Presence and Characterization of Microplastics in Coastal Fish around the Eastern Coast of Thailand
Abstract
:1. Introduction
2. Materials and Methods
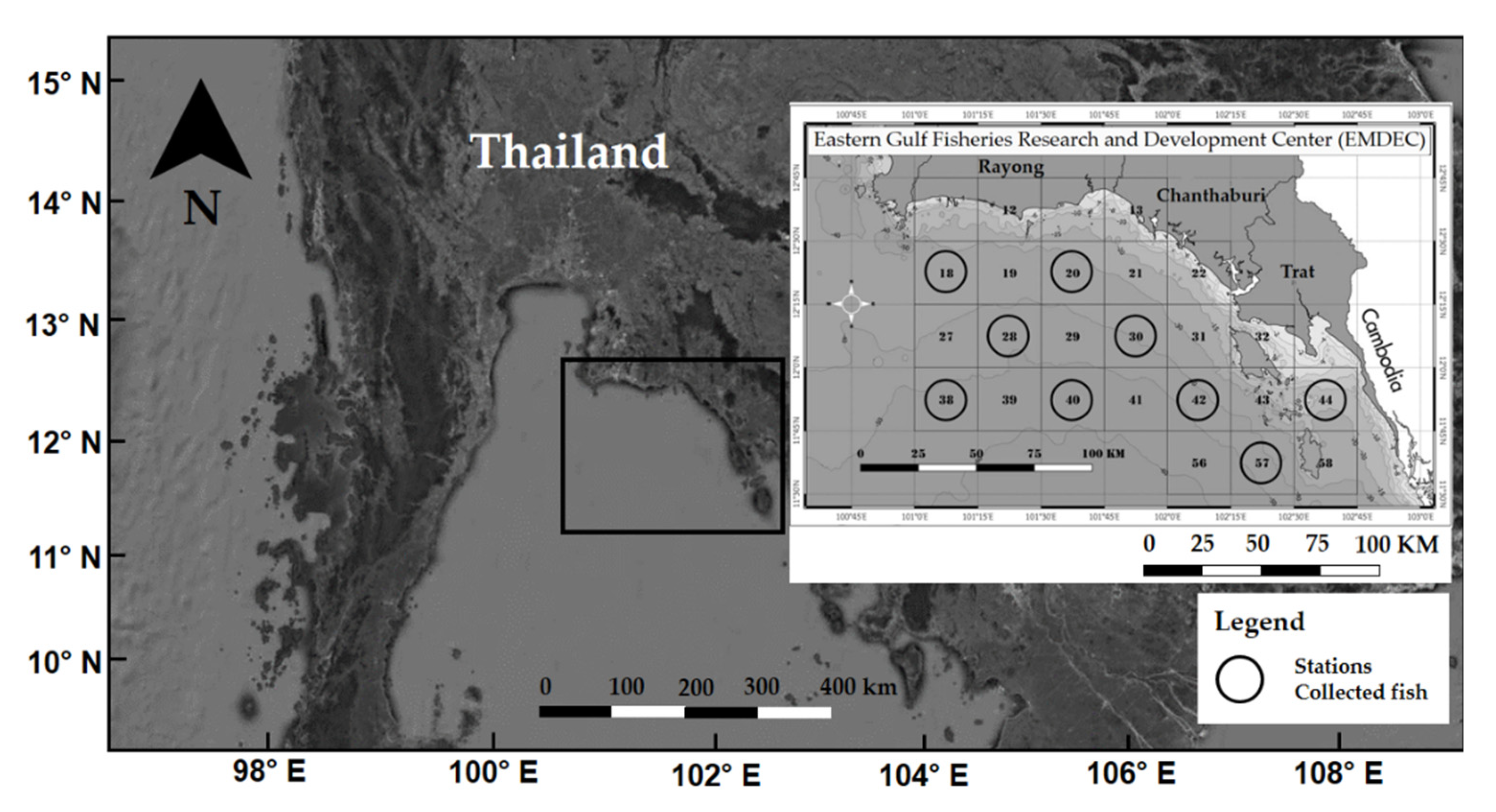
2.1. Study Area and Sample Collection
2.2. Microplastic Extraction
2.3. Prevention of Microplastic Contamination
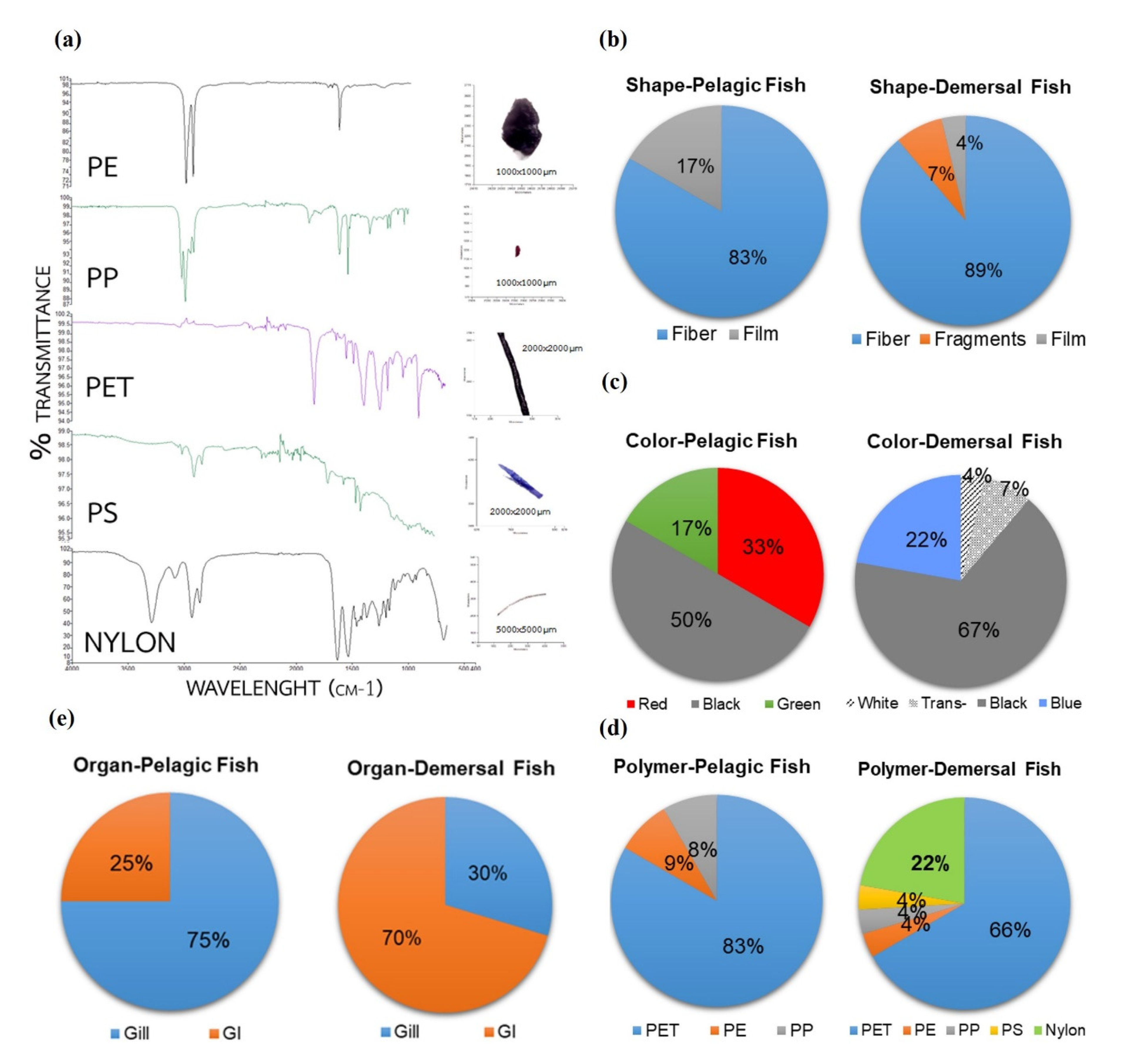
2.4. Identification of Microplastics
2.5. Statistical Analysis
3. Results
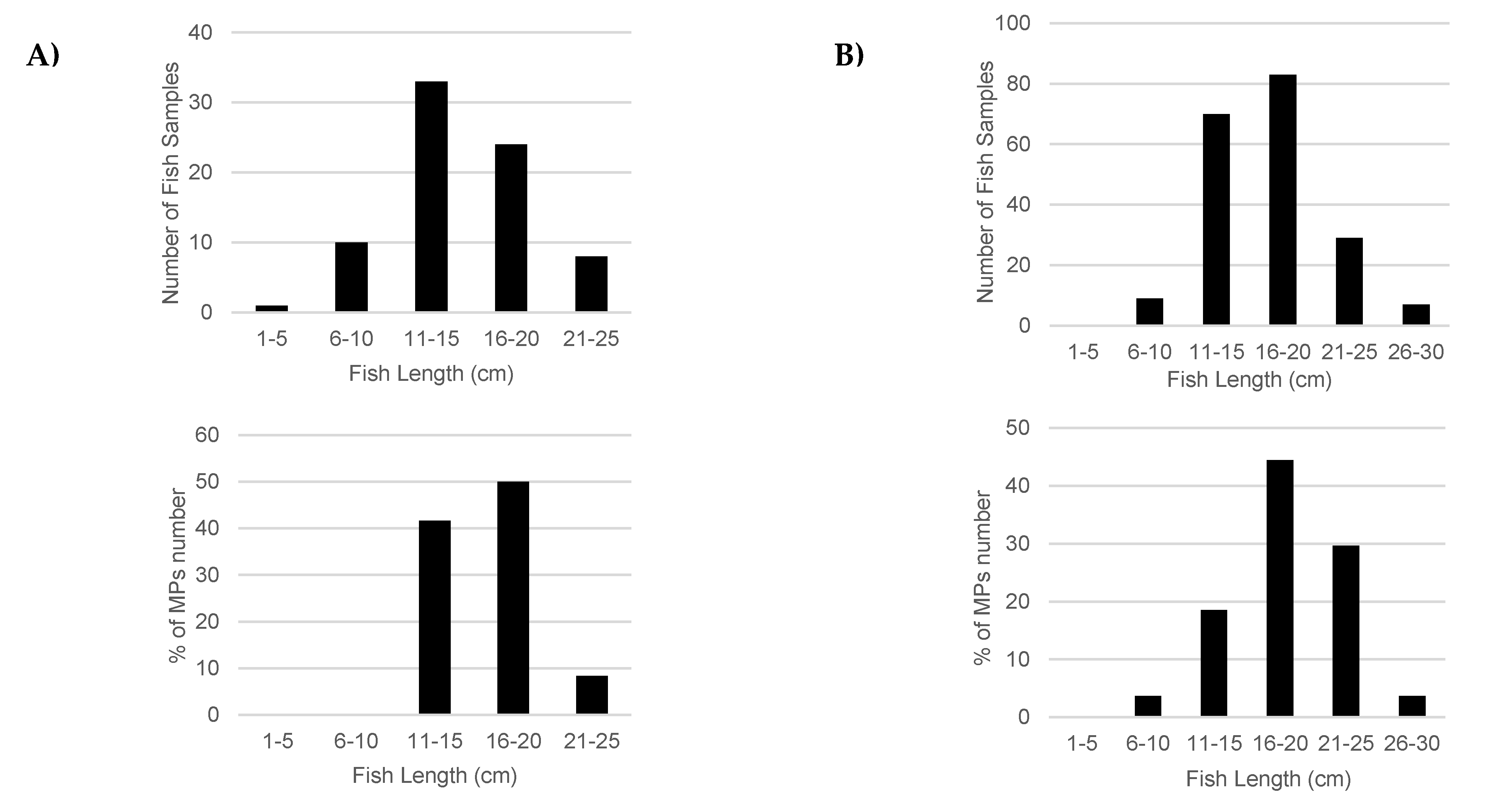
3.1. Characteristic of Fish
3.2. Abundance of Microplastics in Fish and Habitat
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galgani, F.; Leaute, J.; Moguedet, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goraguer, H.; Latrouite, D.; Andral, B.; Cadiou, Y.; et al. Litter on the Sea Floor Along European Coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Milner, P. Drifting plastic and its consequences for sessile organism dispersal in the Atlantic Ocean. Mar. Biol. 2005, 146, 815–825. [Google Scholar] [CrossRef]
- Zarfl, C.; Matthies, M. Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar. Pollut. Bull. 2010, 60, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.Á.; Irigoien, X.; Duarte, C.M. Plastic ac-cumulation in the Mediterranean Sea. PLoS ONE 2015, 10, 0121762. [Google Scholar] [CrossRef] [Green Version]
- Gregory, M.R.; Ryan, P.G. Pelagic plastics and other seaborne persistent synthetic debris: A review of Southern Hemisphere perspectives. In Marine Debris; Coe, J.M., Rogers, D.B., Eds.; Springer Series on Environmental Management; Springer: New York, NY, USA, 1997. [Google Scholar]
- Ryan, P.G.; Moore, C.J.; van Franeker, J.A.; Moloney, C.L. Monitoring the abundance of plastic debris in the marine environ-ment. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1999–2012. [Google Scholar] [CrossRef] [Green Version]
- Lee, J. Economic Valuation of Marine Litter and Microplastic Pollution in the Marine Environment: An Initial Assessment of the Case of the United Kingdom; SOAS-CeFiMS: London, UK, 2015. [Google Scholar]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global ecological, social and economic impacts of marine plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef]
- Abalansa, S.; El Mahrad, B.; Vondolia, G.K.; Icely, J.; Newton, A. The Marine Plastic Litter Issue: A Social-Economic Analysis. Sustainability 2020, 12, 8677. [Google Scholar] [CrossRef]
- Mofijur, M.; Ahmed, S.F.; Ashrafur Rahman, S.M.; Yasir, S.K.; Saiful, I.A.B.M.; Shahabuddin, M.; Ong, H.; Mahlia, T.M.I.; Djavanroodi, F.; Show, P.L. Source, distribution and emerging threat of micro- and na-noplastics to marine organism and human health: Socio-economic impact and management strategies. Environ. Res. 2021, 195, 110857. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global envi-ronments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Hall, N.M.; Berry, K.L.E.; Rintoul, L.; Hoogenboom, M. Microplastic ingestion by scleractinian corals. Mar. Biol. 2015, 162, 725–732. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Li, D. Microplastic in three urban estuaries, China. Environ. Pollut. 2015, 206, 597–604. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef]
- Pequeno, J.; Antunes, J.; Dhimmer, V.; Bessa, F.; Sobral, P. Microplastics in Marine and Estuarine Species from the Coast of Portugal. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in marine biota: A review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef]
- Rios-Mendoza, L.M.; Ontiveros-Cuadras, J.F.; Leon-Vargas, D.; Ruiz-Fernández, A.C.; Rangel-García, M.; Pérez-Bernal, L.H.; Sanchez-Cabeza, J.-A. Microplastic contamination and fluxes in a touristic area at the SE Gulf of California. Mar. Pollut. Bull. 2021, 170, 112638. [Google Scholar] [CrossRef]
- Wootton, N.; Reis-Santos, P.; Gillanders, B.M. Microplastic in fish a global synthesis. Rev. Fish. Biol. Fish. 2021, 31, 753–771. [Google Scholar] [CrossRef]
- Lusher, A.; McHugh, M.; Thompson, R. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity; The Scientific and Technical Advisory Panel GEF. Impacts of Marine Debris on Biodiversity: Current Status and Potential Solutions; Technical Series No. 67; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2012; Available online: https://www.cbd.int/doc/publications/cbd-ts-67-en.pdf (accessed on 25 November 2021).
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Wootton, N.; Reis-Santos, P.; Dowsett, N.; Turnbull, A.; Gillanders, B.M. Low abundance of microplastics in commercially caught fish across southern Australia. Environ. Pollut. 2021, 290, 118030. [Google Scholar] [CrossRef]
- Alnajar, N.; Jha, A.N.; Turner, A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis. Chemosphere 2021, 262, 128290. [Google Scholar] [CrossRef]
- Critchell, K.; Hoogenboom, M.O. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus) (ed Patterson HM). PLoS ONE 2018, 13, e0193308. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, C.; Burton, H. Origins and Biological Accumulation of Small Plastic Particles in Fur Seals from Macquarie Island. AMBIO J. Hum. Environ. 2003, 32, 380–384. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, T.; Li, Y.; He, X.; Wang, R.; Xu, J.; Gao, G. The accumulation of microplastics in fish from an important fish farm and mariculture area, Haizhou Bay, China. Sci. Total Environ. 2019, 696, 133948. [Google Scholar] [CrossRef]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Bissen, R.; Chawchai, S. Microplastics on beaches along the eastern Gulf of Thailand A preliminary study. Mar. Pollut. Bull. 2020, 157, 111345. [Google Scholar] [CrossRef] [PubMed]
- Chinfak, N.; Sompongchaiyakul, P.; Charoenpong, C.; Shi, H.H.; Yeemin, T.; Zhang, J. Abundance, composition, and fate of microplastics in water, sediment, and shellfish in the Tapi-Phumduang River system and Bandon Bay, Thailand. Sci. Total Environ. 2021, 781, 146700. [Google Scholar] [CrossRef] [PubMed]
- Sukhsangchan, R.; Keawsang, R.; Worachananant, S.; Thamrongnawasawat, T.; Phaksopa, J. Suspended microplastics during a tidal cycle in sea-surface waters around Chao Phraya River mouth, Thailand. ScienceAsia 2020, 46, 724. [Google Scholar] [CrossRef]
- Jiwarungrueangkul, T.; Phaksopa, J.; Sompongchaiyakul, P.; Tipmanee, D. Seasonal microplastic variations in estuarine sediments from urban canal on the west coast of Thailand: A case study in Phuket province. Mar. Pollut. Bull. 2021, 168, 112452. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, X.; Peng, C.; Qiao, S.; Wang, T.; Yu, W.; Khokiattiwong, S.; Kornkanitnan, N. Occurrence and distribution of microplastics in surface sediments from the Gulf of Thailand. Mar. Pollut. Bull. 2020, 152, 110916. [Google Scholar] [CrossRef]
- Klangnurak, W.; Chunniyom, S. Screening for microplastics in marine fish of Thailand: The accumulation of microplastics in the gastrointestinal tract of different foraging preferences. Environ. Sci. Pollut. Res. 2020, 27, 27161–27168. [Google Scholar] [CrossRef]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.C. Guidance on Monitoring of Marine Litter in European Seas; European Commission, Joint Research Centre, MSFD Technical Subgroup on Marine Litter (TSG-ML), JRC Technical Report. EUR83985. 2013; Available online: https://mcc.jrc.ec.europa.eu/documents/201702074014.pdf (accessed on 25 November 2021).
- Peters, C.A.; Bratton, S. Urbanization is a major influence on microplastic ingestion by sunfish in the Brazos River Basin, Central Texas, USA. Environ. Pollut. 2016, 210, 380–387. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Med-iterranean Sea, and its occurrence in the gastrointestinal tract of fish Environ. Pollution 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cavalcanti, J.S.; Silva, J.D.; França, E.J.; Araújo, A.C.; Gusmão, F. Microplastics ingestion by a common tropical fresh-water fishing resource. Environ. Pollut. 2017, 221, 218–226. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Possatto, F.E.; Barletta, M.; Costa, M.F.; Sul, J.I.D.; Dantas, D.V. Plastic debris ingestion by marine catfish: An unexpected fisheries impact. Mar. Pollut. Bull. 2011, 62, 1098–1102. [Google Scholar] [CrossRef]
- Pozo, K.; Gomez, V.; Torres, M.; Vera, L.; Nuñez, D.; Oyarzún, P.; Mendonza, G.; Clarke, B.; Fossi, M.C.; Bainic, M.; et al. Presence and characterization of microplastics in fish of commercial importance from the Biobío region in central Chile. Mar. Pollut. Bull. 2019, 140, 315–319. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Hou, C.; Wu, Y.; Teng, J.; Zhang, C.; Tan, H.; Shan, E.; Zhang, W.; Zhao, J. Microplastic uptake in commercial fishes from the Bohai Sea, China. Chemosphere 2021, 263, 127962. [Google Scholar] [CrossRef]
- Rummel, C.D.; Loder, M.G.; Fricke, N.F.; Lang, T.; Griebeler, E.M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and de-mersal fish from the North Sea and Baltic Sea. Mar. Pollut Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.; Ojeda, F.P.; Duarte, C.; Galbán-Malagón, C. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Bessa, F.; Barría, P.; Neto, J.; Frias, J.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastics on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Welden, N.A.; Cowie, P.R. Degradation of common polymer ropes in a sublittoral marine environment. Mar. Pollut. Bull. 2017, 118, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Steer, M.; Cole, M.; Thompson, R.C.; Lindeque, P.K. Microplastic ingestion in fish larvae in the western English Channel. Environ. Pollut. 2017, 226, 250–259. [Google Scholar] [CrossRef]
- Azad, S.M.O.; Towatana, P.; Pradit, S.; Patricia, B.G.; Hue, H.T.T.; Jualaong, S. First Evidence of Existence of Microplastics in Stomach of Some Commercial Fishes in the Lower Gulf of Thailand. Appl. Ecol. Environ. Res. 2018, 16, 7345–7360. [Google Scholar] [CrossRef]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef]
- Naji, A.; Esmaili, Z.; Mason, S.A.; Vethaak, A.D. The occurrence of microplastic contamination in littoral sediments of the Persian Gulf, Iran. Environ. Sci. Pollut. Res. 2017, 24, 20459–20468. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Chavanich, S.; Yakupitiyage, A. Coastal debris analysis in beaches of Chonburi Province, eastern of Thailand as implications for coastal conservation. Mar. Pollut. Bull. 2017, 116, 121–129. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Bour, A.; Avio, C.G.; Gorbi, S.; Regoli, F.; Hylland, K. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. Pollut. 2018, 243, 1217–1225. [Google Scholar] [CrossRef]
- Clark, J.R.; Cole, M.; Lindeque, P.K.; Fileman, E.; Blackford, J.; Lewis, C.; Lenton, T.M.; Galloway, T.S. Marine microplastic debris: A targeted plan for understanding and quantifying interactions with marine life. Front. Ecol. Environ. 2016, 14, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Policy: Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Arsenault-Pernet, E.-J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [Green Version]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
| Fish Species | n | Average ± SD Weight (g) | Average ± SD Length (cm) | No. of Fish Found MPs (No. of MPs) | Average ± SD MPs Items/Ind |
|---|---|---|---|---|---|
| Pelagic | |||||
| Aurigequula fasciata (Lacepède, 1803) | 5 | 64.80 ± 9.73 | 15.10 ± 0.89 | 1 (1) | 0.20 ± 0.45 |
| Leiognathus equulus (Forsskål, 1775) | 6 | 78.23 ± 16.97 | 16.37 ± 2.23 | 0 (0) | ND 1 |
| Stolephorus indicus (Van Hasselt, 1823) | 5 | 19.40 ± 1.52 | 13.25 ± 0.42 | 1 (1) | 0.20 ± 0.45 |
| Lutjanus lutjanus (Bloch, 1790) | 6 | 20.92 ± 27.86 | 10.33 ± 3.33 | 0 (0) | ND 1 |
| Lutjanus madras (Valenciennes, 1831) | 5 | 47.60 ± 10.50 | 14.40 ± 1.34 | 1 (1) | 0.20 ± 0.45 |
| Sphyraena obtusata (Cuvier, 1829) | 6 | 56.82 ± 15.82 | 21.32 ± 1.98 | 1 (1) | 0.17 ± 0.41 |
| Rastrelliger kanagurta (Cuvier, 1816) | 15 | 50.34 ± 41.85 | 15.20 ± 4.94 | 2 (2) | 0.13 ± 0.35 |
| Amblygaster clupeoides (Bleeker, 1849) | 9 | 19.89 ± 2.32 | 12.89 ± 0.60 | 1 (1) | 0.11 ± 0.33 |
| Atule mate (Cuvier, 1833) | 9 | 32.15 ± 15.73 | 12.39 ± 3.93 | 1 (2) | 0.22 ± 0.67 |
| Gerres erythrourus (Bloch, 1791) | 10 | 87.76 ± 32.07 | 16.60 ± 1.98 | 3 (3) | 0.30 ± 0.48 |
| Demersal | |||||
| Nemipterus hexodon (Quoy & Gaimard, 1824) | 40 | 75.71 ± 34.79 | 18.38 ± 6.02 | 0 (0) | ND 1 |
| Scolopsis taenioptera (Cuvier, 1830) | 54 | 58.01 ± 27.02 | 15.87 ± 2.67 | 6 (6) | 0.11 ± 0.32 |
| Saurida elongata (Temminck & Schlegel, 1846) | 35 | 65.34 ± 40.78 | 19.91 ± 3.87 | 13 (15) | 0.43 ± 0.65 |
| Saurida undosquamis (Richardson, 1848) | 17 | 49.59 ± 23.40 | 18.45 ± 2.63 | 1 (1) | 0.06 ± 0.24 |
| Upeneus vittatus (Forsskål, 1775) | 21 | 21.30 ± 11.68 | 16.75 ± 22.59 | 2 (2) | 0.10 ± 0.30 |
| Upeneus tragula (Richardson, 1846) | 5 | 49.24 ± 23.48 | 15.74 ± 3.57 | 1 (1) | 0.20 ± 0.45 |
| Upeneus sulphureus (Cuvier, 1829) | 12 | 30.83 ± 9.70 | 12.65 ± 1.45 | 0 (0) | ND 1 |
| Platycephalus indicus (Linnaeus, 1758) | 5 | 24.00 ± 2.45 | 15.16 ± 0.63 | 1 (1) | 0.20 ± 0.45 |
| Priacanthus tayenus (Richardson, 1846) | 9 | 53.42 ± 56.46 | 14.47 ± 5.95 | 1 (1) | 0.11 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phaksopa, J.; Sukhsangchan, R.; Keawsang, R.; Tanapivattanakul, K.; Thamrongnawasawat, T.; Worachananant, S.; Sreesamran, P. Presence and Characterization of Microplastics in Coastal Fish around the Eastern Coast of Thailand. Sustainability 2021, 13, 13110. https://doi.org/10.3390/su132313110
Phaksopa J, Sukhsangchan R, Keawsang R, Tanapivattanakul K, Thamrongnawasawat T, Worachananant S, Sreesamran P. Presence and Characterization of Microplastics in Coastal Fish around the Eastern Coast of Thailand. Sustainability. 2021; 13(23):13110. https://doi.org/10.3390/su132313110
Chicago/Turabian StylePhaksopa, Jitraporn, Roochira Sukhsangchan, Rangsiwut Keawsang, Kittipod Tanapivattanakul, Thon Thamrongnawasawat, Suchai Worachananant, and Patinya Sreesamran. 2021. "Presence and Characterization of Microplastics in Coastal Fish around the Eastern Coast of Thailand" Sustainability 13, no. 23: 13110. https://doi.org/10.3390/su132313110
APA StylePhaksopa, J., Sukhsangchan, R., Keawsang, R., Tanapivattanakul, K., Thamrongnawasawat, T., Worachananant, S., & Sreesamran, P. (2021). Presence and Characterization of Microplastics in Coastal Fish around the Eastern Coast of Thailand. Sustainability, 13(23), 13110. https://doi.org/10.3390/su132313110






