1. Introduction
Over the past century, anthropogenic activities have greatly impacted and degraded coastal systems, resulting in widespread habitat loss [
1,
2,
3]. The pressures driving coastal habitat loss are myriad, often interconnected, and are both natural and anthropogenic in origin [
4,
5,
6]. Due to these factors, some coastal systems and their foundation species are threatened, with many ecosystems greatly reduced from their historic distributions and abundances [
7,
8,
9,
10]. Therefore, as near-field and far-field pressures continue to increase sea level, sea surface temperatures, and alter regional weather patterns, it is becoming increasingly important to take more holistic approaches to the protection and conservation of coastal ecosystems [
4,
11,
12,
13,
14].
Resource managers have previously addressed the loss of higher trophic level species by using top-down management strategies focusing on a single species, but many of these efforts had limited success [
15]. In many fisheries, management approaches to increase abundance, such as stocking higher trophic level sport fishes, have been shown to only temporarily increase abundance and eventually produce negative cascading effects [
16,
17,
18]. More recently, coastal managers have been transitioning towards more complete ecosystem-based management (EBM) strategies to reduce risk in coastal ecosystems [
19,
20,
21,
22]. Frequently, EBM uses bottom-up approaches to protect entire ecosystems with the goal of increasing sustainability [
23]. Often, these approaches begin with protecting habitats, many of which are considered essential fish habitat (EFH). Essential fish habitat is any habitat utilized by a fish as foraging grounds, breeding grounds, or refugia and many policies have been implemented globally to help ensure their protection [
24,
25,
26,
27]. Essential fish habitat is often created by foundation species (a large or numerically abundant species that provides food and habitat), and frequently, these species are ecosystem engineers that modify habitats and physically alter the environment [
28,
29,
30,
31]. Due to their relatively high abundance, foundation species support a multitude of species that comprise lower levels of the food web. For example, oysters create biogenic habitat used by lower trophic level fishes and invertebrates [
30,
31,
32].
Globally, oysters are crucial ecosystem engineers [
24,
33]. Within estuaries along the east coast of North and South America, eastern oysters (
Crassostrea virginica) create complex 3-dimensional structures used for forage and refugia by numerous organisms [
34,
35]. However, over the past century, eastern oysters have seen an ~85% decrease in filtration capacity in US estuaries, a 64% decline in oyster extent, and an 88% decrease in oyster biomass due to factors such as overharvesting, degraded water quality, and physical disturbances such as boat wakes [
36,
37,
38,
39,
40,
41,
42]. Due to their critical role, a loss of oyster reefs within an estuarine system can have effects throughout the broader food web [
43,
44,
45,
46,
47]. To mitigate decreases in oyster abundance, there have been ongoing efforts to enhance and restore oyster reefs, often involving the physical placement of recruitment substrate, and, where needed, the introduction of additional larvae/spat/broodstock [
33,
48,
49].
The benefits of oyster reef restoration are far-reaching and range from an increase in fish and shellfish abundance to the maintenance of biodiversity to the production of ecosystem services and sediment accretion along estuarine shorelines [
38,
50,
51,
52,
53]. More specifically, oyster reef restoration has enhanced EFH in estuarine systems by improving water quality, increasing habitat available for foraging, and through the creation of complex habitats that can provide refugia from predators, increasing survivorship [
24,
33,
54,
55,
56]. Historically, restoration success has been assessed by quantifying oyster abundance and oyster density; however, more recent studies have started to assess the benefits of habitat restoration accruing at higher trophic levels [
33,
35,
48,
57,
58]. Higher trophic level predators, such as wading birds and sport fish, often use oyster reefs to forage for invertebrates and smaller fishes, but they tend to have relatively high rates of movement and continue to forage over large spatial scales, creating challenges when attempting to understand the role of reef restoration to these more mobile and transient species [
59,
60]. However, some taxa such as invertebrates and small demersal fishes (hereafter reef residents) are relatively sedentary and spend their entire life cycles on or near an individual oyster reef.
Many of these smaller demersal fishes are generalists in both environmental ranges and diet (
Table 1). Reef residents generally occupy intermediate trophic levels within the broader reef food web, acting as predators of spat, copepods, amphipods, and polychaetes, but also serving as prey to higher trophic level predators [
35,
61]. These reef resident taxa are reliant on the 3-dimensional structure of oyster reefs, using interstitial spaces within the live oyster reef matrix as refugia from predators and for foraging and nesting habitat [
35,
53,
54,
55,
62]. Many species of gobies, blennies, and toadfish are territorial, often showing high site fidelity, especially males during the breeding season [
63]. Some blenny species have shown site fidelity across multiple breeding seasons, suggesting lifetime nesting site fidelity [
63]. For example, in one of the most extensive mark-recapture studies on goby and blenny site fidelity, Harding et al. (2020) found that of the individuals recaptured, nearly all individuals (e.g., 94% of breeding males) were captured on the same oyster reefs where they were initially tagged. The findings of this study suggest the spatial scale of movement is less than 40 m for males [
63]. This further indicates that many reef residents rely partially on larval dispersal for the movement of the species from site to site [
64,
65]. In Florida, this dispersal occurs for many of these species during the wet season (i.e., increased precipitation and temperature occur annually from May through October) (
Table 1; [
66,
67]). The larval settlement of these fishes was found to occur downcurrent of reefs next to structured relief [
34,
64,
68,
69]. Similarly, vertical height and quality of interstitial spaces are important factors leading to the recruitment of oyster spat, potentially suggesting a correlation between reef residents and spat settlement [
34]. Hence, reef residents that use oyster habitat for most of their life cycle may be valuable indicators for assessing restoration success or to assess if restored reefs are used similarly as live reefs by higher trophic level species [
35]. The specific habitat features necessary to support these reef residents could have positive impacts on higher trophic level species that rely on them as prey. Thus, determining the oyster metrics that support reef residents (e.g., reef rugosity, oyster density) can be used to help guide and assess the goals of restoration projects [
51,
70].
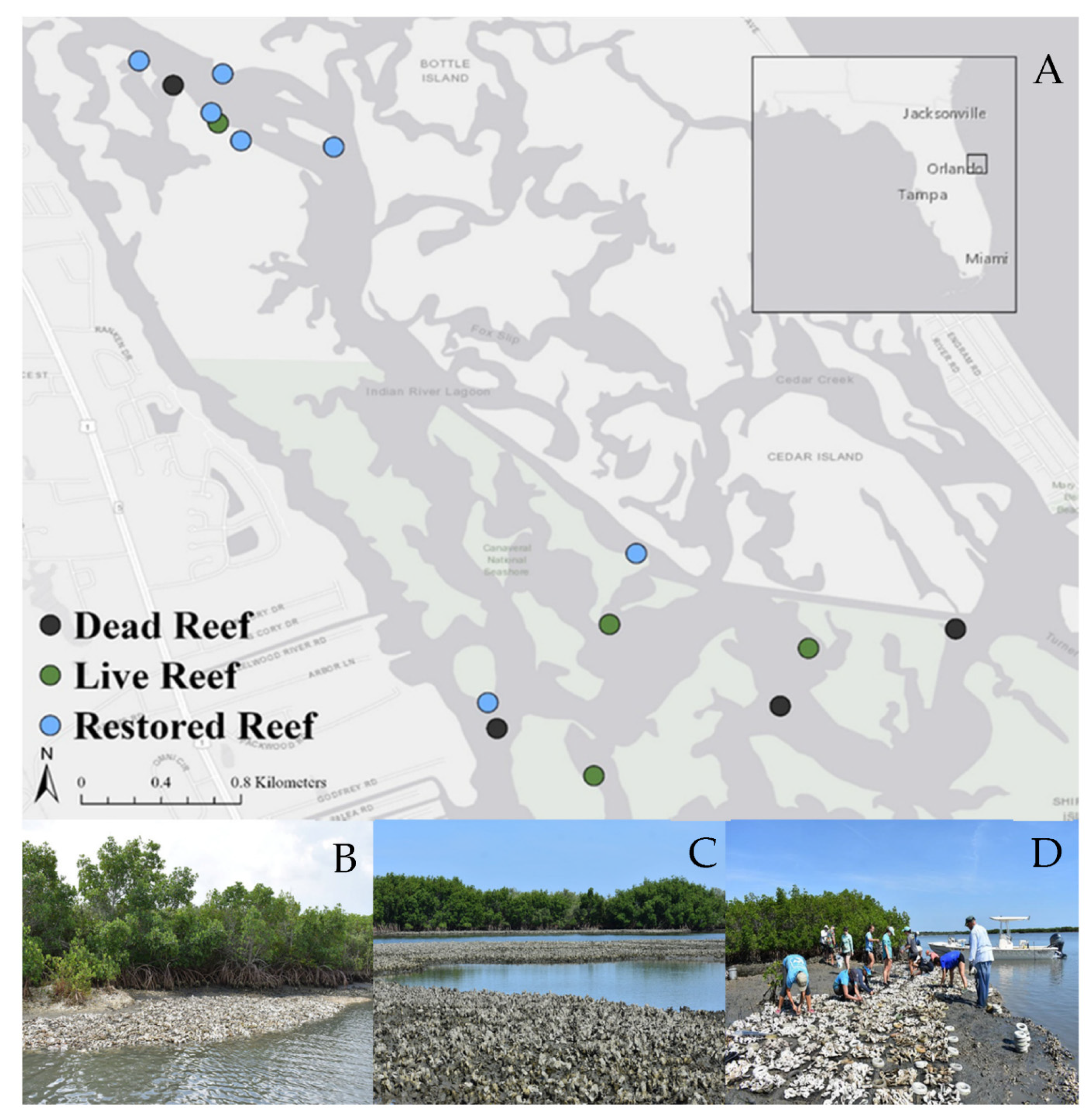
To address this research need, here we examine the efficacy of using the local reef resident fish guild to provide a more holistic understanding of how reef restoration affects higher trophic level species [
35,
57]. More specifically, we explore knowledge gaps regarding how reef resident fish diversity and abundance responds to the restoration of oyster reefs within Mosquito Lagoon, Florida. These ecological data are compared with standard metrics of oyster habitat restoration to assess if habitat use, and the numerical response of reef resident fishes can be used as a higher trophic level indicator of oyster reef restoration success.
4. Discussion
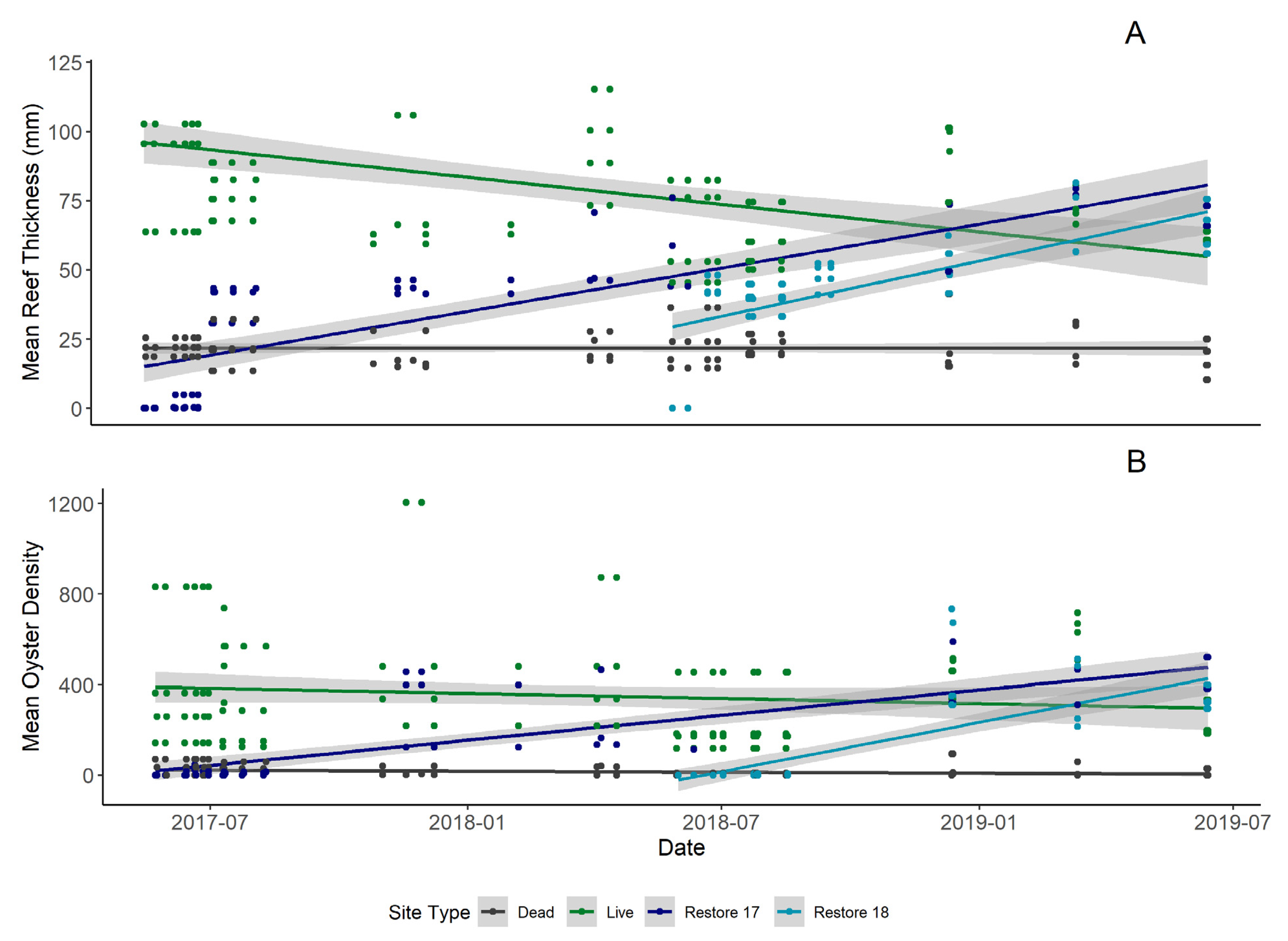
This study highlighted the strong relationship between reef resident fishes and oyster reef metrics (i.e., oyster density and reef thickness). Directly following restoration, reef metrics more closely resembled dead reefs. Over the two years of the study, restored reefs began to more closely resemble live reefs with respect to oyster density and oyster reef thickness (
Figure 2). When assessing the abundance of the reef resident fish guild, more resident fishes were captured at live reefs than dead and restored reefs (
Table 3). The temporal shift in the oyster reefs following restoration (essentially transitioning from a dead reef to a live reef), resulted in low correlations, and reef type being a poor predictor of fish community metrics. However, using oyster density and reef thickness as predictors of reef resident fish metrics gave insight into the response of the resident fish guild to habitat restoration. Reef resident abundance and species richness positively responded to an increase in oyster thickness and count (
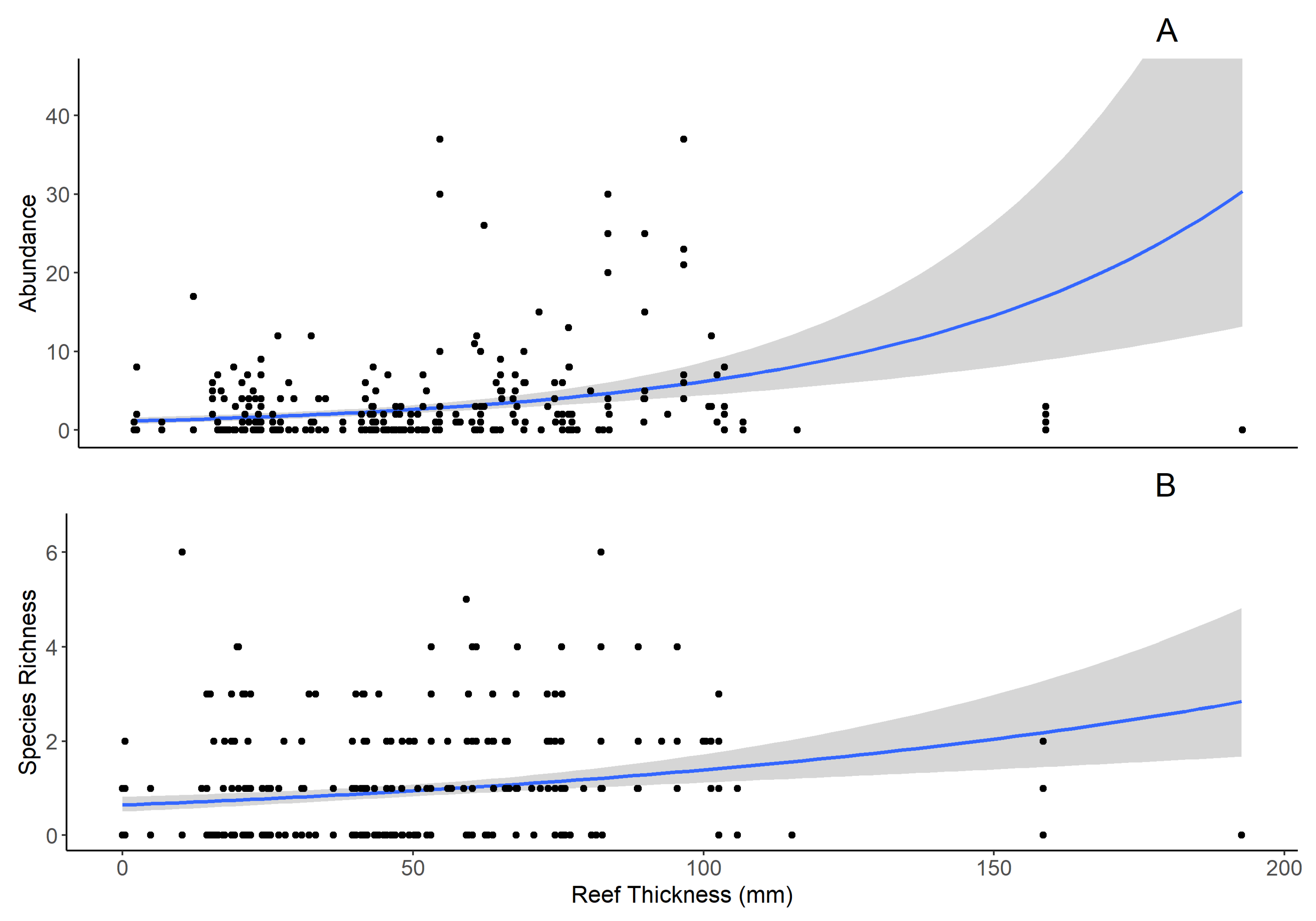
Figure 3). These results align with Tolley and Volety (2005) suggesting it is not necessarily the number of live oysters alone but the increasing habitat complexity over time that supports and attracts reef resident type fishes (gobies and blennies native to that region). These results suggest projects that create oyster settlement habitat and categorize changes in the faunal community using reef type alone, may miss important gradients of change at higher trophic levels and fail to provide evidence of restoration success throughout the food web.
Using oyster reef thickness and oyster density as a proxy for restoration success (as opposed to using reef type in isolation) in generalized linear models is warranted. Generalized linear models predicting reef resident abundances regardless of season identified the oyster reef model combining reef thickness and oyster density as the best fitting model (∆AIC = 0;
Table 4). This model also explained a considerable amount of variation in the resident reef fish data annually (r
2 = 0.43) and during the wet season (r
2 = 0.31). The quadratic temperature model tied with oyster reef metrics during the wet season. This may be indicative of reef resident fishes inhabiting waters closer to their thermal maximum during the wet season which encompasses the warmer summer months. However, reef metrics were still playing a role in reef fish community abundance. Oyster reef thickness was the next best fitting model in both cases and explained more variation than oyster density. Estimates of oyster density indicate annual recruitment pulses occurred at all reef types but reef thickness increased linearly across restored reefs. This implies that for these species, reef complexity may be a stronger driver of total resident fish abundances than oyster counts alone. Furthermore, proximity to source populations on live reefs may be a strong driver of which restored reefs receive settlers via larval dispersal.
Predictors of species richness were more complicated and include oyster metrics and water quality variables at annual and seasonal temporal scales. This trend is potentially explained by the relatively low number of reef resident species in this study (
n = 8), most of which have similar ecological niches (
Table 1). Due to this, common diversity metrics such as richness may not be sufficiently fine scale to determine large shifts in habitat use. For instance, the Frillfin Goby and Florida Blenny were found in markedly higher abundances at the base of dead reefs compared to other reef types. These species are known to lay eggs on clean, disarticulated shells; however, these species only accounted for 3% of total individuals captured and most likely would not impact greatly the relationship between richness and oyster reef metrics [
35,
87,
88].
The community assemblage of reef resident fishes was also similar among reef types, with seven of eight species occurring at all reef types; these species, however, varied greatly in their abundances across reef types (
Table 3). These similarities and differences can potentially be attributed to life history strategies of the reef resident species. Most species in this study have similar recruitment periods (i.e., the wet season (May–October) in Florida [
66,
67]). When looking at individual species, certain species that feed on or brood nests directly on oyster shells, such as the Oyster Toadfish and Naked Gobies, mirrored the recruitment pulses in oyster abundance [
89,
90,
91]. Although sampling efforts were greater in the wet season, when catches were standardized by catch per unit effort, CPUE did increase during the wet season and is most likely due to these recruitment pulses. The preferred reef type of reef residents may change based on season with some species preferring cleaned, disarticulated shell for egg laying and brooding [
64,
88]. Sampling reef residents over multiple wet seasons would more unequivocally elucidate how newly and previously restored reefs are supporting lower trophic level fishes. In order to provide a more complete understanding of how habitat restoration broadly, and oyster reef restoration in particular, benefits coastal food webs will require a concerted effort in future studies to sample and monitor restored reefs for longer than the two years following restoration that was possible in this study.
Quantifying the benefits of oyster reef restoration to more mobile higher trophic level sportfish is inherently complicated. However, smaller resident fishes with lower relative vagility are less likely to disperse among reefs or estuaries and thus may act as a higher trophic level indicator of restoration success at the local reef scale. Future studies exploring dispersal, spatial configuration of local reefs, and how proximity to live or more mature restored oyster reefs impacts the capacity of reefs to support new recruits of reef residents could provide strategic insight regarding the identification of candidate reefs to restored in the future. By identifying reefs with the greatest demographic connectivity to other reefs, restoration activities could be designed to maximize higher rates of settlement and post-settlement recruitment. In this manner, the network of restored reefs could function as a metapopulation [
92,
93], thus increasing the persistence and regional carrying capacity of lower trophic level species that in turn can support greater abundances of higher trophic level species within the reef network.
Coastal restoration efforts have been ongoing in many parts of the world, but these projects have experienced varying levels of success [
94,
95,
96,
97]. Specifically, the restoration of foundation species, large or numerically dominant species such as mangroves, corals, and seagrasses that create habitat or provide food for other species, is increasing, with generally positive results for the related ecosystem and ecosystem services being produced [
6,
31,
98,
99]. While many strategies to assess the efficacy of restoring foundation species exist, these often struggle to capture the indirect benefits of restoration of the habitat conferred to higher trophic level species reliant on their presence for prey and/or habitat. To evaluate the success of oyster reef restoration, multiple oyster metrics including density, rugosity, and oyster size class structure (following restoration) exist. However other potential metrics of restoration success such as nutrient cycling or support of other higher trophic level species such as invertebrates, fishes, and birds are quantified less frequently [
58,
100,
101]. These factors, in combination with the logistical constraints related to post-restoration monitoring, result in few quantitative estimates of the benefit of habitat restoration for higher trophic level species such as fishes. Compared to more transient fishes, reef resident fishes greatly rely on oyster reefs to provide habitat for nesting and refugia, in addition to providing prey [
48]. Furthermore, small resident fish species are often some of the first to inhabit an area that has been restored; in this study, reef resident fish were collected at six of the seven restored sites as early as one-week post-restoration (
n = 21). Prior to restoration, only one fish was collected at these sites in pre-restoration sampling. Therefore, these species could act as relatively rapid indicators of restoration success for mid and lower trophic level fishes [
35].
5. Conclusions
Oyster reef restoration has been used to mitigate impacts from direct harvest, overfishing, boat wakes, and degraded water quality that together have decreased oyster density by nearly 85% [
36,
37,
39,
40]. This study demonstrated how oyster reef restoration creates essential fish habitat for resident fish communities in a relatively short period of time (~12–24 months). The abundances of these species of fish are positively and closely related to oyster reef thickness and abundance (
Figure 3;
Table 4). Therefore, the abundance of reef resident fishes appears to be a practical indicator of restoration success as these species are an important link to higher trophic level fishes [
61,
63,
91]. Adjusting locations of reefs and closely monitoring settlement/recruitment can provide a novel means of gauging the success of shellfish habitat restoration at higher trophic levels. Using the presence of oyster reef resident species, in addition to setting target levels of abundance observed at live oyster reefs, may be a useful metric for monitoring the function of restored reef habitat at higher trophic levels and provide insight beyond the level of the oyster reef itself. Furthermore, future studies are necessary to determine actual distances smaller demersal fishes disperse during larval stages to help quantify how close restored reefs must be to receive settlers from source populations and how settlement is influenced by patch reef size, shape, configuration, and hydrology. In addition, sizes and sexes of fishes should be assessed in future studies to further elucidate specific behavioral trends such as individuals that are present and breeding versus non-breeding individuals. While results here reaffirm the significance of the interactions between reef-associated species and structural complexity of the reef, they moreover suggest that additional time and monitoring are required to accurately assess if and when the reef resident fish assemblage at restored reefs resembles the fish assemblage at live reefs. If oyster reefs within Mosquito Lagoon continue to suffer degradation from recreational boat wakes [
37,
79], reductions in resident reef fish abundance could be indicative of a reduction in oyster habitat quality, with implications for other components of the food web. Future studies utilizing methods such as structural equation models to assess higher trophic level species such as sportfish and wading birds, with special foci on their diets and foraging behavior, should be considered to better understand oyster reef restoration impacts on the broader food web [
102,
103,
104,
105,
106,
107,
108,
109,
110]. Incorporating biotic metrics describing lower trophic level fishes, here a guild of reef resident fishes, can elucidate unseen factors that may result in differences in reef restoration success. In this way, these species can act as indicators of restoration success, and provide insight that can help guide future restoration efforts.