Abstract
Linking wildlife areas with corridors facilitating species dispersal between core habitats is a key intervention to reduce the deleterious effects of population isolation. Large heterogeneous networks of areas managed for wildlife protection present site- and species-scale complexity underpinning the scope and performance of proposed corridors. In Southern Africa, the Kavango-Zambezi Transfrontier Conservation Area seeks to link Kafue National Park to a cluster of wildlife areas centered in Namibia and Botswana. To assess and identify potential linkages on the Zambian side, we generated a high-resolution land cover map and combined empirical occurrence data for Lions (Panthera leo), Leopards (Panthera pardus) and Spotted Hyena (Crocuta crocuta) to build habitat suitability maps. We then developed four connectivity models to map potential single and multi-species corridors between Kafue and the Zambezi River border with Namibia. Single and multi-species connectivity models selected corridors follow broadly similar pathways narrowing significantly in central-southern areas of the Kafue-Zambezi interface, indicating a potential connectivity bottleneck. Capturing the full extent of human disturbance and barriers to connectivity remains challenging, suggesting increased risk to corridor integrity than modelled here. Notwithstanding model limitations, these data provide important results for land use planners at the Kafue-Zambezi Interface, removing much speculations from existing connectivity narratives. Failure to control human disturbance and secure corridors will leave Kafue National Park, Zambia’s majority component in the Kavango-Zambezi Transfrontier Conservation Area, isolated.
1. Introduction
Expanding human pressures on natural resources and conversion of wildlife habitats to farmlands and rangelands are driving rapid fragmentation with often detrimental effects to native wildlife species, due to population isolation and decline [1,2]. Native large carnivores are particularly sensitive to anthropogenic pressures due to their dependence on prey availability and exhibit elevated extinction risk with population isolation [3,4]. More than 75% of terrestrial large carnivores are now experiencing declines in population size and distribution due to anthropogenic pressures related to habitat fragmentation and loss [5]. Maintaining free-ranging large carnivore populations is contingent on provision of sufficient habitat and prey species and limiting the deleterious effects of human disturbances [6].
Given the importance of maintaining structural and functional connectivity between wildlife managed areas, large scale conservation networks, including Transfrontier Conservation Areas, are being developed to address issues surrounding fragmentation and isolation of wildlife populations [7,8]. These initiatives have important implications for many large carnivore populations [9], the ecological functionality of the landscapes within which they reside [5], and the development of wildlife-based land uses and economies [10].
Understanding and promoting species-level connectivity between wildlife managed areas is a key objective of the Kavango-Zambezi Transfrontier Conservation Area Programme that spans five neighboring countries and ~70 wildlife managed areas across ~520,000 km2 of central southern Africa [11]. The Kavango-Zambezi Transfrontier Conservation Area represents the largest and arguably most important and ambitious conservation initiative in Africa, covering a wide range of threatened species, habitats and ecosystem services that provide critical resources and natural wealth to the regions people. However, much success with key objectives in this program is dependent on maintaining and expanding landscape connectivity. Connecting wildlife subpopulations through protecting and developing movement corridors within and between wildlife managed area networks is an increasingly important component in wildlife conservation [8]. The emergence of Transboundary networks and connectivity narratives in Southern Africa represents important political acknowledgement of the prerequisite to manage many vulnerable species at appropriate scales in human-dominated landscapes [12]. Yet, with the boundaries of many TFCAs drawn in accordance with subjective, socio-political priorities, much work remains in order to understand and implement management strategies that will better serve the movement requirements of target species given limited resources [13]. A recent study by Hoffman et al. [14] demonstrates the need for planning at transboundary scale for restoring connectivity and landscape permeability for highly mobile species.
Yet, few of the proposed wildlife managed area linkages in the Kavango-Zambezi Transfrontier Conservation Area Programme have been sufficiently studied at the species and scale of interest to provide planners and managers with the empirical evidence necessary for the informed decision making critical to effective natural resource management and the development of wildlife-based land uses [15,16].
Connectivity between Kafue National Park in central western Zambia and the core cluster of wildlife managed areas centred on Chobe National Park in Botswana represents one of the largest questions (and challenges) surrounding natural resource management in the Kavango-Zambezi Transfrontier Conservation Area Programme. If connectivity cannot be established or developed, Zambia’s majority component in the program will effectively be isolated from the broader landscape, with significant implications for low density, wide-ranging species in Kafue National Park and the potential and promise of the Kavango-Zambezi Transfrontier Conservation Area Programme as a whole.
However, promoting connectivity and species-level movement at large spatial scales in heterogenous landscapes is dependent on high quality data of system states and species level responses to key drivers [17]. The main aim of this study is to assess landscape connectivity at the large carnivore scale at the interface of Kafue National Park and adjacent wildlife managed areas, through the Kafue-Simalaha Wildlife Dispersal Corridor and surrounds. Specifically, the objectives of this study are: (a) to investigate and model the effect of environmental and anthropogenic factors on the occurrence three large carnivores between Kafue National Park and the Simalaha Wildlife Recovery Sanctuary; and (b) to assess the potential of the proposed Kafue-Simalaha Wildlife Dispersal Corridor for single and multi-species landscape-level connectivity planning.
Our final output has broad applied value for corridor planning and the development of wildlife-based land uses throughout the Kavango-Zambezi Transfrontier Conservation Area and beyond, supporting the knowledge base for long term persistence of large carnivores, the ecological integrity of systems in which they reside and the promotion of sustainable wildlife-based land uses for the landscapes’ rural poor.
2. Methods
2.1. Study Area
The Kavango-Zambezi Transfrontier Conservation Area (KAZA TFCA) encompasses a matrix of over 70 wildlife managed areas in IUCN I-VI and Not Reported categories [18], spanning the borders of Angola, Botswana, Namibia, Zambia and Zimbabwe in central southern Africa, extending over c.520,000 km2 [16]. Spatially, these wildlife managed areas fall into three major clusters and five periphery clusters, with Kafue National Park and surrounding wildlife managed areas representing the major northern cluster (Figure 1).
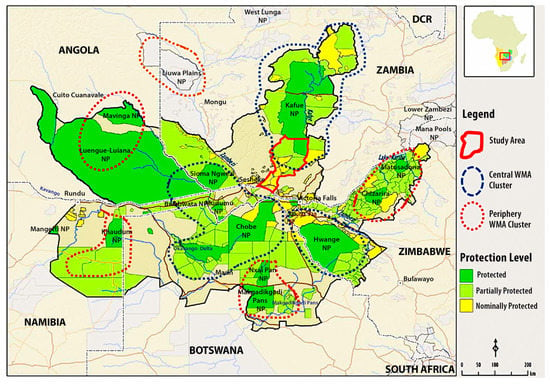
Figure 1.
The Kavango-Zambezi Transfrontier Conservation Area landscape, indicating study area, clusters of wildlife managed areas (WMAs) and their degrees of protection. Protected = National Parks IUCN II; Partially Protected = IUCN III-VI; Nominally Protected = IUCN Not Reported (adapted from [19]).
Kafue National Park is Zambia’s oldest and largest protected area, the largest National Park in the Kavango-Zambezi Transfrontier Conservation Area and the 2nd largest National Park in Africa at 22,480 km2 [18]. In concert with nine surrounding IUCN category VI Game Management Areas, the effective unfenced area known as the Greater Kafue System covers c.68,000 km2, representing 9% of Zambia’s land area [20].
Zambia maintains a wide spectrum of protected areas by category, status, designation and management effectiveness [21]. New protected areas are emerging e.g., Simalaha Communal Conservancy, while some existing areas e.g., Bombwe Forest Reserve are subject to down listing or degazettement from the National protected area estate due to the extent of human impacts. To reflect the uncertainty surrounding unclassified areas [22], emerging or failing protected areas have been designated a Nominally or Potentially protected category. The Greater Kafue System (GKS) has been included as Zambia’s majority component in the Kavango-Zambezi Transfrontier Conservation Area programme, encompassing c.25% of the programs’’ wildlife estate [16]. Terrestrial connectivity to adjacent clusters of wildlife managed areas comprises a minor potential linkage routing west-southwest to Sioma National Park, spanning ≥210 km, and a major potential linkage routing south-southwest towards Chobe National Park, at the heart of the Kavango-Zambezi Transfrontier Conservation Area—the focus of this study. This linkage, termed the Kafue-Simalaha Wildlife Dispersal Corridor [16], passes through a mosaic of nominally, potentially and possibly protected wildlife managed areas including Mulobezi and Sichifulo Game Management Areas, Nachitwe, Martin and Machili Forest Reserves, the Nyawa communal areas, and Simalaha Communal Conservancy (Figure 2). In concert, these wildlife managed areas extend the Greater Kafue System to around 7.3 m ha and provide a contiguous matrix of wildlife managed areas spanning 140–170 km from the Kafue National Park border south-southwest towards the Zambezi River at the confluence of Zambia, Namibia and Botswana, broadly following the Machili watershed [23]. Periphery areas, considered outside of any formal wildlife protection category, are known as ‘Open’ Communal Areas, and fall under statutorily land tenure authority of the local Chiefdom [24].
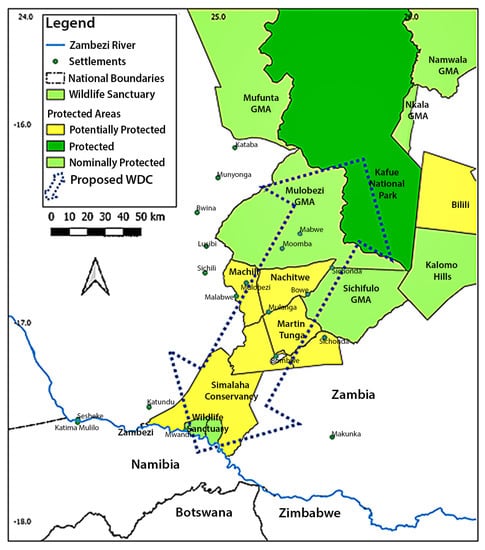
Figure 2.
Wildlife managed areas within study area, indicating proposed Simalaha-Kafue Wildlife Dispersal Corridor (WDC).
In order to achieve the objectives of the study, we produced a landcover map for 3.8 m ha using high resolution satellite imagery, combined with ground truth data. Then we incorporated empirical occurrence data for Lions (Panthera leo), Leopards (Panthera pardus) and Spotted Hyena (Crocuta crocuta) throughout ten wildlife managed areas covering ~1 m ha to produce species-level habitat suitability maps. Finally, we constructed connectivity models to investigate potential for single and multi-species corridors in support of conservation and land use planning for this vast, heterogenous and increasingly human-impacted landscape.
2.2. Landcover Mapping
A landcover map of the study area was produced using a time-series of Sentinel 2 images in an object-oriented image analysis (OBIA) environment using the software eCognition [25]. Sentinel 2 is a constellation of two polar-orbiting satellites launched by ESA under the program Copernicus [26]. Due to the large size of the study area (32,812 km2), and the fact that it fell within two paths of the Sentinel 2 orbit, the study area was split in two parts and analyzed separately.
The eastern part was mapped using a time series of three images acquired in January, April and August 2016 while the western part was mapped using a time series of three images acquired in May, September and December 2016. The reason for using a time series rather than a single image for classification was in order to capture the phenological variation of the vegetation imposed by the apparent seasonality of precipitation and the respected vegetation responses. In total, 24 image tiles were processed which cover the entire study area. The images were geometrically and atmospherically corrected prior to segmentation and classification. During the classification process various spectral ratios were calculated including Normalized Difference Vegetation Index (NDVI;), Normalized Difference Wetness Index (NDWI), Normalized Difference Moisture Index (NDMI), Near InfraRed/Red (NIR).
The first step in any image analysis using the OBIA approach is the creation of meaningful image objects by using segmentation algorithms that group neighboring pixels according to certain criteria of homogeneity. The size and shape of the objects depend primarily on the selected scale parameter (which determines the degree of homogeneity in the resulted objects) as well as by the shape and compactness criterion. Various scale parameters were tested, with the aim of generating objects that have low internal variation and low spatial autocorrelation, and the results were visually inspected. The adopted scale parameter was set at 100 while the color and homogeneity criterion were set at 0.3 and 0.5, respectively. The minimum mapping unit was set at 0.25 ha.
The training data set for the semi-automatic image classification was provided by in-situ observations. Six classes were identified in the field resulting in a training data set consisting of 172 in-situ observations. The identified classes were Arable land, Kalahari, Mopane and Teak woodland, seasonally flooded grasslands and permanent water. A vector dataset containing the settlements (comprising areas encompassing borders of housing aggregations), which were digitized manually using Google Earth, was integrated in the final classification product in a GIS environment [27].
Random Forests was the classifier which was found to perform better in this study area and with the data used, compared to Classification Trees, Support Vector Machines and K Nearest Neighbor.
The two land cover maps that resulted from the classification of the two separate parts of the study area were then merged in eCognition and the accuracy assessment was performed for the entire land cover map. The method proposed by [28] was employed for the accuracy assessment which uses an error matrix based on a TTA mask.
2.3. Habitat Suitability Modelling
To map the suitability of the GKS landscape for providing habitat for the three carnivore species, we relied on statistical habitat suitability modelling. This type of modelling relates environmental factors (physical and human) to the presence or absence of the species; the resulting model can be projected and mapped as a cartographic layer depicting the density or probability of species occurrence [29]. The habitat suitability maps for lions, leopards and spotted hyaena were generated using MaxEnt [30], as this software has been found to perform well compared to other modelling techniques that use presence only data [31], and has been repeatedly used to model the distribution of large carnivores in diverse geographical contexts (e.g., [32,33,34,35]). We incorporated empirically generated occurrence data from on-going research and monitoring in the area, from [23], In total, 102 × 4 km transects, optimized for site conditions, were surveyed on foot three times by the first author and two experienced local trackers from the safari hunting industry, amounting to 1224 km of spoor transects during the dry season of May–October 2015. To account for any sampling bias, we spatially rarefied occurrence records for all species by thinning (using a 500 × 500 m pixel-size grid of the area). In total, 43 occurrence records were used for lions, 84 for leopards and 78 for spotted hyenas. While we sought to incorporate occurrence data for the entire extant large carnivore guild known from the GKS, sample sizes were too small for cheetahs (Acinonyx jubatus) and African wild dogs (Lycaon pictus) to include in final analyses. Occurrence data for lions, leopards and spotted hyenas are shown in the Supplementary Figure S1.
The full set of predictor variables utilized is presented in Table 1. All vegetation-related variables (except Enhanced Vegetation Index), as well as proportion of arable land, distance to and proportion of settlements, and distance to water were derived from the land cover map generated using the Sentinel imagery described above. Roads layers, provided by Peace Parks Foundation, were modified using ground truthing, and distances to roads calculated in [36]. Bioclimatic parameters exhibit little spatial variability across the study area, so we did not use them as predictor variables [37]. To account for collinearity, we retained all uncorrelated variables (r < 0.7). In our case, only variables related to anthropogenic pressures and elevation were correlated (Table 1).
In terms of variable selection, to keep models as simple as possible to aid in the interpretation of the species’ distribution and its environment drivers, we strived for “parsimonious and interpretable models” [38]. In all models, through a backwards stepwise process, we excluded all variables that when added in the models did not produce any increase in AUC. For the same reasons (parsimony and interpretability), we run models using only linear and quadratic features (as suggested by [38]). Following standard practice (e.g., [39,40,41,42]), we trained the models using a randomly selected sample (70%) of the occurrence data, and we used the remaining 30% of the occurrence data to evaluate the models. Fifty model iterations were run, with 10,000 background points. The resulting habitat suitability map provides a habitat suitability value between 0 and 1 for each grid cell/pixel.


Table 1.
Predictor variables used in the model building process. Source key: (1) [43], (2) Expert knowledge/ground truthing, (3) Landcover map (this paper), (4) ASTER Global DEM, (5) MODIS Vegetation Index Products, (5) From Sentinel 2.
Table 1.
Predictor variables used in the model building process. Source key: (1) [43], (2) Expert knowledge/ground truthing, (3) Landcover map (this paper), (4) ASTER Global DEM, (5) MODIS Vegetation Index Products, (5) From Sentinel 2.
| Predictor Variables | |||
|---|---|---|---|
| 1 | Proportion of arable land (3) | 10 | Distance to minor roads (unpaved) (1,2) |
| 2 | Proportion of Kalahari woodland (3) | 11 | Distance to major and minor roads, combined (1,2) |
| 3 | Proportion of Mopane woodland (3) | 12 | Distance to major, minor roads & rail, combined (1,2) |
| 4 | Proportion of Teak woodland (3) | 13 | Distance to train tracks (1) |
| 5 | Proportion of grassland (3) | 14 | Altitude (DEM) (4) |
| 6 | Proportion of settlements (at 1, 2 and 3 km radius) (3) | 15 | Topographic Position Index (4) |
| 7 | Distance to water (4) | 16 | Slope (4) |
| 8 | Distance to settlements (4) | 17 | Enhanced Vegetation Index (5) |
| 9 | Distance to major roads (paved) (2) | ||
2.4. Connectivity Modelling
To provide insights into scope, scale and any species-level overlap of potential ecological flows in the Kafue-Simalaha Wildlife Dispersal Corridor we developed three individual species, and one multi-species model using Linkage Mapper [44], as this software provides robust estimates of connectivity for large carnivores in heterogenous landscapes [45,46,47]. The Pathway Tool was chosen to identify and model potential linkages between core areas, graphically displaying least-cost corridors where species would encounter more features facilitating, and less features impeding, movement between core areas based on predetermined site and species-specific resistance layers—the inversed habitat suitability maps generated by MaxEnt [44]. Parameterising Linkage Mapper requires the identification of paired nodes between which patch-based graphic analyses of connectivity can be undertake. Given Kafue National Park represents a single source population of all three target species, with a border extending c120 km within the study area, we tested the effect of multiple source nodes along the Kafue National Park border on least-cost path outcomes, finally settling on three roughly equidistant nodes at the northwest, central and southwestern points bordering a representative cross-section of adjacent Game Management Areas, implying target species could move between any of these nodes to the southern node at the Simalaha Wildlife Recovery Sanctuary.
3. Results
3.1. Land Cover Mapping
The final landcover map (Figure 3) had an overall classification accuracy of 91.6% and a Kappa Statistic of 0.88, indicating an excellent classification performance. The dominant land cover is Kalahari woodland (56.2%), followed by Mopane woodland (15.6%) characteristic of south-central floodplains. Seasonally flooded grassland (13.0%) dominates the floodplains and drainage lines and are closely associated with water (0.2% and arable land (4.6%). Teak woodland (10.3%), formerly representing extensive closed-canopy forest tracts, has been heavily denuded by extractive activities, and further suppressed by fire [48]. Settlements (0.1%) are closely associated with drainage lines (available surface water), grasslands and agricultural areas. Surface water is depicted here for dry season (May-Nov). After heavy rains most all grassland areas are commonly inundated for many months.
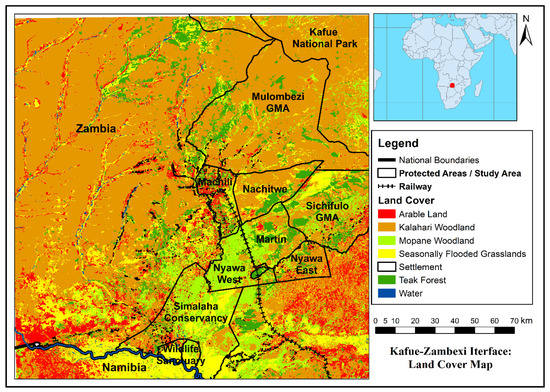
Figure 3.
Landcover map of the wider study area, indicating wildlife managed areas.
3.2. MaxEnt Habitat Suitability Models
Three habitat suitability models were built, one for each species (Figure 4). The models for lion had the best AUC (0.81), followed by leopards (0.80) and spotted hyenas (0.79). All models identified Kafue National Park as core habitat with extensive areas of Mulobezi and Sichifulo Game Management Areas, and to decreasing extent the Forest reserves of Nachitwe and Martin. Machili Forest Reserve has been identified as largely unsuitable for all three species. Further south, increased variability was exhibited between wildlife managed areas and species, though western areas of Simalaha present poor suitability. Interestingly, east of Simalaha was identified as common suitable habitat.
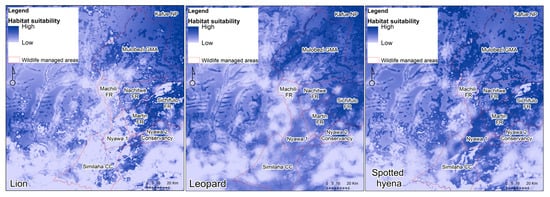
Figure 4.
Habitat Suitability Models by species.
According to model output, the three species distributions are affected by different variables (see Table 2). Notably the proportion of settlement, a proxy of human population density, had a negative effect on all species. The proportion of arable land had strong negative effects on leopard and hyena while linear anthropogenic features, such as road and/or rail infrastructures have negative impact on all three species. The proportion of Kalahari woodland cover was positively associated with all species, with Lion positively associated with proportion of all woodland landcover classifications. The proportion of grasslands (lion and hyena) and distance to water (leopard and hyena) were also notable predictor variables.

Table 2.
Variable importance for the Maxent models, based on AUC using a single variable. Light grey represents variables with a negative contribution to relative habitat suitability.
3.3. Connectivity Modelling
Pathways analyses indicates broadly similar spatial routing for all three species, with least-cost corridors consistently following the Kafue National Park border, indicating optimal (core) habitat within Kafue National Park (Figure 5). Irrespective of source nodes, all models produced least-cost corridor exiting Kafue National Park at the southern border, at the closest spatial point to the southern node, routing through eastern Mulobezi Game Management Areas, Sichifulo Game Management Areas, the border areas of Martin Forest Reserve and Nyawa Communal Areas, then through the eastern section of Simalaha Communal Conservancy, extending notably east of Simalaha in the case of Lion into Open Communal Areas. The Spotted Hyena model provided the largest spatial extent of moderate to highly suitable corridor area (depicted as yellow/green/blue shading), followed by Leopard then Lion.
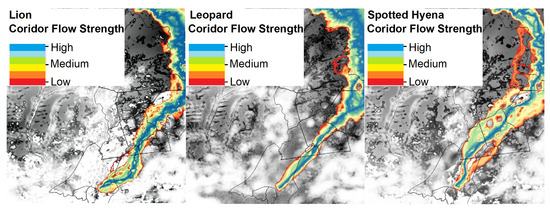
Figure 5.
Connectivity modelling outputs by species.
Minimum corridor width, identified as moderately to highly suitable areas, was 5.1 km for lion, 4.3 km for Leopard and 3.2 km Spotted Hyena. All three species’ paths narrowed noticeably in southern areas, and all reaching the Simalaha Wildlife Recovery Sanctuary at the northeast corner.
The derived multi-species model (Figure 6) exhibits broadly similar corridor routing and spatial extent through Kafue National Park and eastern Mulobezi Game Management Areas into Sichifulo Game Management Areas, before displaying greater variability and a reduction in common corridor routing and current flow strength in central southern areas. A ~25 km long stretch of low current flow, starting at the interface of the Nyawa West Communal Area and the adjacent Open Communal Area, indicate potential constraints to species movement. Moderate to highly suitable current flow, though narrow (<2 km width), converges again within ~10 km from the northeast corner of the Simalaha Wildlife Recovery Sanctuary.
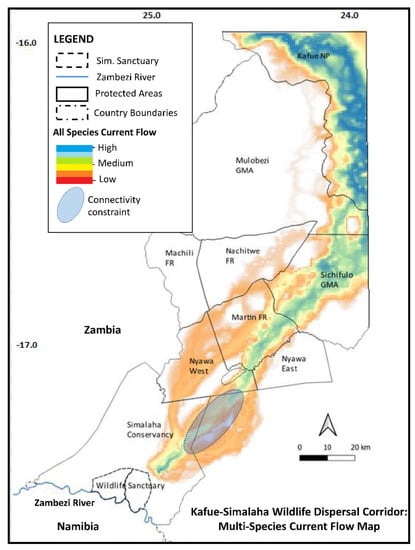
Figure 6.
Composite species connectivity model, indicating potential connectivity constraint.
4. Discussion
Connectivity is an increasingly threatened ecological process and predicted to deteriorate due primarily to issues surrounding human population growth and encroachment around core wildlife areas [49], with severe implications for many wide-ranging, low-density species [5,50]. Maintaining and promoting connectivity in wildlife managed area networks is thus considered an important intervention for the conservation of free-ranging large carnivore populations, elevating the value of the proposed Kafue-Simalaha Wildlife Dispersal Corridor, and providing a test case for explicit goals and outcomes within the Kavango-Zambezi Transfrontier Conservation Area Programme.
Developing linkages in heterogenous landscapes presents practical and theoretical challenges surrounding the identification of land that will best meet the requirements for focal species to move between core wildlife managed areas, and how to translate resource selection measurements into resistance [51]. By choosing a multi-species approach that focuses on wildlife with high susceptibility to human disturbance and resource competition, and those driving broader ecological processes, our approach serves as a model for a collective umbrella under which a wider range of species and processes are factored in, creating a broadly applicable approach pertinent to the wider Kavango-Zambezi Transfrontier Conservation Area landscape and beyond. Considering that our approach is based on often available species occurrence data and free to use Sentinel images, it could serve as a general approach in other data-poor regions in Central Southern Africa and beyond.
The current study reveals the negative impact of all anthropogenic structures (settlements, agricultural land, road and rail network) on the distribution of large carnivores in the proposed Kafue-Simalaha Wildlife Dispersal Corridor, with important implications for landscape and wildlife management in the area. While well documented case studies highlight the occurrence of resident Spotted Hyena and Leopard populations in and around human settlements, in our case the area under study is not heavily urbanized nor populated as in other cases in the literature [52,53]. Our findings indicate that the effects of settlements are context dependent, demonstrating the importance of understanding broader area and management characteristics [54,55]. Road and rail infrastructure in various combinations impacts all species. While literature indicates wide-ranging impacts of transport infrastructure on vertebrate populations [56,57], there are significant limits to our understanding of how transport links affect carnivore populations, though by providing people access to remote areas there are likely broader issues surrounding negative synergistic effects of access at larger scales [58].
With all species being habitat generalists, we had expected a strong relationship to a cross-section of habitat types. Lion exhibit a strong preference for woodland cover, a localized characteristic found by [59], and likely a response partly driven by spatial avoidance of humans [60,61] and preferred prey availability [62]. The more catholic diet of leopard and hyena allows both species to exploit a wider range of habitats and food sources [63]. The relationship of leopard and hyena to dry season surface water poses questions surrounding disturbance competition by seasonal shifting pastoralist activities at water points, including elevated poaching pressure [10], charcoal production [64] and impacts of fire [48].
Our modelled corridor routing for single and multi-species models broadly compliments existing outputs [65,66], indicating limited habitat suitability in southern and central areas, increasing habitat suitability towards Kafue National Park, and also preferential corridor routing along eastern areas of the proposed Kafue-Simalaha Wildlife Dispersal Corridor. At the wildlife managed area scale our output indicated elevated habitat suitability within Kafue National Park which is intuitively encouraging given Kafue National Park is a significant core wildlife area, and specifically for the region’s large carnivores [67,68,69]. Given extensive areas of northern and eastern Mulobezi Game Management Area are ostensibly free of direct and much indirect human pressure (Lines, expert opinion) there was an expectation for elevated current flow in these areas, though evidently least-cost path models prioritized Kafue National Park and proximity to the destination node, though this doesn’t preclude availability of suitable habitat and prey in remote areas of Mulobezi Game Management Area [23]. Further south, beyond the Forest Reserves and into the Communal Areas, current flow closely matched expectations, with constrained current flow for all species, and the possibility of a connectivity bottleneck south of Bombwe, representing an area where movement is funneled, curtailed or severed, and connectivity diminished or lost [70], in support of the hypothesis offered by [23].
Notwithstanding expectations, and given likely compounded error through occurrence, land cover and habitat suitability analyses, the corridor modelling, both at species and multi-species level, clearly reflects reduced connectivity moving south from Kafue National Park into more human disturbed areas. From a connectivity management perspective this is a particularly important area to keep intact, as loss of this area might disproportionally compromise connectivity [46]. A greater understanding of implications concerning species-level demography on movement would be a valuable addition to these outputs.
Our integrated approach has extended the analytical framework to provide higher resolution understanding of localized corridor characteristics—notably overlap in multi-species corridor routing and spatial constraints in habitat suitability for central-southern extents of the proposed Kafue-Simalaha Wildlife Dispersal Corridor. This has important implications for practical corridor planning and interventions to counter effects limiting movement. While landscape dynamics will affect individual vs population level movements [71], and species-scale demographics will likely influence dispersal mechanisms [59], our approach has clearly provided valuable supplemental detail and understanding to existing broader-scale, species-specific models.
What is clear from the land cover map is the extensive settlement and agricultural development along the majority of watercourses, even deep into Game Management Areas where settlement and agriculture should be precluded from core wildlife zones. The eastern areas of Sichifulo Game Management Area, almost all of the Machili Forest Reserve and eastern Nyawa areas have been heavily affected by slash and burn agricultural development as with the central and western sections of Simalaha. Intensive settlement and agricultural development are occurring in all periphery areas bordering the proposed Kafue-Simalaha Wildlife Dispersal Corridor with the exception of the western extents of the Open Communal area bordering Nyawa East and Simalaha. We urge additional research and support in this area as a priority addition to wildlife-based land use planning for the Kafue-Simalaha Wildlife Dispersal Corridor.
5. Conclusions
Integration of land cover, habitat suitability and corridor modelling at the landscape level produced an output broadly in line with existing literature and implying constriction of connectivity for all three species approaching the southern areas of the Kafue-Simalaha Wildlife Dispersal Corridor, but identified a potentially important new area in which to focus conservation efforts. Additionally, at the Kavango-Zambezi Transfrontier Conservation Area scale, the fencing of the Simalaha Wildlife Recovery Sanctuary and effects of the Zambezi River on wildlife movement south to the central cluster of wildlife managed areas centered on Zambezi Region in Namibia and Chobe National Park in Botswana have not been modelled.
With the human population increasing, and expanding conversion of wildlife habitat for agriculture, any existing bottlenecks hindering wildlife movement, both for large carnivores and their prey, will likely increase in the absence of effective land use planning and implementation, including control of poaching and human-wildlife conflict. Nonetheless, our analyses have produced the first empirical data for evidence-based corridor planning for Zambia’s key linkage in the Kavango-Zambezi Transfrontier Conservation Area Programme.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su132212886/s1, Figure S1: Species Occurrence Data.
Author Contributions
Conceptualization, R.L. and J.T.; methodology, R.L., D.B., P.X. and J.T.; validation, R.L.; formal analysis, R.L., D.B., P.X. and J.T.; validation, R.L.; investigation, R.L.; data curation, R.L., D.B., P.X.; writing—original draft preparation, R.L., D.B., P.X. and J.T.; visualization, R.L., D.B., P.X.; supervision, J.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: (1) University of Kent 50th Anniversary Scholarship, (2) WWF Namibia, (3) Humane Society International, (4) Westwood.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the School of Anthropology and Conservation, University of Kent. Permission to contact research in Zambia was provided by the Zambia Wildlife Authority.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank A. Nambota for study development, P. Moss, J. Hanks and M. Musgrave for insights into Kafue National Park and surrounding Game Management Areas, the Chilanga, Ngoma, Mulobezi and Mulanga offices of the Department of Parks and Wildlife Management (formerly the Zambia Wildlife Authority) for permissions and field support. HRH Inyambo Yeta of the Lozi and the late HRH Moomba of the Nkoya, for granting smooth passage through their Chiefdoms.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E. Biodiversity: The ravages of guns, nets and bulldozers. Nat. News 2016, 546, 143. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, M.; Boitani, L.; Mallon, D.; Hoffmann, M.; Iacucci, A.; Meijaard, E.; Visconti, P.; Schipper, J.; Rondinini, C. A retrospective evaluation of the global decline of carnivores and ungulates. Conserv. Biol. 2014, 28, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. Proc. Biol. Sci. 2000, 267, 1947–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardillo, M.; Mace, G.M.; Jones, K.E.; Bielby, J.; Bininda-Emonds, O.R.; Sechrest, W.; Orme, C.D.; Purvis, A. Multiple causes of high extinction risk in large mammal species. Science 2005, 309, 1239–1241. [Google Scholar] [CrossRef] [Green Version]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P. Status and ecological effects of the world’s largest carnivores. Science 2014, 343, 124–148. [Google Scholar] [CrossRef] [Green Version]
- Wolf, C.; Ripple, W.J. Range contractions of the world’s large carnivores. R. Soc. Open Sci. 2017, 4, 170052. [Google Scholar] [CrossRef] [Green Version]
- Cushman, S.A.; McRae, B.; Adriaensen, F.; Beier, P.; Shirley, M.; Zeller, K. Biological corridors and connectivity. In Key Topics in Conservation Biology 2; Macdonald, D.W., Willis, K.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 384–404. [Google Scholar]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; Rondinini, C.; Boitani, L. Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Philosophical Transactions of the Royal Society B. Biol. Sci. 2011, 366, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Funston, P.J.; Groom, R.J.; Lindsey, P.A. Insights into the management of large carnivores for profitable wildlife-based land uses in African savannas. PLoS ONE 2013, 8, e59044. [Google Scholar] [CrossRef]
- KAZA. Treaty Between the Governments of Angola, Botswana, Namibia, Zambia and Zimbabwe on the Establishment of the Kavango–Zambezi Transfrontier Conservation Area; Kavango–Zambezi TFCA Secretariat: Kasane, Botswana, 2011. [Google Scholar]
- Cumming, D.H.M.; Anderson, J.A.; de Garine-Wichatitsky, M.; Dzingirai, V.; Giller, K.E. Whither TFCAs and people on the edge in Southern Africa? In Transfrontier Conservation Areas: People Living on the Edge; Andersson, J.A., de Garine-Wichatitsky, M., Cumming, D., Dzingirai, V., Giller, K., Eds.; Taylor & Francis: London, UK, 2017; pp. 192–203. [Google Scholar]
- Pullinger, M.G.; Johnson, C.J. Maintaining or restoring connectivity of modified landscapes: Evaluating the least-cost path model with multiple sources of ecological information. Landsc. Ecol. 2010, 25, 1547–1560. [Google Scholar] [CrossRef]
- Hofmann, D.D.; Behr, D.M.; McNutt, J.W.; Ozgul, A.; Cozzi, G. Bound within boundaries: Do protected areas cover movement corridors of their most mobile, protected species? J. Appl. Ecol. 2021, 58, 1133–1144. [Google Scholar] [CrossRef]
- Cumming, D.H. Large Scale Conservation Planning and Priorities for the Kavango-Zambezi Transfrontier Conservation Area; Conservation International: Arlington, VA, USA, 2008. [Google Scholar]
- ΚAΖA. Master Integrated Development Plan; KAZA Secretariat. Victoria Falls: Kasane, Botswana, 2014. [Google Scholar]
- Worboys, G.; Francis, W.L.; Lockwood, M. Connectivity Conservation Management: A Global Guide (with Particular Reference to Mountain Connectivity Conservation); Earthscan: London, UK; Washington, DC, USA, 2010. [Google Scholar]
- UNEP-WCMC. United Nations Environment Programmes World Conservation Monitoring Centre. Protected Planet Database. 2015. Available online: https://www.protectedplanet.net/ (accessed on 1 May 2015).
- PPF. Integrated Development Plan for the Zambian Component of the Kavango-Zambezi Transfrontier Conservation Area; Peace Parks Foundation: Stellenbosch, South Africa, 2008. [Google Scholar]
- ZAWA. General Management Plan for Kafue National Park; ZAWA: Chilanga, Zambia, 2010. [Google Scholar]
- Geldmann, J.; Manica, A.; Burgess, N.D.; Coad, L.; Balmford, A. A global-level assessment of the effectiveness of protected areas at resisting anthropogenic pressures. Proc. Natl. Acad. Sci. USA 2019, 116, 23209–23215. [Google Scholar] [CrossRef]
- IUCN; UNEP-WCMC. The World Database on Protected Areas (WDPA). Available online: https://www.protectedplanet.net/ (accessed on 1 May 2015).
- Lines, R.; Tzanopoulos, J.; MacMillan, D. Status of terrestrial mammals at the Kafue–Zambezi interface: Implications for transboundary connectivity. Oryx 2018, 53, 764–773. [Google Scholar] [CrossRef]
- Machina, H. Land is Life. In Land Policy and Administration in Zambia; 2005; pp. 1–5. [Google Scholar]
- eCognition Developer, T. 9.0 User Guide; Trimble Germany GmbH: Munich, Germany, 2014.
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P. Sentinel-2: ESA’s optical high-resolution mission for GMES operational services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- ESRI. Environmental Systems Research Institute.ArcGIS Release 10.1; ESRI: Redlands, CA, USA, 2012. [Google Scholar]
- Congalton, R.G. A review of assessing the accuracy of classifications of remotely sensed data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Ortigosa, G.R.; De Leo, G.A.; Gatto, M. VVF: Integrating modelling and GIS in a software tool for habitat suitability assessment. Environ. Model. Softw. 2000, 15, 1–12. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Jackson, C.R.; Marnewick, K.; Lindsey, P.A.; Røskaft, E.; Robertson, M.P. Evaluating habitat connectivity methodologies: A case study with endangered African wild dogs in South Africa. Landsc. Ecol. 2016, 31, 1433–1447. [Google Scholar] [CrossRef]
- Di Minin, E.; Macmillan, D.C.; Goodman, P.S.; Escott, B.; Slotow, R.; Moilanen, A. Conservation businesses and conservation planning in a biological diversity hotspot. Conserv. Biol. 2013, 27, 808–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelieri, C.C.S.; Adams-Hosking, C.; Barros, D.; Micchi, K.M.P.; de Souza, M.P.; McAlpine, C.A. Using species distribution models to predict potential landscape restoration effects on puma conservation. PLoS ONE 2016, 11, e0145232. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Nezami Balouchi, B.; Jowkar, H.; Hemami, M.; Fadakar, D.; Malakouti-Khah, S.; Ostrowski, S. Combining landscape suitability and habitat connectivity to conserve the last surviving population of cheetah in Asia. Divers. Distrib. 2017, 23, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Team, Q.D. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2016. Available online: http://qgis.osgeo.org (accessed on 20 December 2016).
- Fitzpatrick, M.C.; Gotelli, N.J.; Ellison, A.M. MaxEnt versus MaxLike: Empirical comparisons with ant species distributions. Ecosphere 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Parolo, G.; Rossi, G.; Ferrarini, A. Toward improved species niche modelling: Arnica montana in the Alps as a case study. J. Appl. Ecol. 2008, 45, 1410–1418. [Google Scholar] [CrossRef]
- Chen, F.; Du, Y.; Niu, S.; Zhao, J. Modeling forest lightning fire occurrence in the Daxinganling Mountains of Northeastern China with MAXENT. Forests 2015, 6, 1422–1438. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Amat, E.; Mateo, R.G.; Nieto-Lugilde, D.; Morueta-Holme, N.; Svenning, J.C.; García-Amorena, I. Impact of model complexity on cross-temporal transferability in Maxent species distribution models: An assessment using paleobotanical data. Ecol. Model. 2015, 312, 308–317. [Google Scholar] [CrossRef]
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. MaxEnt modeling for predicting the current and future potential geographical distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef] [Green Version]
- PPF. In House Dataset for the Zambian Component of the Kavango-Zambezi Transfrontier Conservation Area. Unpublished Data; Peace Parks Foundation: Stellenbosch, South Africa, 2016. [Google Scholar]
- McRae, B.H.; Kavanagh, D.M. Linkage Mapper Connectivity Analysis Software; The Nature Conservancy: Seattle, WA, USA, 2011. [Google Scholar]
- Wolf, C.; Ripple, W.J. Rewilding the world’s large carnivores. R. Soc. Open Sci. 2018, 5, 172–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castilho, C.S.; Hackbart, V.C.; Pivello, V.R.; dos Santos, R.F. Evaluating landscape connectivity for Puma concolor and Panthera onca among Atlantic forest protected areas. Environ. Manag. 2015, 55, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.; McRAE, B.H.; Brookes, A. Use of linkage mapping and centrality analysis across habitat gradients to conserve connectivity of gray wolf populations in western North America. Conserv. Biol. 2012, 26, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, M. Scale, Governance and Change in Zambezi Teak Forests: Sustainable Development for Commodity and Community; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2016. [Google Scholar]
- Wittemyer, G.; Elsen, P.; Bean, W.T.; Burton, A.C.; Brashares, J.S. Accelerated human population growth at protected area edges. Science 2008, 321, 123–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, F.G.; Becker, M.S.; Milanzi, J.; Nyirenda, M. Human encroachment into protected area networks in Zambia: Implications for large carnivore conservation. Reg. Environ. Chang. 2015, 15, 415–429. [Google Scholar] [CrossRef]
- Beier, P.; Majka, D.R.; Spencer, W.D. Forks in the road: Choices in procedures for designing wildland linkages. Conserv. Biol. 2008, 22, 36–851. [Google Scholar] [CrossRef]
- Yirga, G.; Leirs, H.; De Iongh, H.H.; Asmelash, T.; Gebrehiwot, K.; Deckers, J.; Bauer, H. Spotted hyena (Crocuta crocuta) concentrate around urban waste dumps across Tigray, northern Ethiopia. Wildl. Res. 2016, 42, 563–569. [Google Scholar] [CrossRef]
- Athreya, V.; Odden, M.; Linnell, J.D.; Krishnaswamy, J.; Karanth, U. Big cats in our backyards: Persistence of large carnivores in a human dominated landscape in India. PLoS ONE 2013, 8, e57872. [Google Scholar] [CrossRef] [Green Version]
- Woodroffe, R. Predators and people: Using human densities to interpret declines of large carnivores. Anim. Conserv. Forum 2000, 3, 165–173. [Google Scholar] [CrossRef]
- Linnell, J.D.; Thomassen, J.; Jones, K. Wildlife-Human Interactions: From Conflict to Coexistence in Sustainable Landscapes; Norsk Institutt for Naturforskning (NINA): Trondheim, Norway, 2011. [Google Scholar]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Coffin, A.W. From roadkill to road ecology: A review of the ecological effects of roads. J. Transp. Geogr. 2007, 15, 396–406. [Google Scholar] [CrossRef]
- Forman, R.T.; Sperling, D.; Bissonette, J.A.; Clevenger, A.P.; Cutshall, C.D.; Dale, V.H.; Fahrig, L.; France, R.L.; Heanue, K.; Goldman, C.R. Road Ecology: Science and Solutions; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Elliot, N.B.; Cushman, S.A.; Loveridge, A.J.; Mtare, G.; Macdonald, D.W. Movements vary according to dispersal stage, group size, and rainfall: The case of the African lion. Ecology 2014, 95, 2860–2869. [Google Scholar] [CrossRef]
- Oriol-Cotterill, A.; Valeix, M.; Frank, L.G.; Riginos, C.; Macdonald, D.W. Landscapes of coexistence for terrestrial carnivores: The ecological consequences of being downgraded from ultimate to penultimate predator by humans. Oikos 2015, 124, 1263–1273. [Google Scholar] [CrossRef]
- Loveridge, A.J.; Valeix, M.; Elliot, N.B.; Macdonald, D.W. The landscape of anthropogenic mortality: How African lions respond to spatial variation in risk. J. Appl. Ecol. 2017, 54, 815–825. [Google Scholar] [CrossRef]
- Schaller, G.B. The Serengeti Lion: A Study of Predator-Prey Relations; University of Chicago Press: Chicago, IL, USA, 1976. [Google Scholar]
- Hayward, M.W.; Kerley, G.I. Prey preferences and dietary overlap amongst Africa’s large predators. Afr. J. Wildl. Res. 2008, 38, 93–109. [Google Scholar] [CrossRef]
- Munthali, S.M.; Smart, N.; Siamundaala, V.; Mtsambiwa, M.; Harvie, E. Integration of Ecological and Socioeconomic Factors in securing Wildlife Dispersal Corridors in the Kavango-Zambezi Transfrontier Conservation Area, Southern Africa; Selected Studies in Biodiversity; Sen, B., Ed.; IntechOpen, 2018; Available online: https://www.intechopen.com/chapters/56778 (accessed on 1 May 2021).
- Cushman, S.A.; Elliot, N.B.; Bauer, D.; Kesch, K.; Bothwell, H.; Flyman, M.; Mtare, G.; Macdonald, D.W.; Loveridge, A.J. Prioritizing core areas, corridors and conflict hotspots for lion conservation in southern Africa. PLoS ONE 2018, 13, e0196213. [Google Scholar] [CrossRef]
- Cushman, S.A.; Elliot, N.B.; Macdonald, D.W.; Loveridge, A.J. A multi-scale assessment of population connectivity in African lions (Panthera leo) in response to landscape change. Landsc. Ecol. 2016, 31, 1337–1353. [Google Scholar] [CrossRef]
- IUCN. Conservation Strategy for the Lion in Eastern and Southern Africa; IUCN SSC Cat Specialist Group: Gland, Switzerland; Cambridge, UK, 2006. [Google Scholar]
- Jacobson, A.P.; Gerngross, P.; Lemeris, J.R., Jr.; Schoonover, R.F.; Anco, C.; Breitenmoser-Würsten, C.; Durant, S.M.; Farhadinia, M.S.; Henschel, P.; Kamler, J.F.; et al. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ 2016, 4, e1974. [Google Scholar] [CrossRef] [Green Version]
- RWCP; IUCN/SSC. Regional Conservation Strategy for the Cheetah and African Wild Dog in Southern Africa; Revised and Updated, August 2015; IUCN: Gland, Switzerland, 2015. [Google Scholar]
- Berger, J.; Cain, S.L.; Berger, K.M. Connecting the dots: An invariant migration corridor links the Holocene to the present. Biol. Lett. 2006, 2, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Olson, K.A.; Dressler, G.; Leimgruber, P.; Fuller, T.K.; Nicolson, C.; Novaro, A.J.; Bolgeri, M.J.; Wattles, D.; DeStefano, S. How landscape dynamics link individual-to population-level movement patterns: A multispecies comparison of ungulate relocation data. Glob. Ecol. Biogeogr. 2011, 20, 683–694. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).