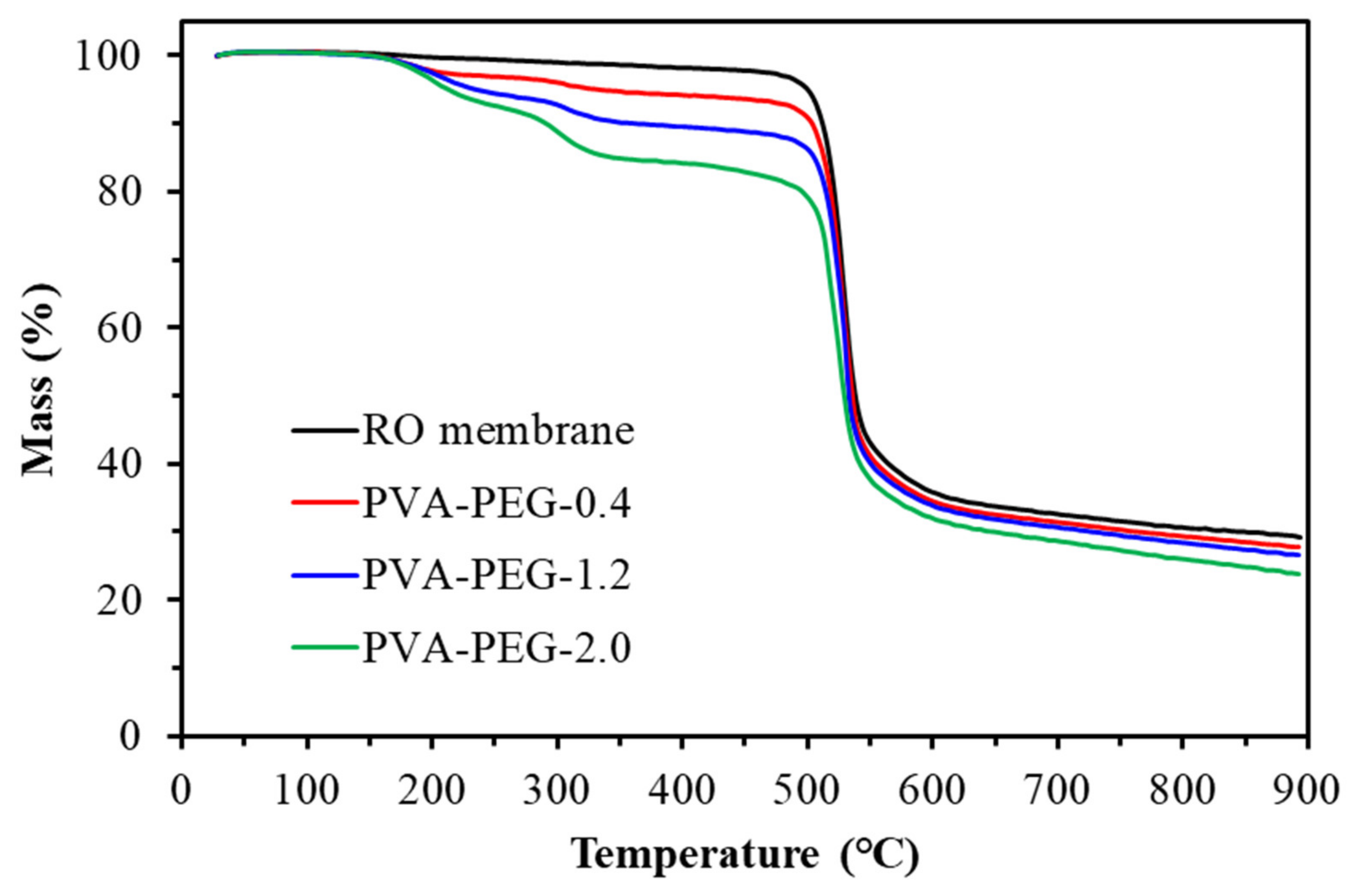
Figure 10.
TGA diagram of polyvinyl alcohol-polyethylene glycol composite membranes.
Figure 10.
TGA diagram of polyvinyl alcohol-polyethylene glycol composite membranes.
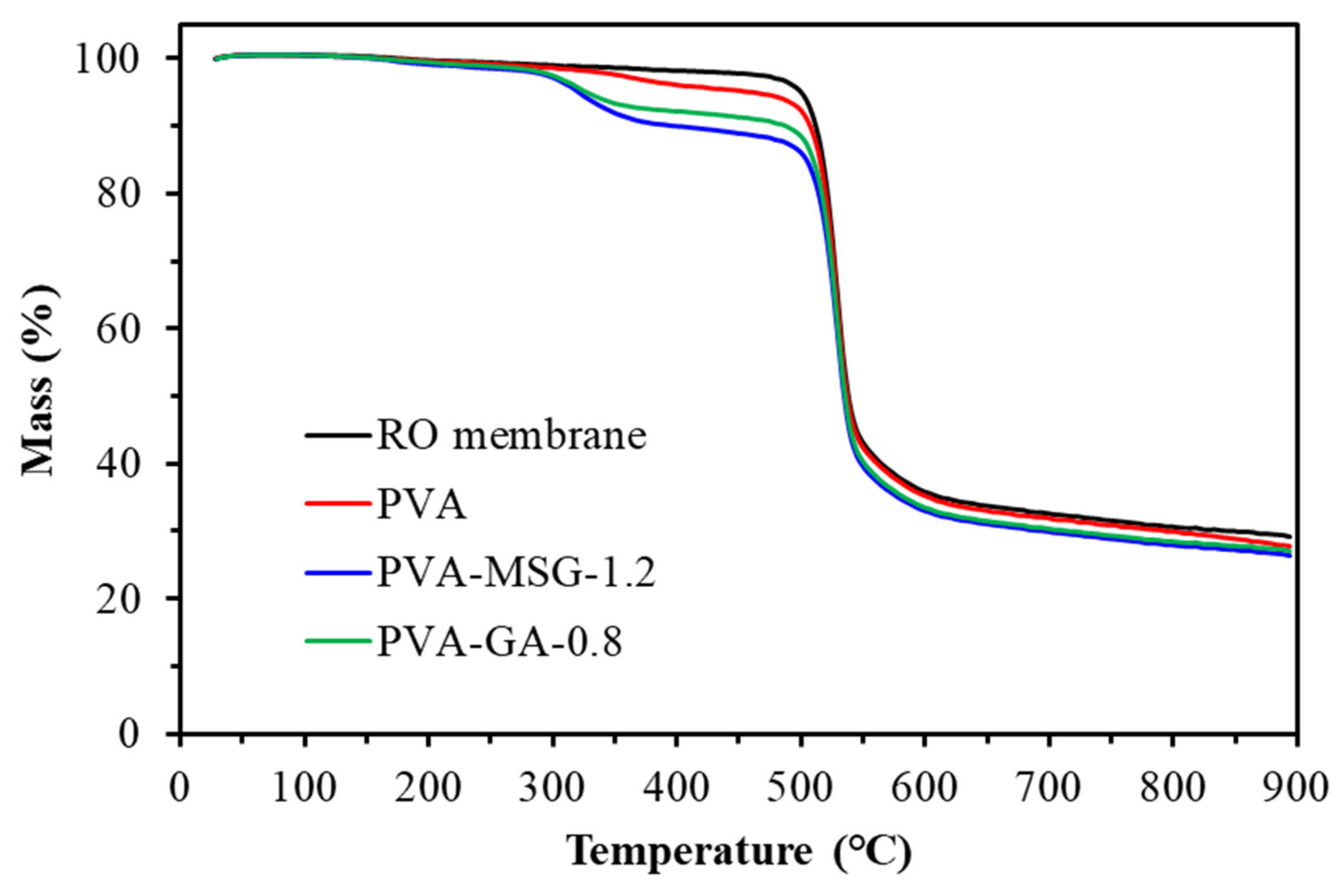
Figure 11.
TGA diagram of polyvinyl alcohol-glutamic acid (salt) composite membranes.
Figure 11.
TGA diagram of polyvinyl alcohol-glutamic acid (salt) composite membranes.
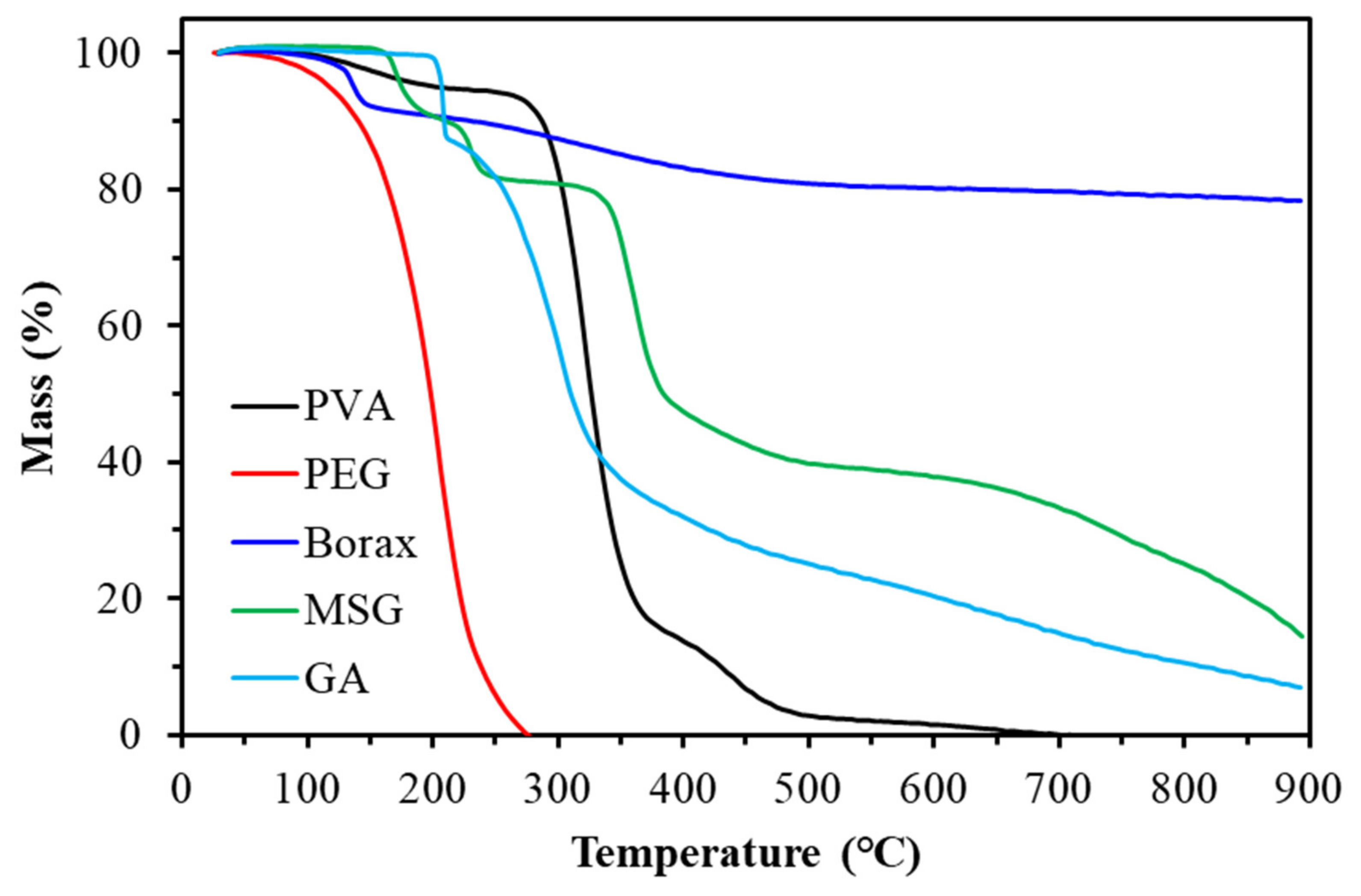
Figure 12.
TGA diagram of experimental chemicals.
Figure 12.
TGA diagram of experimental chemicals.
Figure 13.
SEM diagrams of (A) reverse osmosis film and (B) PVA-B-2 coated film.
Figure 13.
SEM diagrams of (A) reverse osmosis film and (B) PVA-B-2 coated film.
Figure 14.
SEM diagrams of different PEG content PVA-PEG composite membranes: (A) no additional PEG, (B) 10 wt%, (C) 18 wt%, (D) 24 wt%, (E) 30 wt%, and (F) 35 wt%.
Figure 14.
SEM diagrams of different PEG content PVA-PEG composite membranes: (A) no additional PEG, (B) 10 wt%, (C) 18 wt%, (D) 24 wt%, (E) 30 wt%, and (F) 35 wt%.
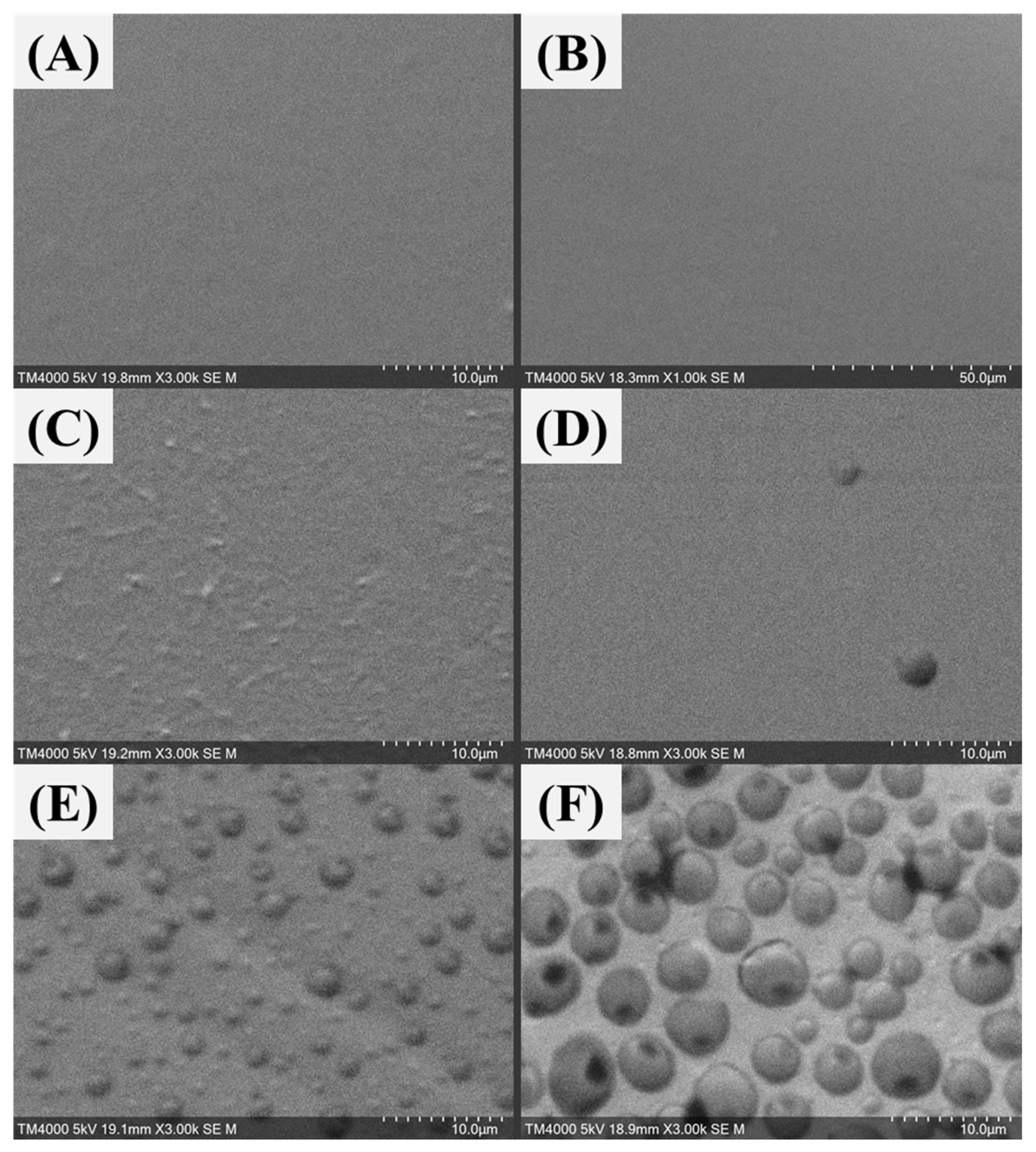
Figure 15.
SEM diagrams of different MSG content PVA-MSG composite membranes: (A) no additional MSG, (B) 2 wt%, (C) 5 wt%, (D) 9 wt%, (E) 17 wt%, and (F) 23 wt%.
Figure 15.
SEM diagrams of different MSG content PVA-MSG composite membranes: (A) no additional MSG, (B) 2 wt%, (C) 5 wt%, (D) 9 wt%, (E) 17 wt%, and (F) 23 wt%.
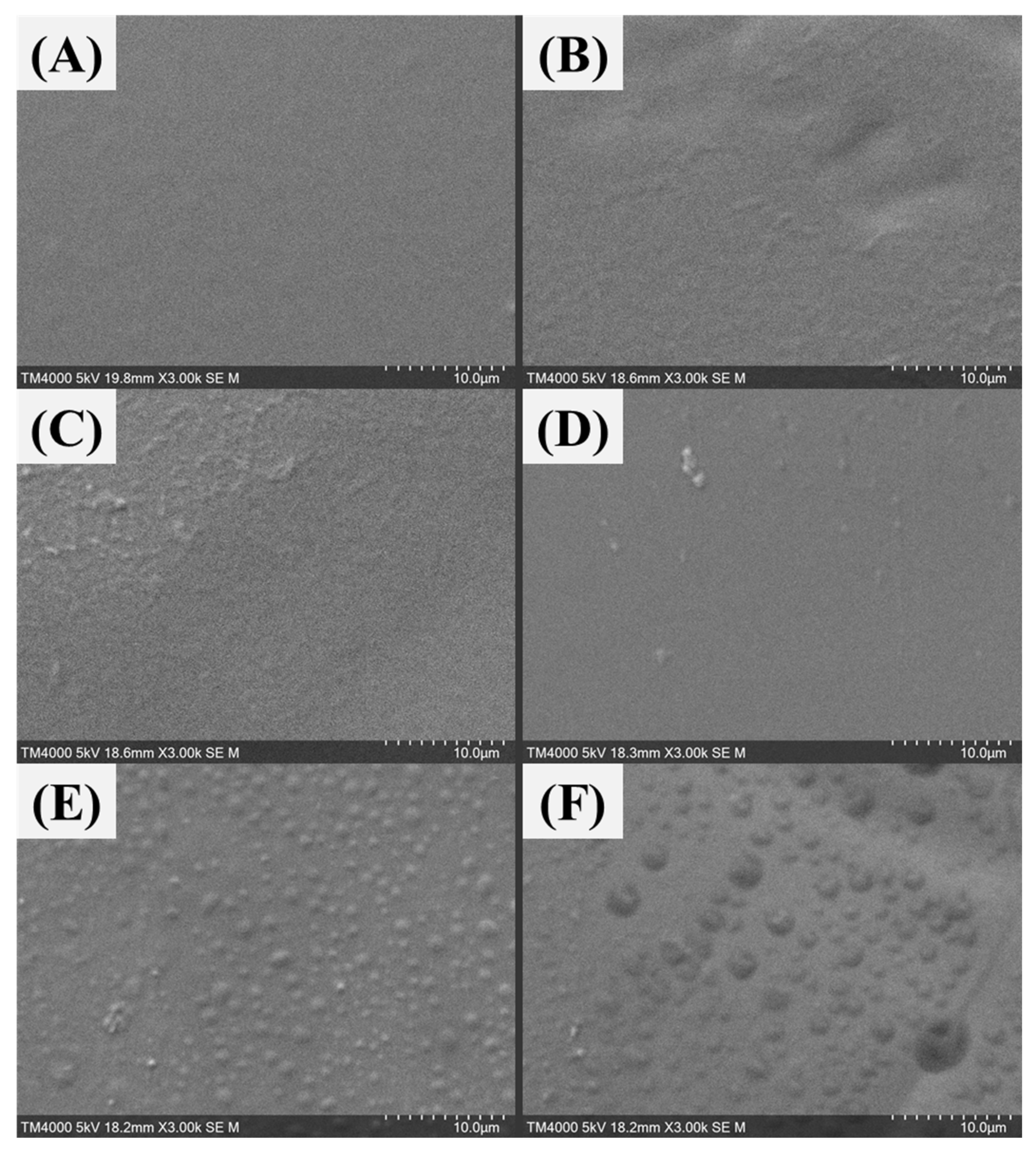
Figure 16.
SEM diagrams of different GA content PVA-GA composite membranes: (A) no additional GA, (B) 2 wt%, (C) 5 wt%, (D) 9 wt%, (E) 13 wt%, and (F) 17 wt%.
Figure 16.
SEM diagrams of different GA content PVA-GA composite membranes: (A) no additional GA, (B) 2 wt%, (C) 5 wt%, (D) 9 wt%, (E) 13 wt%, and (F) 17 wt%.
Figure 17.
Effect of PEG content on the gas permeability of composite membrane in a dry or humid environment.
Figure 17.
Effect of PEG content on the gas permeability of composite membrane in a dry or humid environment.
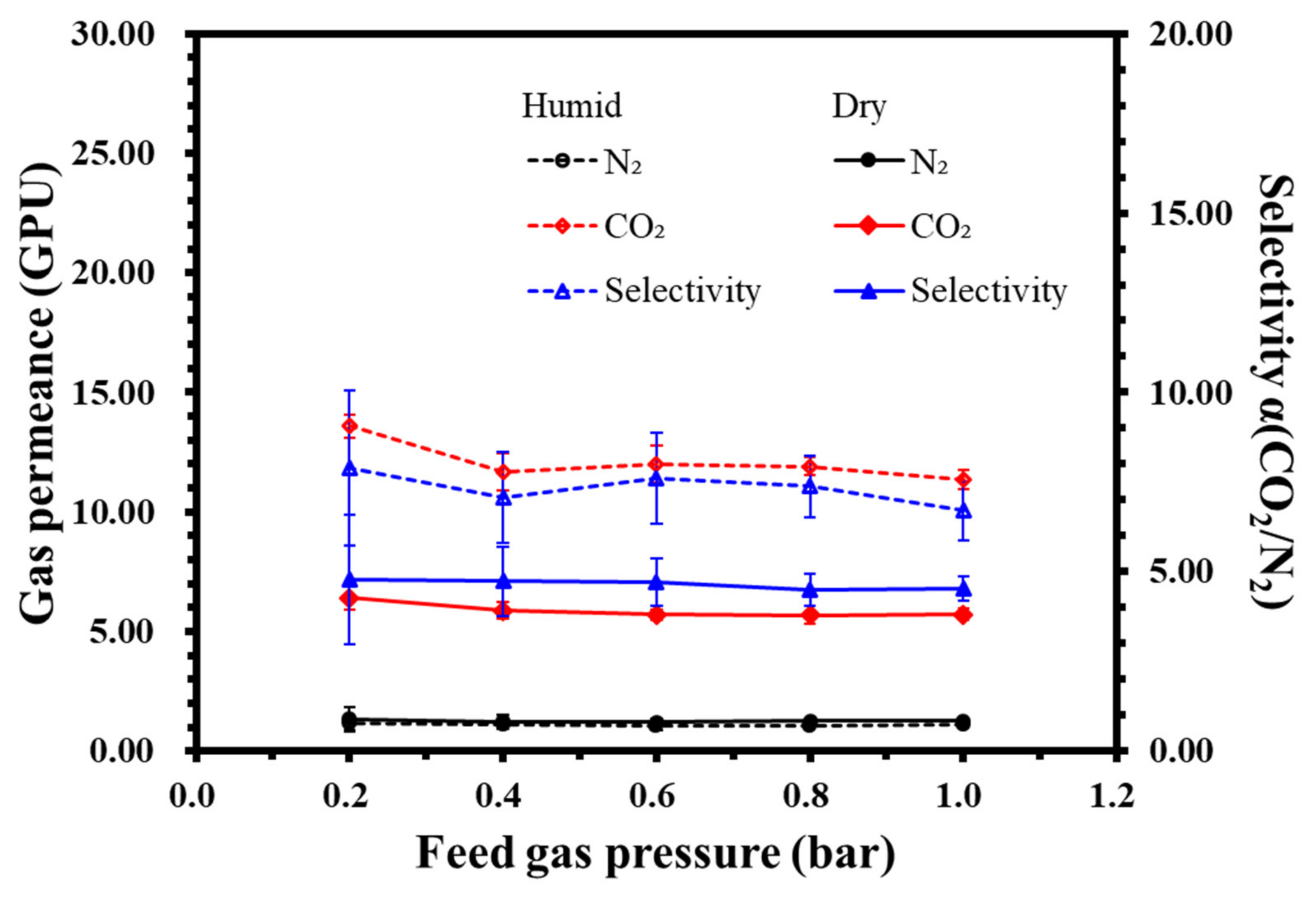
Figure 18.
The relationship between gas permeability/selectivity and the pressure of the PVA-PEG-1.2 composite membrane in a dry or humid environment.
Figure 18.
The relationship between gas permeability/selectivity and the pressure of the PVA-PEG-1.2 composite membrane in a dry or humid environment.
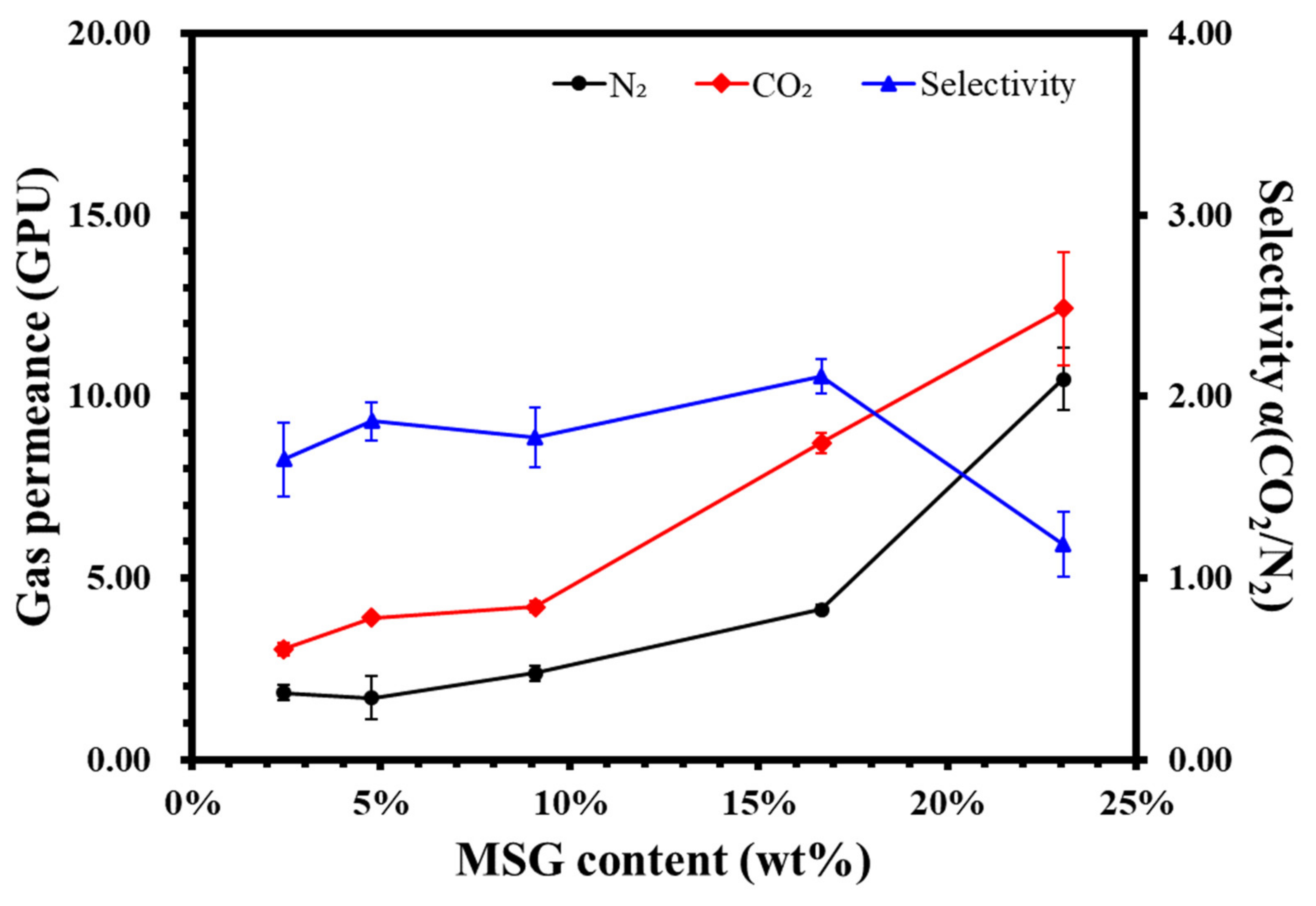
Figure 19.
Effect of sodium glutamate content on the gas permeability of composite membrane.
Figure 19.
Effect of sodium glutamate content on the gas permeability of composite membrane.
Figure 20.
Effect of glutamic acid content on the gas permeability of composite membrane.
Figure 20.
Effect of glutamic acid content on the gas permeability of composite membrane.
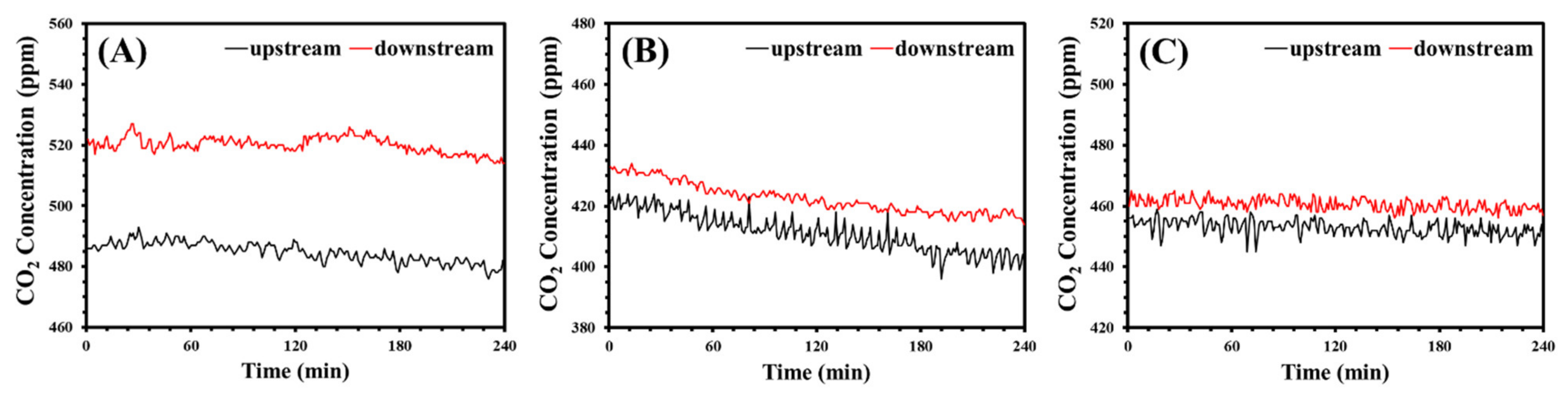
Figure 21.
The concentration difference of CO2 in the composite membranes: (A) PVA-PEG-1.2, (B) PVA-MSG-0.8, and (C) PVA-GA-0.4.
Figure 21.
The concentration difference of CO2 in the composite membranes: (A) PVA-PEG-1.2, (B) PVA-MSG-0.8, and (C) PVA-GA-0.4.
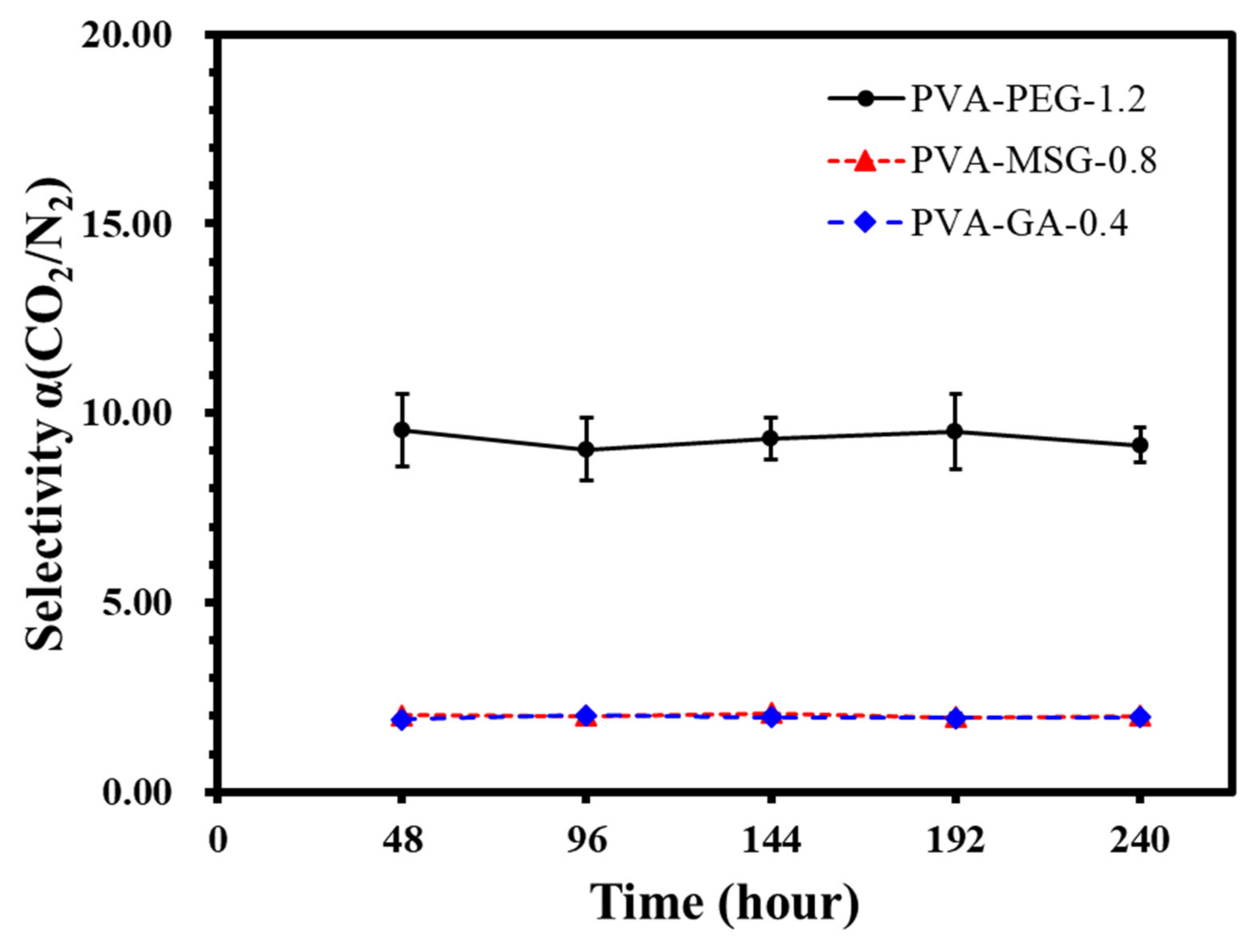
Figure 22.
The selectivity of PVA-PEG-1.2, PVA-MSG-0.8, and PVA-GA-0.4 composite membranes.
Figure 22.
The selectivity of PVA-PEG-1.2, PVA-MSG-0.8, and PVA-GA-0.4 composite membranes.
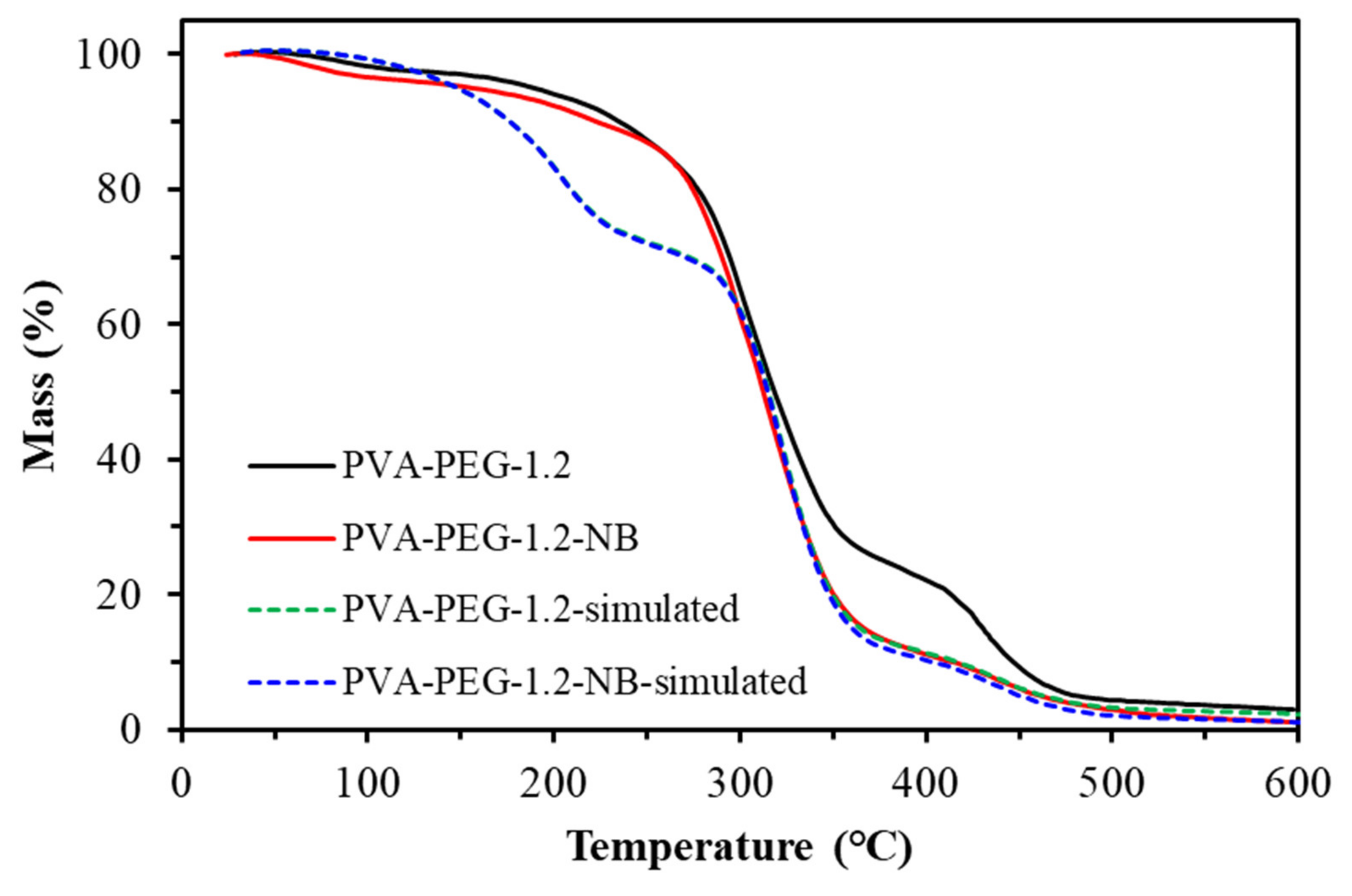
Figure 23.
Thermal mass of the PVA-PEG-1.2 composite membrane.
Figure 23.
Thermal mass of the PVA-PEG-1.2 composite membrane.
Table 1.
Peak assignments of PVA and cross-linked PVA.
Table 1.
Peak assignments of PVA and cross-linked PVA.
| Peak Position (cm−1) | Peak Assignment |
|---|
| PVA | PVA-B-Series |
|---|
| 3100–3600 | 3100–3600 | O-H stretching |
| 2850–2950 | 2850–2950 | C-H symmetric/asymmetric stretching |
| 1730 | 1730 | C=O stretching |
| 1350 | 1350 | C-H bending |
| 1140 | 1140 | C-O stretching |
| 1090 | 1090 | C-O stretching |
| | 1286 | B-O-C stretching |
| | 663 | O-B-O stretching |
Table 2.
Peak assignments of composite membrane.
Table 2.
Peak assignments of composite membrane.
| Peak Position (cm−1) | Peak Assignment |
|---|
| 3600–3000 | O-H stretching |
| 2966 | C-H stretching |
| 2950–2850 | C-H symmetric asymmetric stretching |
| 1585 | C=C stretching |
| 1560 | N-H stretching |
| 1500 | C=C stretching |
| 1487 | C=C stretching |
| 1236 | C-O-C stretching |
| 1149 | O-S-O stretching |
| 1050 | C-O stretching |
| 1014 | C-H stretching |
| 831 | C-H stretching |
| 690 | C-S-C stretching |
Table 3.
Peak assignments of PVA-X composite membranes.
Table 3.
Peak assignments of PVA-X composite membranes.
| Peak Position (cm−1) | Peak Assignment |
|---|
| PVA-PEG | PVA-MSG | PVA-GA | |
|---|
| 3000–3600 | 3000–3600 | 3000–3600 | O-H stretching |
| 2800–2900 | 2950–3050 | 2950–3050 | C-H stretching |
| | | 1635 | N-H stretching |
| | 1602 | | C-O stretching |
| | | 1500 | N-H stretching, symmetric |
| | | 1400 | COOH stretching, symmetric |
| | 1395 | | C-O stretching |
| 1350 | | | C-O stretching |
| 1090 | | | C-O stretching |
Table 4.
Thermal gravimetric analysis.
Table 4.
Thermal gravimetric analysis.
| Sample | 1st Degradation | 2nd Degradation | 3rd Degradation |
|---|
| | T1 (°C) | Weight Loss (%) | T2 (°C) | Weight Loss (%) | T3 (°C) | Weight Loss (%) |
|---|
| RO | 500–550 | 50 | - | - | - | - |
| PVA-PEG-2.0 | 150–250 | 7.3 | 275–350 | 5.8 | 500–550 | 45.8 |
| PVA-MSG-1.2 | 275–375 | 7.7 | 500–550 | 51.1 | - | - |
| PVA-GA-0.8 | 275–375 | 5.9 | 500–550 | 54.0 | - | - |
Table 5.
Gas permeability and selectivity in a dry environment *.
Table 5.
Gas permeability and selectivity in a dry environment *.
| Sample | PEG (wt%) | Permeance (GPU) | Selectivity (CO2/N2) |
|---|
| CO2 | N2 |
|---|
| PVA-B-2 | 0 | 0.71 ± 0.12 | 0.26 ± 0.12 | 2.80 ± 1.36 |
| PVA-PEG-0.4 | 10 | 1.10 ± 0.16 | 0.48 ± 0.12 | 2.26 ± 0.63 |
| PVA-PEG-0.8 | 18 | 3.83 ± 0.28 | 0.87 ± 0.12 | 4.41 ± 0.68 |
| PVA-PEG-1.2 | 24 | 5.72 ± 0.25 | 1.26 ± 0.08 | 4.53 ± 0.34 |
| PVA-PEG-1.6 | 30 | 8.42 ± 0.70 | 1.94 ± 0.16 | 4.34 ± 0.51 |
| PVA-PEG-2.0 | 35 | 10.86 ± 0.54 | 2.65 ± 0.19 | 4.10 ± 0.36 |
Table 6.
Gas permeability and selectivity in a humid environment *.
Table 6.
Gas permeability and selectivity in a humid environment *.
| Sample | PEG (wt%) | Permeance (GPU) | Selectivity (CO2/N2) |
|---|
| CO2 | N2 |
|---|
| PVA-B-2 | 0 | 0.92 ± 0.15 | 0.15 ± 0.08 | 6.00 ± 3.16 |
| PVA-PEG-0.4 | 10 | 2.42 ± 0.25 | 0.31 ± 0.08 | 7.92 ± 2.14 |
| PVA-PEG-0.8 | 18 | 5.18 ± 0.27 | 0.64 ± 0.12 | 8.12 ± 1.55 |
| PVA-PEG-1.2 | 24 | 11.34 ± 0.41 | 1.13 ± 0.13 | 10.05 ± 1.22 |
| PVA-PEG-1.6 | 30 | 13.46 ± 0.15 | 1.68 ± 0.15 | 8.00 ± 0.73 |
| PVA-PEG-2.0 | 35 | 14.54 ± 0.58 | 2.17 ± 0.12 | 6.71 ± 0.45 |
Table 7.
The gas permeability and selectivity of PVA-PEG-1.2 membrane in varied pressure.
Table 7.
The gas permeability and selectivity of PVA-PEG-1.2 membrane in varied pressure.
| Pressure (bar) | Permeance (GPU) | Selectivity |
|---|
| Dry | Humid | Dry | Humid |
|---|
| CO2 | N2 | CO2 | N2 | (CO2/N2) |
|---|
| 1.00 | 5.72 ± 0.25 | 1.26 ± 0.08 | 11.34 ± 0.41 | 1.13 ± 0.13 | 4.53 ± 0.34 | 10.05 ± 1.22 |
| 0.80 | 5.69 ± 0.37 | 1.27 ± 0.09 | 11.91 ± 0.39 | 1.08 ± 0.12 | 4.49 ± 0.44 | 11.07 ± 1.29 |
| 0.60 | 5.71 ± 0.22 | 1.21 ± 0.16 | 11.99 ± 0.79 | 1.05 ± 0.16 | 4.71 ± 0.66 | 11.39 ± 1.89 |
| 0.40 | 5.88 ± 0.33 | 1.24 ± 0.25 | 11.67 ± 0.78 | 1.10 ± 0.18 | 4.73 ± 0.98 | 10.61 ± 1.90 |
| 0.20 | 6.41 ± 0.48 | 1.34 ± 0.49 | 13.58 ± 0.49 | 1.15 ± 0.31 | 4.79 ± 1.80 | 11.83 ± 3.25 |
Table 8.
Gas permeability of polyvinyl alcohol-sodium glutamate composite membranes *.
Table 8.
Gas permeability of polyvinyl alcohol-sodium glutamate composite membranes *.
| Sample | MSG (wt%) | Permeance (GPU) | Selectivity (CO2/N2) |
|---|
| CO2 | N2 |
|---|
| PVA-MSG-0.1 | 2 | 3.03 ± 0.16 | 1.84 ± 0.20 | 1.65 ± 0.20 |
| PVA-MSG-0.2 | 5 | 3.91 ± 0.08 | 1.69 ± 0.60 | 1.86 ± 0.10 |
| PVA-MSG-0.4 | 9 | 4.21 ± 0.15 | 2.37 ± 0.20 | 1.77 ± 0.16 |
| PVA-MSG-0.8 | 17 | 8.72 ± 0.27 | 4.13 ± 0.13 | 2.11 ± 0.09 |
| PVA-MSG-1.2 | 23 | 12.42 ± 1.55 | 10.48 ± 0.85 | 1.18 ± 0.18 |
Table 9.
Gas permeability of polyvinyl alcohol/glutamic acid composite membranes *.
Table 9.
Gas permeability of polyvinyl alcohol/glutamic acid composite membranes *.
| Sample | GA (wt%) | Permeance (GPU) | Selectivity (CO2/N2) |
|---|
| CO2 | N2 |
|---|
| PVA-GA-0.1 | 2 | 2.78 ± 0.19 | 1.76 ± 0.08 | 1.58 ± 0.13 |
| PVA-GA-0.2 | 5 | 2.83 ± 0.15 | 1.63 ± 0.56 | 1.46 ± 0.12 |
| PVA-GA-0.4 | 9 | 5.43 ± 0.20 | 2.81 ± 0.12 | 1.94 ± 0.11 |
| PVA-GA-0.6 | 13 | 7.06 ± 0.27 | 3.85 ± 0.12 | 1.83 ± 0.09 |
| PVA-GA-0.8 | 17 | 7.88 ± 0.20 | 6.15 ± 0.23 | 1.28 ± 0.06 |
Table 10.
Separation efficiency of the composite membranes.
Table 10.
Separation efficiency of the composite membranes.
| Sample | Pressure Difference (mmHg) | CO2 Concentration (ppm) |
|---|
| blank | - | 2.43 ± 2.08 |
| reverse osmosis membrane | 700 | 2.73 ± 1.43 |
| PVA-PEG-1.2 | 700 | 35.37 ± 3.15 |
| PVA-MSG-0.8 | 700 | 11.32 ± 3.32 |
| PVA-GA-0.4 | 700 | 7.70 ± 2.76 |
Table 11.
Water absorption measurement of the self-supporting membranes.
Table 11.
Water absorption measurement of the self-supporting membranes.
| Sample | Water Uptake (%) |
|---|
| PVA | 52.3 ± 1.4 |
| PVA-B-2 | 60.7 ± 1.3 |
| PVA-PEG-1.2 | 72.1 ± 1.6 |
| PVA-MSG-0.8 | 39.9 ± 1.3 |
| PVA-GA-0.4 | N/A |
Table 12.
The selectivity α(CO2/N2) of PVA-X composite membranes.
Table 12.
The selectivity α(CO2/N2) of PVA-X composite membranes.
| Time (hour) | PVA-PEG-1.2 | PVA-MSG-0.8 | PVA-GA-0.4 |
|---|
| 48 | 9.59 ± 0.97 | 2.10 ± 0.08 | 1.91 ± 0.09 |
| 96 | 9.56 ± 0.95 | 2.02 ± 0.13 | 2.01 ± 0.04 |
| 144 | 9.04 ± 0.83 | 2.01 ± 0.15 | 1.97 ± 0.11 |
| 192 | 9.33 ± 0.54 | 1.97 ± 0.09 | 1.95 ± 0.14 |
| 240 | 9.51 ± 0.99 | 2.01 ± 0.10 | 1.97 ± 0.09 |
Table 13.
The composition of samples with or without Borax.
Table 13.
The composition of samples with or without Borax.
| Sample | PVA (g) | PEG (mL) | Borax (g) |
|---|
| PVA-PEG-1.2 | 4.00 | 1.20 | 0.08 |
| PVA-PEG-1.2-NB | 4.00 | 1.20 | - |
Table 14.
The gas separation and adsorption properties of samples with or without Borax.
Table 14.
The gas separation and adsorption properties of samples with or without Borax.
| Sample | Permeance (GPU) | Selectivity
(CO2/N2) | Water Uptake (%) |
|---|
| CO2 | N2 |
|---|
| PVA-PEG-1.2 | 10.76 ± 0.61 | 1.05 ± 0.12 | 10.05 ± 1.22 | 72.1 ± 1.6 |
| PVA-PEG-1.2-NB | 13.95 ± 0.42 | 1.66 ± 0.12 | 8.42 ± 0.65 | 66.9 ± 1.7 |
Table 15.
Gas dynamic radius.
Table 15.
Gas dynamic radius.
| Molecule | Kinetic Diameter (Å) |
|---|
| CO2 | 3.30 |
| N2 | 3.64 |