Effects of Experimental Warming and Canada Goldenrod Invasion on the Diversity and Function of the Soil Nematode Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Soil Sample Collection and Preparation
2.3. Soil Property Measurements
2.4. Soil Nematode Extraction, Classification, and Analyses
2.5. Statistical Analyses
3. Results
3.1. Soil Properties
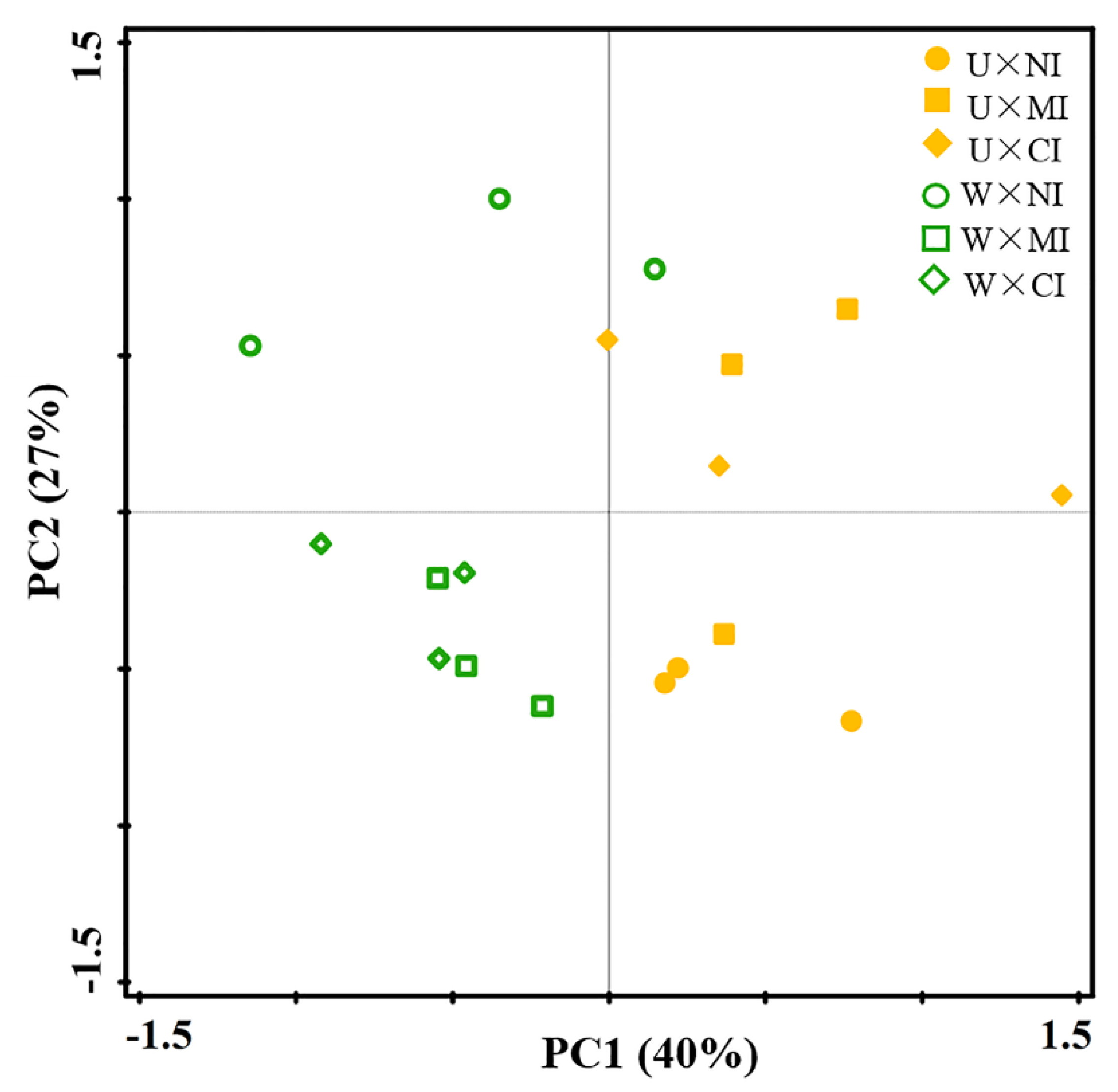
3.2. Soil Nematode Diversity and Functional Indices
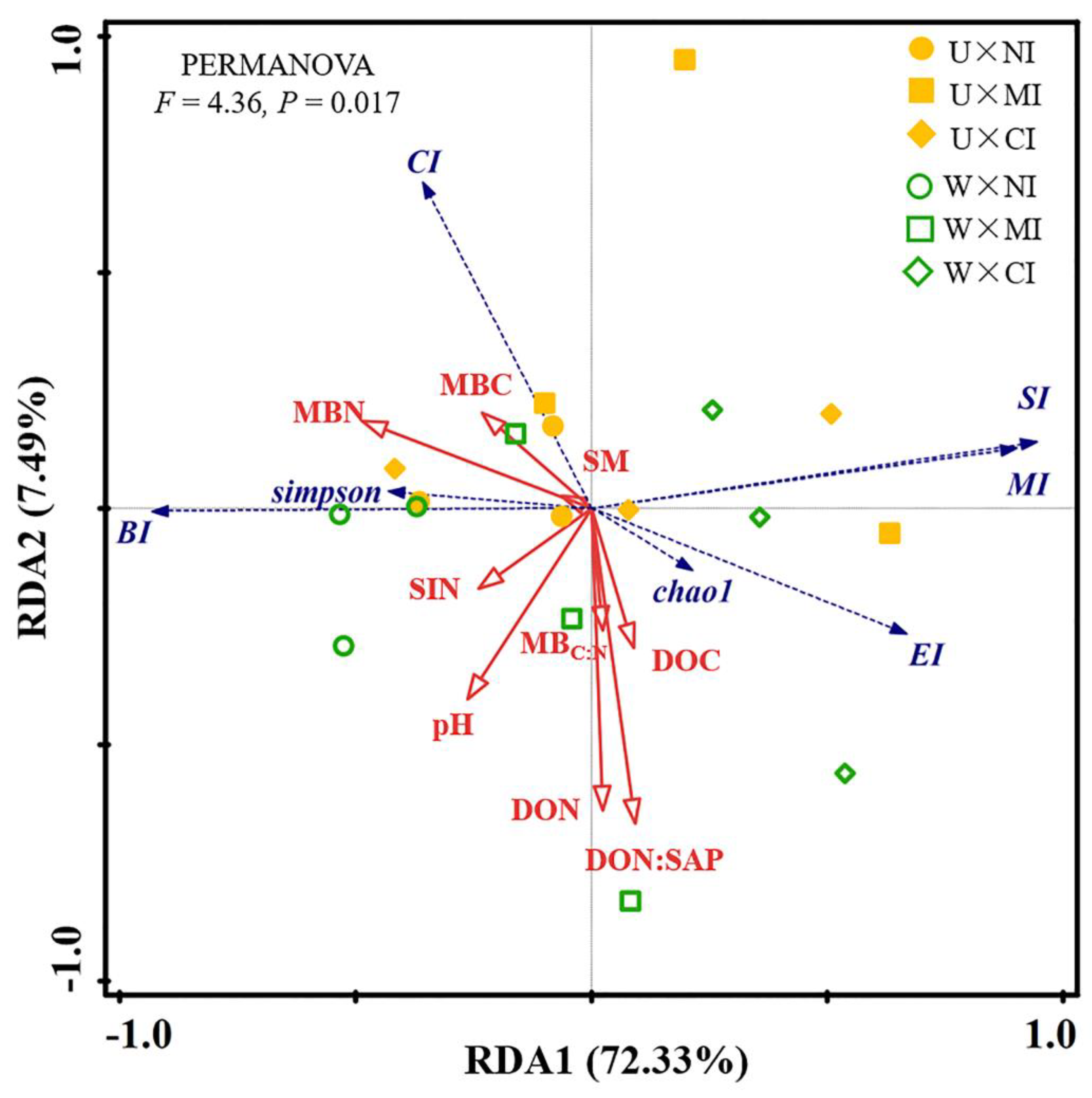
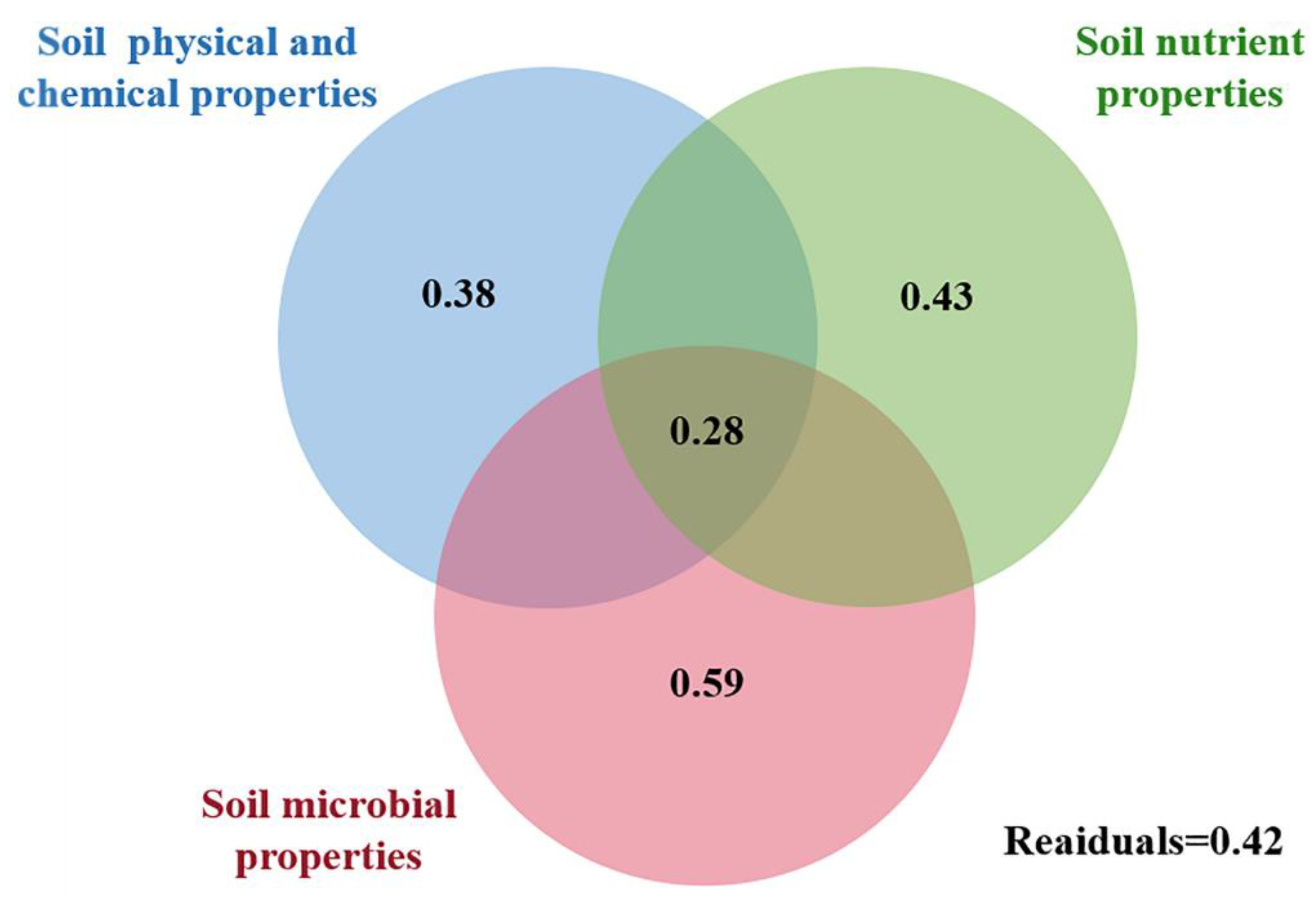
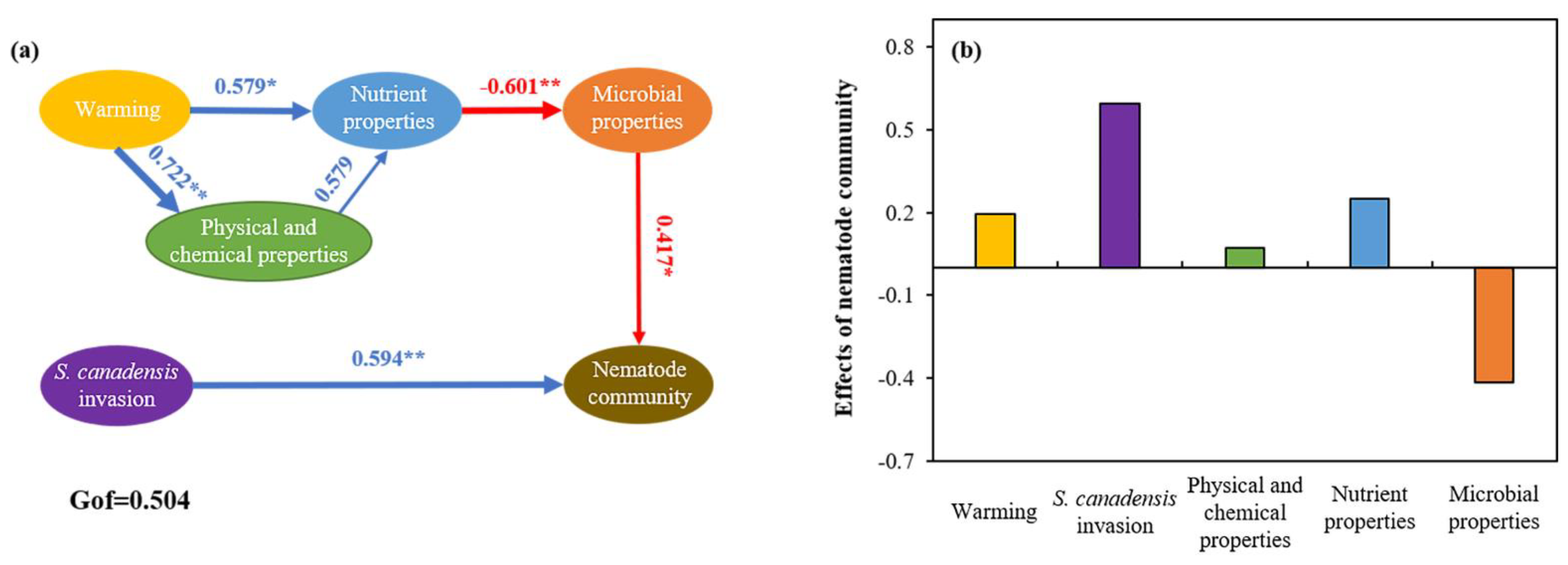
3.3. Relationship among Soil Properties and Ecological Function Indexes of Soil Nematode Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schleuning, M.; Frund, J.; Garcia, D. Predicting ecosystem functions from biodiversity and mutualistic networks: An extension of trait-based concepts to plant animal interactions. Ecography 2015, 38, 380–392. [Google Scholar] [CrossRef]
- Del-Claro, K.; Torezan-Silingardi, H.M. Plant-Mediated Above-Belowground Interactions: A Phytobiome Story. In Plant-Animal Interactions Source of Biodiversity; Springer Nature Switzerland: Cham, Switzerland, 2021; pp. 205–232. [Google Scholar] [CrossRef]
- Krupa, J.J.; Hopper, K.R.; Harwood, J.D. Plant animal interactions between carnivorous plants, sheetweb spiders, and ground-running spiders as guild predators in a wet meadow community. Ecol. Evol. 2020, 10, 4762–4772. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S. Nematodes as Drivers of Plant Performance in Natural Systems. Trends Plant Sci. 2021, 26, 237–247. [Google Scholar] [CrossRef]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar]
- Hu, C.; Xia, X.G.; Han, X.M.; Chen, Y.F.; Qiao, Y.; Liu, D.H.; Li, S.L. Soil nematode abundances were increased by an incremental nutrient input in a paddy-upland rotation system. Helminthologia 2018, 55, 322–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.; Bhandari, S.; Bhusal, D.R. Variation of nematode indices under contrasting pest management practices in a tomato growing agro-ecosystem. Heliyon 2019, 5, e02621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maina, S.; Karuri, H.; Ng’endo, R.N. Nematode metabolic footprints, ecological and functional indices in tropical maize-beans agro-ecosystems under different farming practices. Acta Oecol. 2020, 108, 103622. [Google Scholar] [CrossRef]
- Guo, X.H.; Endler, A.; Poll, C.; Marhan, S.; Ruess, L. Independent effects of warming and altered precipitation pattern on nematode community structure in an arable field. Agric. Ecosyst. Environ. 2021, 316, 107467. [Google Scholar] [CrossRef]
- Coffey, V.; Otfinowski, R. Legacies of afforestation on soil nematode community composition, structure, and diversity in a northern Canadian prairie. Plant Soil. 2019, 435, 437–447. [Google Scholar] [CrossRef]
- Darby, B.J.; Neher, D.A.; Housman, D.C.; Belnap, J. Few apparent short-term effects of elevated soil temperature and increased frequency of summer precipitation on the abundance and taxonomic diversity of desert soil micro- and meso-fauna. Soil Biol. Biochem. 2011, 43, 1474–1481. [Google Scholar] [CrossRef]
- Reczuga, M.K.; Lamentowicz, M.; Mulot, M.; Mitchell, E.A.D.; Buttler, A.; Chojnicki, B.; Slowinski, M.; Binet, P.; Chiapusio, G.; Gilbert, D.; et al. Predator-prey mass ratio drives microbial activity under dry conditions in Sphagnum peatlands. Ecol. Evol. 2018, 8, 5752–5764. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.M.; Yan, D.H.; Song, X.S.; Yu, Z.L.; Peng, D.; Ting, X.; Weng, B.S. Community structure of soil nematodes under different drought conditions. Geoderma 2018, 325, 110–116. [Google Scholar] [CrossRef]
- Belnap, J.; Phillips, S.L.; Sherrod, S.K.; Moldenke, A. Soil biota can change after exotic plant invasion: Does this affect ecosystem processes. Ecology 2005, 86, 3007–3017. [Google Scholar] [CrossRef]
- Renco, M.; Balezentiene, L. An analysis of soil free-living and plant-parasitic nematode communities in three habitats invaded by Heracleum sosnowskyi in central Lithuania. Biol. Invasions 2015, 17, 1025–1039. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Pennings, S.C.; Li, B.; Wu, J.H. Biotic homogenization of wetland nematode communities by exotic Spartina alterniflora in China. Ecology 2019, 100, e02596. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, L.; Mazza, G.; d’Errico, G.; Fabiani, A.; Giuliani, C.; Inghilesi, A.F.; Lagomarsino, A.; Landi, S.; Lastrucci, L.; Pastorelli, R.; et al. How ecosystems change following invasion by Robinia pseudoacacia: Insights from soil chemical properties and soil microbial, nematode, microarthropod and plant communities. Sci. Total Environ. 2018, 622, 1509–1518. [Google Scholar] [CrossRef]
- Qin, Z.; Xie, J.F.; Quan, G.M.; Zhang, J.E.; Mao, D.J.; Wang, J.X. Changes in the soil meso- and micro-fauna community under the impacts of oxotic Ambrosia artemisiifolia. Environ. Res. 2019, 34, 265–276. [Google Scholar] [CrossRef]
- Cerevkova, A.; Miklisova, D.; Bobul’ska, L.; Renco, M. Impact of the invasive plant Solidago gigantea on soil nematodes in a semi-natural grassland and a temperate broadleaved mixed forest. J. Helminthol. 2019, 94, e15. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, F. Canada Goldenrod Solidago canadensis L. In Biological Invasions and Its Management in China; Springer: Singapore, 2017; pp. 143–151. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Li, J.T.; Shi, K.W.; Ren, G.Q.; Dai, Z.C.; Sun, J.F.; Zheng, X.J.; Zhou, Y.W.; Zhang, J.Q.; Li, G.L.; et al. Effects of Canada Goldenrod Invasion on Soil Extracellular Enzyme Activities and Ecoenzymatic Stoichiometry. Sustainability 2021, 13, 3768. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Goncalves, P.; Copeland, E.; Qi, S.S.; Dao, Z.C.; Li, G.L.; Wang, C.Y.; Du, D.L.; Thomas, T. Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol. Biochem. 2020, 143, 107739. [Google Scholar] [CrossRef]
- Li, Q.W.; Liu, Y.; Gu, Y.F.; Guo, L.; Huang, Y.Y.; Zhang, J.; Xu, Z.F.; Tan, B.; Zhang, L.; Chen, L.H.; et al. Ecoenzymatic stoichiometry and microbial nutrient limitations in rhizosphere soil along the hailuogou glacier forefield chronosequence. Sci. Total Environ. 2020, 704, 135413. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorous. In Methods of Soil Analysis, Part 2, Chemical and Microbial Properties; SSSA and ASA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, R.L. Nitrogen-inorganic Forms. In Methods of Soil Analysis. Part 3, Chemical Methods; SSSA and ASA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass. C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Viglierchio, D.R.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Biermann funnel modifications. J. Nematol. 1983, 15, 438–444. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Kitagami, Y.; Kanzaki, N.; Matsuda, Y. Distribution and community structure of soil nematodes in coastal Japanese pine forests were shaped by harsh environmental conditions. Appl. Soil Ecol. 2017, 119, 91–98. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Vienna, Austria. 2013. Available online: https://www.R-project.org (accessed on 20 December 2020).
- Stevnbak, K.; Scherber, C.; Gladbach, D.J.; Beier, C.; Mikkelsen, T.N.; Christensen, S. Interactions between above- and belowground organisms modified in climate change experiments. Nat. Clim. Chang. 2012, 2, 805–808. [Google Scholar] [CrossRef]
- Castanho, A.D.A.; Coe, M.T.; Brando, P.; Macedo, M.; Baccini, A.; Walker, W.; Andrade, E.M. Potential shifts in the aboveground biomass and physiognomy of a seasonally dry tropical forest in a changing climate. Environ. Res. Lett. 2020, 15, 034053. [Google Scholar] [CrossRef]
- Shi, Y.; Xiang, X.J.; Shen, C.C.; Chu, H.Y.; Neufeld, J.D.; Walker, V.K.; Grogan, P. Vegetation-Associated Impacts on Arctic Tundra Bacterial and Microeukaryotic Communities. Appl. Environ. 2015, 81, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.R.; Cheng, X.L.; Shu, X.; Liu, Y.; Zhang, Q.F. Linking soil bacterial and fungal communities to vegetation succession following agricultural abandonment. Plant Soil 2018, 431, 19–36. [Google Scholar] [CrossRef]
- Chen, L.L.; Baoyin, T.; Minggagud, H. Effects of mowing regimes on above- and belowground biota in semi-arid grassland of northern China. J. Environ. 2021, 277, 111441. [Google Scholar] [CrossRef] [PubMed]
- Song, S.S.; Zhang, C.; Gao, Y.; Zhu, X.Y.; Wang, R.H.; Wang, M.D.; Zheng, Y.L.; Hou, L.J.; Liu, M.; Wu, D.M. Responses of wetland soil bacterial community and edaphic factors to two-year experimental warming and Spartina alterniflora invasion in Chongming Island. J. Clean. Prod. 2020, 250, 119502. [Google Scholar] [CrossRef]
- Zhang, G.G.; Sui, X.; Li, Y.; Jia, M.Q.; Wang, Z.W.; Han, G.D.; Wang, L.C. The response of soil nematode fauna to climate drying and warming in Stipa breviflora desert steppe in Inner Mongolia, China. J. Soils Sediments 2020, 20, 2166–2180. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.L.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Li, Q.; Zhang, H.Y.; Hu, Y.Y.; Hou, S.L.; Wei, H.W.; Yin, J.X.; Lu, X.T. The impacts of nutrient addition and livestock exclosure on the soil nematode community in a degraded grassland. Land Degrad Dev. 2019, 30, 1574–1583. [Google Scholar] [CrossRef]
- Ma, Q.H.; Yu, H.Y.; Liu, X.D.; Xu, Z.Z.; Zhou, G.S.; Shi, Y.H. Climatic warming shifts the soil nematode community in a desert steppe. Clim. Chang. 2018, 150, 243–258. [Google Scholar] [CrossRef]
- Peralta, G.; Schon, N.L.; Dickie, I.A.; St John, M.G.; Orwin, K.H.; Yeates, G.W.; Peltzer, D.A. Contrasting responses of soil nematode communities to native and non-native woody plant expansion. Oecologia 2019, 190, 891–899. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Xiao, H.G.; Wu, B.D.; Jiang, K.; Du, D.L.; Wang, C.Y. Stand-alone or co-occurring invasive plant species do not modify the diversity of the soil N-2-fixing bacterial community. Plant Ecol Divers. 2020, 13, 277–287. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bodenhausen, N.; Bunemann, E.K.; Brussaard, L.; Geisen, S.; Mader, P.; Quist, C.W.; Walser, J.C.; de Goede, R.G.M. Reduced tillage, but not organic matter input, increased nematode diversity and food web stability in European long-term field experiments. Mol. Ecol. 2019, 28, 4987–5005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, M.A.; Stinson, K.A.; Moore, J.A.M.; Frey, S.D. Plant invasion impacts on fungal community structure and function depend on soil warming and nitrogen enrichment. Oecologia 2020, 194, 659–672. [Google Scholar] [CrossRef] [PubMed]
| Unwarmed | Warming | Main Effects and Interactions | |||||
|---|---|---|---|---|---|---|---|
| NI | MI | CI | NI | MI | CI | ||
| Simpson | 4.11 ± 0.09abc | 2.50 ± 0.29c | 6.53 ± 1.06c | 6.00 ± 0.50ab | 3.93 ± 1.49bc | 2.36 ± 0.63c | W × I ** |
| Chao1 | 10.67 ± 1.67bc | 9.33 ± 1.20bc | 12.90 ± 0.92ab | 11.07 ± 1.55bc | 8.83 ± 1.09c | 15.67 ± 0.83a | I ** |
| Maturity Index | 2.16 ± 0.07bc | 2.62 ± 0.16a | 2.46 ± 0.23ab | 1.99 ± 0.02c | 2.14 ± 0.05bc | 2.75 ± 0.21a | I * |
| Basal Index | 57.53 ± 6.12ab | 31.92 ± 7.26b | 47.01 ± 21.23b | 82.78 ± 5.57a | 53.36 ± 9.28ab | 24.97 ± 6.90b | I * |
| Enrichment Index | 25.94 ± 10.07a | 33.55 ± 6.62a | 32.34 ± 15.02a | 15.35 ± 6.41a | 28.83 ± 15.23a | 40.20 ± 1.38a | ns |
| Structure Index | 24.68 ± 9.112cd | 61.67 ± 9.70ab | 46.65 ± 19.46abc | 2.33 ± 1.25d | 30.24 ± 0.61bcd | 69.33 ± 9.78a | I ** |
| Channel Index | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a | 99.38 ± 0.62a | 85.48 ± 14.52a | 79.63 ± 14.99a | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wang, J.; Zhang, J.; Li, Y.; Liu, E.; Yu, Y.; Iqbal, B.; Dai, Z.; Jia, H.; Li, J.; et al. Effects of Experimental Warming and Canada Goldenrod Invasion on the Diversity and Function of the Soil Nematode Community. Sustainability 2021, 13, 13145. https://doi.org/10.3390/su132313145
Li G, Wang J, Zhang J, Li Y, Liu E, Yu Y, Iqbal B, Dai Z, Jia H, Li J, et al. Effects of Experimental Warming and Canada Goldenrod Invasion on the Diversity and Function of the Soil Nematode Community. Sustainability. 2021; 13(23):13145. https://doi.org/10.3390/su132313145
Chicago/Turabian StyleLi, Guanlin, Jingquan Wang, Jiaqi Zhang, Yingnan Li, Enxi Liu, Yuechen Yu, Babar Iqbal, Zhicong Dai, Hui Jia, Jian Li, and et al. 2021. "Effects of Experimental Warming and Canada Goldenrod Invasion on the Diversity and Function of the Soil Nematode Community" Sustainability 13, no. 23: 13145. https://doi.org/10.3390/su132313145
APA StyleLi, G., Wang, J., Zhang, J., Li, Y., Liu, E., Yu, Y., Iqbal, B., Dai, Z., Jia, H., Li, J., & Du, D. (2021). Effects of Experimental Warming and Canada Goldenrod Invasion on the Diversity and Function of the Soil Nematode Community. Sustainability, 13(23), 13145. https://doi.org/10.3390/su132313145











