Sorption Efficiency of Potentially Toxic Elements onto Low-Cost Materials: Peat and Compost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Compost and Peat Characterization
2.3. Sorption Equilibrium Studies
2.4. Sorption Kinetic Studies
2.4.1. Pseudo-First-Order (PFO)
2.4.2. Pseudo-Second-Order (PSO)
2.4.3. Elovich Kinetic Model
2.4.4. Mathews and Weber (M&W)
2.4.5. Weber and Morris (W&M)
2.5. Bioaccessibility Test
- (i)
- To simulate the gastric phase (first phase): 50 mL of 0.4 M glycine solution adjusted to pH 1.5 with HCl was added to 0.5 g of dry peat. The solution was maintained at 37 °C and stirred occasionally for 1 h, and then an aliquot of the supernatant (10 mL) was sampled and filtered;
- (ii)
- To simulate passage from the gastric to the intestinal phase (second phase): the pH of the remaining extract was elevated using NaHCO3; the solid-solution ratio (1:100) was maintained constant by adding glycine; the suspension was maintained at 37 °C and stirred occasionally for 3 h; and an aliquot of the supernatant was sampled and filtered (filter paper similar to the first phase).
3. Results
3.1. Compost and Peat Properties
3.2. Sorption Results
3.2.1. Equilibrium Studies
3.2.2. Kinetic Studies
3.3. Human Bioaccessibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, A.M.O.; Paleologos, E.K.; Rodrigues, V.G.S.; Singh, D.N. Fundamentals of Geoenvironmental Engineering: Understanding Soil, Water, and Pollutant Interaction and Transport; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128051450. [Google Scholar]
- Förstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1983. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press LLC: Boca Raton, MA, USA; London, UK; New York, NY, USA, 2011. [Google Scholar]
- Boscov, M.E.G. Geotecnia Ambiental; Oficina de Textos: São Paulo, Brazil, 2008. [Google Scholar]
- Bosso, S.T.; Enzweiler, J. Bioaccessible lead in soils, slag, and mine wastes from an abandoned mining district in Brazil. Environ. Geochem. Health 2007, 30, 219–229. [Google Scholar] [CrossRef]
- Ettler, V.; Tomášová, Z.; Komárek, M.; Mihaljevič, M.; Šebek, O.; Michálková, Z. The pH-dependent long-term stability of an amorphous manganese oxide in smelter-polluted soils: Implication for chemical stabilization of metals and metalloids. J. Hazard. Mater. 2015, 286, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V.; Cihlová, M.; Jarošíková, A.; Mihaljevič, M.; Drahota, P.; Kříbek, B.; Vaněk, A.; Penížek, V.; Sracek, O.; Klementová, M.; et al. Oral bioaccessibility of metal(loid)s in dust materials from mining areas of northern Namibia. Environ. Int. 2019, 124, 205–215. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Lima, J.Z.; Sakamoto, I.K.; Varesche, M.B.A.; Trofino, J.C.; Rodrigues, V.G.S. Soil contamination assessment for Pb, Zn and Cd in a slag disposal area using the integration of geochemical and microbiological data. Environ. Monit. Assess. 2016, 188, 1–24. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Papa, T.B.R.; Sígolo, J.B.; Rodrigues, V.G.S. Assessment of the mobility, bioaccessibility, and ecological risk of Pb and Zn on a dirt road located in a former mining area-Ribeira Valley-Brazil. Environ. Monit. Assess. 2019, 191, 101. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Sakamoto, I.K.; Varesche, M.B.A.; Rodrigues, V.G.S. Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci. Total Environ. 2019, 675, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Helser, J.; Cappuyns, V. Trace elements leaching from Pb-Zn mine waste (Plombières, Belgium) and environmental implications. J. Geochem. Explor. 2021, 220, 106659. [Google Scholar] [CrossRef]
- Orgiazzi, A.; Bardgett, R.D.; Barrios, E.; Behan-Pelletier, V.; Briones, M.J.I.; Chotte, J.-L.; De Deyn, G.B.; Eggleton, P.; Fierer, N.; Fraser, T.; et al. Global Soil Biodiversity Atlas; European Commission, Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- World Health Organization (WHO). Environmental Health Criteria 221 ZINC; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Agency for Toxic Substances and Diseases Registry (ATSDR). Toxicological Profile for Zinc; ATSDR: Atlanta, GA, USA, 2005.
- Agency for Toxic Substances and Diseases Registry (ATSDR). Toxicological Profile for Lead; ATSDR: Atlanta, GA, USA, 2020.
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Diseases Registry (ATSDR). Toxicological Profile for Cadmium; ATSDR: Atlanta, GA, USA, 2012.
- Tian, J.; Li, Z.; Wang, L.; Qiu, D.; Zhang, X.; Xin, X.; Cai, Z.; Lei, B. Metabolic signatures for safety assessment of low-level cadmium exposure on human osteoblast-like cells. Ecotoxicol. Environ. Saf. 2021, 207, 111257. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (US EPA). Abandoned Mine Site Characterization and Cleanup Handbook; US EPA: Washington, DC, USA, 2000.
- Božić, D.; Stanković, V.; Gorgievski, M.; Bogdanović, G.; Kovačević, R. Adsorption of heavy metal ions by sawdust of deciduous trees. J. Hazard. Mater. 2009, 171, 684–692. [Google Scholar] [CrossRef]
- Alhogbi, B.G. Potential of coffee husk biomass waste for the adsorption of Pb(II) ion from aqueous solutions. Sustain. Chem. Pharm. 2017, 6, 21–25. [Google Scholar] [CrossRef]
- Adewuyi, A.; Pereira, F.V. Underutilized Luffa cylindrica sponge: A local bio-adsorbent for the removal of Pb(II) pollutant from water system. Beni Suef Univ. J. Basic Appl. Sci. 2017, 6, 118–126. [Google Scholar] [CrossRef]
- Çelebi, H.; Gök, G.; Gök, O. Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Milani, P.A.; Debs, K.B.; Labuto, G.; Carrilho, E.N.V.M. Agricultural solid waste for sorption of metal ions: Part I—characterization and use of lettuce roots and sugarcane bagasse for Cu(II), Fe(II), Zn(II), and Mn(II) sorption from aqueous medium. Environ. Sci. Pollut. Res. 2018, 25, 35895–35905. [Google Scholar] [CrossRef]
- Raimondi, I.M.; Rodrigues, V.G.S.; Lima, J.Z.; Marques, J.P.; Vaz, L.A.A. The Potential Use of Pressmud as Reactive Material for Cd2+ Removal: Adsorption Equilibrium, Kinetics, Desorption, and Bioaccessibility. Water Air Soil Pollut. 2020, 231, 365. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.A.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Bundschuh, J.; et al. Exploring the arsenic removal potential of various biosorbents from water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef]
- Lima, J.Z.; Ferreira da Silva, E.; Patinha, C.; Durães, N.; Vieira, E.M.; Silvestre Rodrigues, V.G. Sorption of arsenic by composts and biochars derived from the organic fraction of municipal solid wastes: Kinetic, isotherm and oral bioaccessibility study. Environ. Res. 2021, 204, 111988. [Google Scholar] [CrossRef]
- Khoshand, A.; Fall, M. Geotechnical characterization of peat-based landfill cover materials. J. Rock Mech. Geotech. Eng. 2016, 8, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Marques, J.P.; Rodrigues, V.G.S.; Raimondi, I.M.; Lima, J.Z. Increase in Pb and Cd Adsorption by the Application of Peat in a Tropical Soil. Water Air Soil Pollut. 2020, 231, 365. [Google Scholar] [CrossRef]
- Raimondi, I.M.; Rodrigues, V.G.; Lima, J.Z.; Marques, J.P.; Vaz, L.A.A.; Vieira, E.M. Assessment of the use of tropical peats as local alternative materials for the adsorption of Pb, Zn and Cd: An equilibrium study. Earth Sci. Res. J. 2021, 25, 29–40. [Google Scholar] [CrossRef]
- Couillard, D. The use of peat in wastewater treatment. Water Res. 1994, 28, 1261–1274. [Google Scholar] [CrossRef]
- Petroni, S.L.G. Adsorption Studies of Zinc and Cadmium on Peat. Potential Use of a Natural Biosorbent in Wastewater Treatment. Master’s Thesis, Nuclear and Energy Research Institute (IPEN), Associated to the University of São Paulo (USP), São Paulo, Brazil, 1999. [Google Scholar]
- Brown, P.A.; Gill, S.A.; Allen, S.J. Metal removal from wastewater using peat. Water Res. 2000, 34, 3907–3916. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Strömvall, A.M.; Steenari, B.M. Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. J. Hazard. Mater. 2008, 152, 885–891. [Google Scholar] [CrossRef]
- Abat, M.; McLaughlin, M.J.; Kirby, J.K.; Stacey, S.P. Adsorption and desorption of copper and zinc in tropical peat soils of Sarawak, Malaysia. Geoderma 2012, 175–176, 58–63. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, M.E.; Chung, J.W.; Park, J.H.; Huh, K.Y.; Jun, G.I. Immobilization of lead from Pb-contaminated soil amended with peat moss. J. Chem. 2013, 2013, 509520. [Google Scholar] [CrossRef] [Green Version]
- Egene, C.E.; Van Poucke, R.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci. Total Environ. 2018, 626, 195–202. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Zhang, Q.; Li, J.; Zhang, F.; Liu, Z. Effects of peat on plant growth and lead and zinc phytostabilization from lead-zinc mine tailing in southern China: Screening plant species resisting and accumulating metals. Ecotoxicol. Environ. Saf. 2019, 176, 42–49. [Google Scholar] [CrossRef]
- Crescêncio Júnior, F. A Study in Lab of Peats as a Reactive Barrier for Remediation of Aquifers; Federal University of Rio de Janeiro (UFRJ): Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Koivula, M.P.; Kujala, K.; Rönkkömäki, H.; Mäkelä, M. Sorption of Pb(II), Cr(III), Cu(II), As(III) to peat, and utilization of the sorption properties in industrial waste landfill hydraulic barrier layers. J. Hazard. Mater. 2009, 164, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.G.; Pokrovsky, O.S.; Beike, A.K.; Reski, R.; Di Palma, A.; Adamo, P.; Giordano, S.; Angel Fernandez, J. Metal and proton adsorption capacities of natural and cloned Sphagnum mosses. J. Colloid Interface Sci. 2016, 461, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wen, Q.; Hu, L.; Gong, M.; Tang, Z. Feasibility study on the application of coal gangue as landfill liner material. Waste Manag. 2017, 63, 161–171. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Adamcová, D.; Winkler, J.; Koda, E.; Petrželová, L.; Maxianová, A. Alternative method of composting on a reclaimed municipal waste landfill in accordance with the circular economy: Benefits and risks. Sci. Total Environ. 2020, 723, 137971. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Jara-Samaniego, J.; Pérez-Murcia, M.D.; Bustamante, M.A.; Paredes, C.; Pérez-Espinosa, A.; Gavilanes-Terán, I.; López, M.; Marhuenda-Egea, F.C.; Brito, H.; Moral, R. Development of organic fertilizers from food market waste and urban gardening by composting in Ecuador. PLoS ONE 2017, 12, e0181621. [Google Scholar] [CrossRef] [Green Version]
- Kocasoy, G.; Güvener, Z. Efficiency of compost in the removal of heavy metals from the industrial wastewater. EnGeo 2009, 57, 291–296. [Google Scholar] [CrossRef]
- Farrell, M.; Jones, D.L. Use of composts in the remediation of heavy metal contaminated soil. J. Hazard. Mater. 2010, 175, 575–582. [Google Scholar] [CrossRef]
- Farrell, M.; Jones, D.L. Food waste composting: Its use as a peat replacement. Waste Manag. 2010, 30, 1495–1501. [Google Scholar] [CrossRef]
- Farrell, M.; Perkins, W.T.; Hobbs, P.J.; Griffith, G.W.; Jones, D.L. Migration of heavy metals in soil as influenced by compost amendments. Environ. Pollut. 2010, 158, 55–64. [Google Scholar] [CrossRef]
- Paradelo, R.; Barral, M.T. Evaluation of the potential capacity as biosorbents of two MSW composts with different Cu, Pb and Zn concentrations. Bioresour. Technol. 2012, 104, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.Z.; Raimondi, I.M.; Schalch, V.; Rodrigues, V.G.S. Assessment of the use of organic composts derived from municipal solid waste for the adsorption of Pb, Zn and Cd. J. Environ. Manag. 2018, 226, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Abou Jaoude, L.; Garau, G.; Nassif, N.; Darwish, T.; Castaldi, P. Metal(loid)s immobilization in soils of Lebanon using municipal solid waste compost: Microbial and biochemical impact. Appl. Soil Ecol. 2019, 143, 134–143. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Epstein, E. The Science of Composting; CRC Press LLC: Boca Raton, FL, USA, 1997. [Google Scholar]
- Hooda, P.S. Trace Elements in Soils. Trace Elem. Soils 2010. [Google Scholar] [CrossRef]
- Interstate Technology & Regulatory Council (ITRC). Permeable Reactive Barriers: Lessons Learned/New Directions Technical/Regulatory Guidelines; ITRC: Washington, DC, USA, 2005. [Google Scholar]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Semple, K.T.; Megharaj, M.; Juhasz, A.L.; Bolan, N.S.; Gupta, S.K.; Clothier, B.E.; Schulin, R. Chapter 3 Bioavailability: Definition, assessment and implications for risk assessment. Dev. Soil Sci. 2008, 32, 39–51. [Google Scholar] [CrossRef]
- Paustenbach, D.J. The practice of exposure assessment: A state-of-the-art review. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 179–291. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.R. In-Vitro Methods for the Measurement of the Oral Bioaccessibility of Selected Metals and Metalloids in Soils: A Critical Review; Environment Agency: Bristol, UK, 2003.
- National Research Council; Division on Earth and Life Studies; Water Science and Technology Board; Committee on Bioavailability of Contaminants in Soils and Sediments. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Ruby, M.V.; Davis, A.; Link, T.E.; School, R.; Chaney, R.L.; Freeman, G.B.; Bergstrom, P. Development of an in Vitro Screening Test To Evaluate the in Vivo Bioaccessibility of Ingested Mine-Waste Lead. Environ. Sci. Technol 1993, 27, 2870–2877. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Cave, M.; Li, H.B.; Ma, L.Q. Lead bioaccessibility in 12 contaminated soils from China: Correlation to lead relative bioavailability and lead in different fractions. J. Hazard. Mater. 2015, 295, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollson, C.J.; Smith, E.; Scheckel, K.G.; Betts, A.R.; Juhasz, A.L. Assessment of arsenic speciation and bioaccessibility in mine-impacted materials. J. Hazard. Mater. 2016, 313, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, M.; Millward, G.; Henderson, S.; Blake, W. Bioaccessibility of U, Th and Pb in solid wastes and soils from an abandoned uranium mine. J. Environ. Radioact. 2017, 173, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.M.; Cruz, N.; Carvalho, L.; Duarte, A.C.; Pereira, E.; Boim, A.G.F.; Alleoni, L.R.F.; Römkens, P.F.A.M. Evaluation of a single extraction test to estimate the human oral bioaccessibility of potentially toxic elements in soils: Towards more robust risk assessment. Sci. Total Environ. 2018, 635, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Soo Lee, S.; Yang, J.E.; Ro, H.M.; Han Lee, Y.; Sik Ok, Y. Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol. Environ. Saf. 2012, 79, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Udovic, M.; McBride, M.B. Influence of compost addition on lead and arsenic bioavailability in reclaimed orchard soil assessed using Porcellio scaber bioaccumulation test. J. Hazard. Mater. 2012, 205–206, 144–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; McBride, M.B.; Li, K. Bioaccessibility of Ba, Cu, Pb, and Zn in urban garden and orchard soils. Environ. Pollut. 2016, 208, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Brasil. Manual de Métodos Analíticos Oficiais para Fertilizantes e Corretivos; Ministério da Agricultura, Pecuária e Abastecimento (MAPA): Brasília, Brazil, 2017.
- Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA). Manual de Métodos de Análise de Solo, 2nd ed.; EMBRAPA: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Kiehl, E.J. Fertilizantes Orgânicos; Editora Agronômica “Ceres” Ltd.: Piracicaba, Brazil, 1985. [Google Scholar]
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Empresa Brasileira de Pesquisa Agropecuária: Brasília, Brazil, 2009. [Google Scholar]
- Alcarde, J.C. Manual de Análise de Fertilizantes; FEALQ: Piracicaba, Brazil, 2009. [Google Scholar]
- American Society for Testing and Materials (ASTM). D4646: Standard Test Method for 24-h Batch-Type Measurement of Contaminant Sorption by Soils and Sediments; ASTM: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Raimondi, I.M.; Lima, J.Z.; Rodrigues, V.G.S. The Characterization of Tropical Peats for Potentially Toxic Metals Adsorption Purposes in an Abandoned Mine Area. In Proceedings of the IAEG/AEG Annual Meeting Proceedings, San Francisco, CA, USA, 17–21 September 2018; pp. 129–134. [Google Scholar] [CrossRef]
- Lim, S.-F.; Lee, A.Y.W. Kinetic study on removal of heavy metal ions from aqueous solution by using soil. Environ. Sci. Pollut. Res. 2015, 22, 10144–10158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. The sorption of lead(II) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.S.; Wase’, D.; Forster, C.F. Removal of lead ions from aqueous solution using sphagnum moss peat as adsorbent. Water SA 1996. [Google Scholar] [CrossRef]
- Ho, Y.S. Isotherms for the Sorption of Lead Onto Peat: Comparison of Linear and Non-Linear Methods. Polish J. Environ. Stud. 2006, 15, 81–86. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Inyang, H.I.; Onwawoma, A.; Bae, S. The Elovich equation as a predictor of lead and cadmium sorption rates on contaminant barrier minerals. Soil Tillage Res. 2016, 155, 124–132. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, H.J. Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution. J. Environ. Manag. 2018, 209, 382–392. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. Characteristics, performances, equilibrium and kinetic modeling aspects of heavy metal removal using algae. Bioresour. Technol. Rep. 2019, 5, 261–279. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Elovich, S.Y.; Larinov, O.G. Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions. Izv. Akad. Nauk. SSSR Otd. Khim. Nauk. 1962, 2, 209–216. [Google Scholar]
- Mckay, G. The Adsorption of basic dye onto silica from aqueous solution-solid diffusion model. Chem. Eng. Sci. 1984, 39, 129–138. [Google Scholar] [CrossRef]
- Mathews, A.P.; Weber, W. Effects of external mass transfer and intraparticle diffusion on adsorption rates in slurry reactors. AIChE Symp. Ser. 1977, 73, 91–98. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Poggio, L.; Vrščaj, B.; Schulin, R.; Hepperle, E.; Ajmone Marsan, F. Metals pollution and human bioaccessibility of topsoils in Grugliasco (Italy). Environ. Pollut. 2009, 157, 680–689. [Google Scholar] [CrossRef]
- Jorge Mendoza, C.; Tatiana Garrido, R.; Cristian Quilodrán, R.; Matías Segovia, C.; José Parada, A. Evaluation of the bioaccessible gastric and intestinal fractions of heavy metals in contaminated soils by means of a simple bioaccessibility extraction test. Chemosphere 2017, 176, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Valente, B.S.; Xavier, E.G.; Morselli, T.B.G.A.; Jahnke, D.S.; Brum, B.S., Jr.; Cabrera, B.R.; Moraes, P.O.; Lopes, D.C.N. Fatores que afetam o desenvolvimento da compostagem de resíduos orgânicos. Arch. Zootec. 2009, 58, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Instituto de Pesquisas Tecnológicas do Estado de São Paulo (IPT). Estudo das Possibilidades de Aproveitamento de Turfa no Estado de São Paulo. Relatório 12.761; IPT: São Paulo, Brazil, 1979. [Google Scholar]
- Dick, D.P.; Novotny, E.H.; Dieckow, J.; Bayer, C. Química da matéria orgânica no solo. In Química e Mineralogia do Solo; Mello, J.W., Alleoni, L.R., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2009; pp. 1–68. [Google Scholar]
- American Society for Testing and Materials (ASTM). D4427: Standard Classification of Peat Samples by Laboratory Testing; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Sparks, D.L. Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Companhia Ambiental do Estado de São Paulo (CETESB). Decisão de Diretoria 256/2016/E. Relatório de Estabelecimento de Valores Orientadores para Solos e Águas Subterrâneas no Estado de São Paulo; CETESB: São Paulo, Brazil, 2016.
- Venegas, A.; Rigol, A.; Vidal, M. Viability of organic wastes and biochars as amendments for the remediation of heavy metal-contaminated soils. Chemosphere 2015, 119, 190–198. [Google Scholar] [CrossRef]
- Simantiraki, F.; Gidarakos, E. Comparative assessment of compost and zeolite utilisation for the simultaneous removal of BTEX, Cd and Zn from the aqueous phase: Batch and continuous flow study. J. Environ. Manag. 2015, 159, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Guermandi, J.I. Evaluation of Physical, Chemical and Microbiological Parameters of na Organic Fertilizer Produced by Composting and Vermicomposting of Organic Fraction of Municipal Solid Wastes Collected in São Carlos City. Master’s Thesis, São Carlos School of Engineering (EESC), University of São Paulo (USP), São Carlos, Brazil, 2015. [Google Scholar]
- Faverial, J.; Boval, M.; Sierra, J.; Sauvant, D. End-product quality of composts produced under tropical and temperate climates using different raw materials: A meta-analysis. J. Environ. Manag. 2016, 183, 909–916. [Google Scholar] [CrossRef]
- Petroni, S.L.G. Kinetic and Equilibrium Evaluation of the Adsorption Process of Cadmium, Copper and Nickel Metal Ions in Peat. Ph.D. Thesis, Nuclear and Energy Research Institute (IPEN), Associated to the University of São Paulo (USP), São Paulo, Brasil, 2004. [Google Scholar]
- Gündoǧan, R.; Acemioǧlu, B.; Alma, M.H. Copper (II) adsorption from aqueous solution by herbaceous peat. J. Colloid Interface Sci. 2004, 269, 303–309. [Google Scholar] [CrossRef]
- Batista, A.P.S.; Romão, L.P.C.; Arguelho, M.L.P.M.; Garcia, C.A.B.; Alves, J.P.H.; Passos, E.A.; Rosa, A.H. Biosorption of Cr(III) using in natura and chemically treated tropical peats. J. Hazard. Mater. 2009, 163, 517–523. [Google Scholar] [CrossRef]
- Swift, R.S. Organic Matter Characterization. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1011–1068. [Google Scholar]
- Chwastowski, J.; Staroń, P.; Kołoczek, H.; Banach, M. Adsorption of hexavalent chromium from aqueous solutions using Canadian peat and coconut fiber. J. Mol. Liq. 2017, 248, 981–989. [Google Scholar] [CrossRef]
- Soobhany, N.; Gunasee, S.; Rago, Y.P.; Joyram, H.; Raghoo, P.; Mohee, R.; Garg, V.K. Spectroscopic, thermogravimetric and structural characterization analyses for comparing Municipal Solid Waste composts and vermicomposts stability and maturity. Bioresour. Technol. 2017, 236, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.; Guo, X.; Wang, H. Comparison of the effects of different maturity composts on soil nutrient, plant growth and heavy metal mobility in the contaminated soil. J. Environ. Manag. 2019, 250, 109525. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry (IUPAC). Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Franchi, J.G. The Utilization of Peat as Heavy Metal Adsorbent. The Example of the Contamination of Ribeira do Iguape River Catchment by Lead and Associated Minerals. Ph.D. Thesis, University of São Paulo (USP), São Paulo, Brazil, 2004. [Google Scholar]
- Qin, F.; Wen, B.; Shan, X.Q.; Xie, Y.N.; Liu, T.; Zhang, S.Z.; Khan, S.U. Mechanisms of competitive adsorption of Pb, Cu, and Cd on peat. Environ. Pollut. 2006, 144, 669–680. [Google Scholar] [CrossRef]
- Bartczak, P.; Norman, M.; Klapiszewski, Ł.; Karwańska, N.; Kawalec, M.; Baczyńska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2018, 11, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.C.; Forster, C.F. Removal of Hexavalent Chromium Using Sphagnum Moss Peat. Water Res. 1993, 27, 1201–1208. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Modeling multi-metal ion exchange in biosorption. Environ. Sci. Technol. 1996, 30, 2921–2927. [Google Scholar] [CrossRef]
- Ho, Y.S. Adsorption of Heavy Metals from Waste Streams by Peat; School of Chemical Engineering, Faculty of Engineering, University of Birmingham: Birmingham, UK, 1995. [Google Scholar]
- Gosset, T.; Trancart, J.L.; Thévenot, D.R. Batch Metal Removal by Peat—Kinetics and Thermodynamics. Water Res. 1986, 20, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Ong, H.L.; Swanson, V.E. Adsorption of copper by peat, lignite, and bituminous coal. Econ. Geol. 1966, 61, 1214–1231. [Google Scholar] [CrossRef]
- McKay, G.; Porter, J.F. Equilibrium Parameters for the Sorption of Copper, Cadmium and Zinc Ions onto Peat. J. Chem. Tech. Biotechnol. 1997, 69, 309–320. [Google Scholar] [CrossRef]
- Alleoni, L.R.; Mello, J.W.V.; Rocha, W.S.D. Eletroquímica, adsorção e troca iônica no solo. In Química e Mineralogia do Solo; Mello, J.W., Alleoni, L.R., Eds.; Brazilian Soil Science Society: Viçosa, Brazil, 2009; pp. 69–130. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part I: Fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Raimondi, I.M.; Vieira, E.M.; Vaz, L.A.A.; Rodrigues, V.G.S. Comparison of sugarcane pressmud with traditional low-cost materials. Int. J. Environ. Sci. Technol. 2021, 1–18. [Google Scholar] [CrossRef]
- Shah, G.M.; Imran, U.M.; Bakhat, H.F.; Hammad, H.M.; Ahmad, I.; Rabbani, F.; Khan, Z.U.H. Kinetics and equilibrium study of lead bio-sorption from contaminated water by compost and biogas residues. Int. J. Environ. Sci. Technol. 2019, 16, 3839–3850. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Wan, Y. Comparative study of calcium alginate, ball-milled biochar, and their composites on aqueous methylene blue adsorption. Environ. Sci. Pollut. Res. 2019, 26, 11535–11541. [Google Scholar] [CrossRef]
- Rizzuti, A.M.; Newkirk, C.R.; Wilson, K.A.; Cosme, L.W.; Cohen, A.D. Biosorption of hexavalent chromium from aqueous solutions using highly characterised peats. Mires Peat 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Lima, J.Z.; Raimondi, I.M.; Rodrigues, V.G.S. Cd Adsorption by Chemically Activated Tropical Peat. In Proceedings of the Geotechnical Engineering in the XXI Century: Lessons Learned and Future Challenges, Cancun, Mexico, 17–20 November 2019; Lópes-Costa, N.P., Martínez-Hernánde, E., Espinosa-Santioago, A.L., Promotor, J.A., López, A.O., Eds.; IOS Press: Cancun, Mexico, 2019. [Google Scholar]
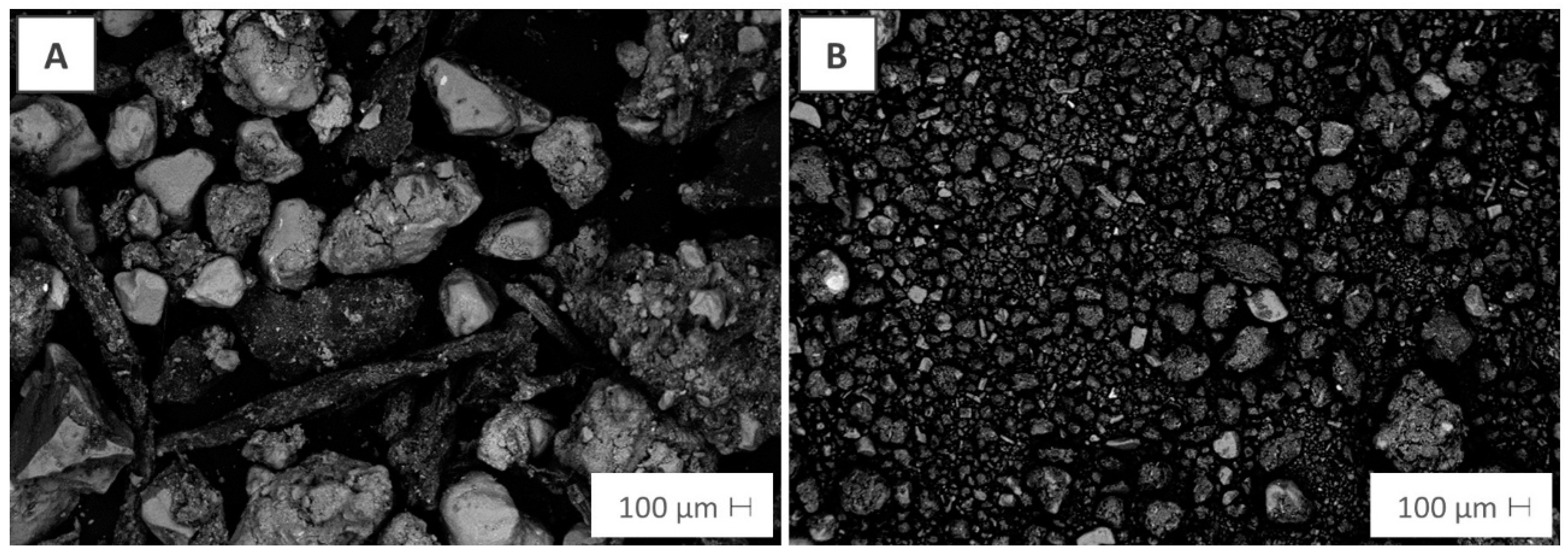
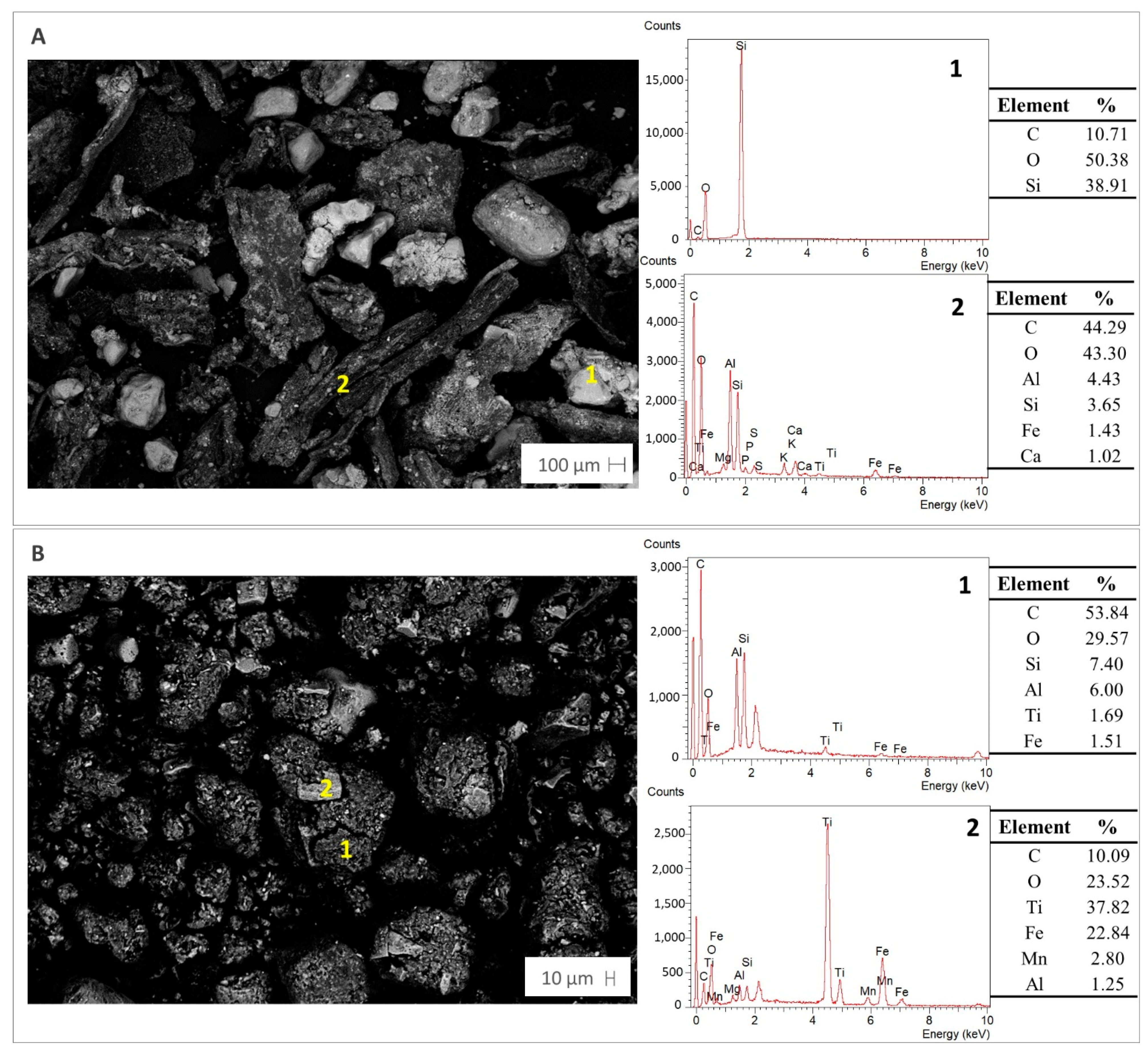
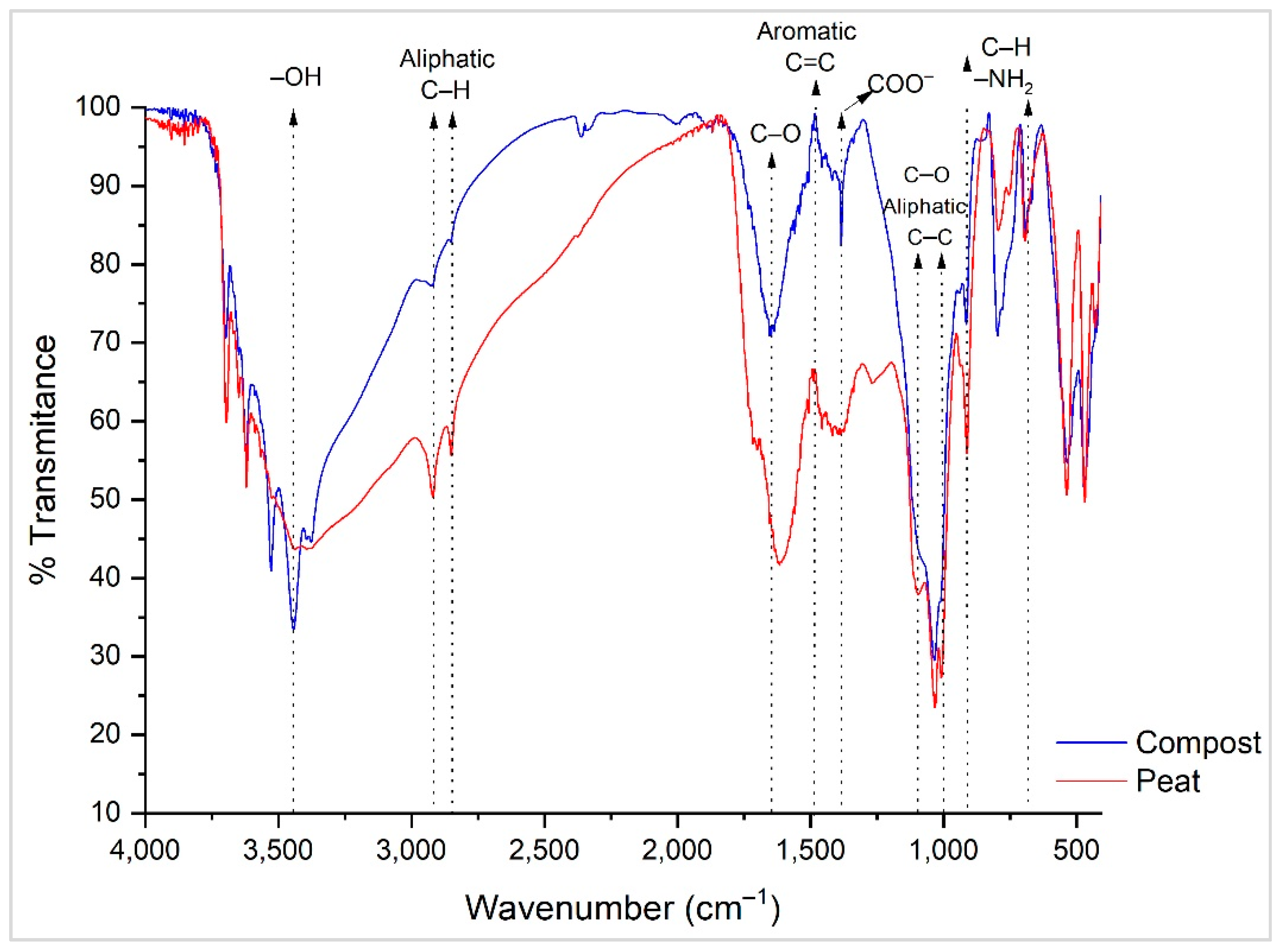




| Parameter | Compost | Peat |
|---|---|---|
| pH: CaCl2 0.01 M | 5.4 | 3.9 |
| pH: H2O | 7.4 | 5.0 |
| Eh (mV) | +268 | +432 |
| EC (µS cm−1) | 665 | 448 |
| Moisture: 65 °C (%) | 36.78 | 7.99 |
| Moisture: 110 °C (%) | 1.52 | 2.29 |
| Total Moisture (%) | 38.30 | 10.28 |
| WRC (%) | 65.26 | 36.28 |
| CEC (mmolc kg−1) | 340.00 b | 910.00 c |
| OM a (g kg−1) | 186.60 | 447.60 |
| IMR a (g kg−1) | 695.30 | 417.60 |
| SMR a (g kg−1) | 94.00 | 110.50 |
| TMR a (g kg−1) | 789.30 | 527.60 |
| C a (%) | 8.32 | 23.36 |
| Na (%) | 0.82 | 1.26 |
| C/N | 10 | 19 |
| P a (g kg−1) | 2.01 b | 0.66 |
| K a (g kg−1) | 1.58 b | 0.58 |
| Ca a (g kg−1) | 28.60 b | 1.80 |
| Mg a (g kg−1) | 1.30 b | 1.10 |
| S a (g kg−1) | 0.30 b | 0.80 |
| Cd a (mg kg−1) | <5 | <5 |
| Pb a (mg kg−1) | 24.50 | <5 |
| Zn a (mg kg−1) | 57.50 | <5 |
| XRF | SiO2 (44.5%); | SiO2 (33.9%); |
| Al2O3 (11.0%); | Al2O3 (12.3%); | |
| Fe2O3 (5.8%); | Fe2O3 (2.2%); | |
| CaO (1.6%) | TiO2 (2.0%) |
| Parameter | Compost | Peat |
|---|---|---|
| SSA (m2 g−1) | ||
| (i) BET | 5.689 | 3.451 |
| (ii) BJH | ||
| (a) Sorption cumulative | 6.636 | 5.828 |
| (b) Desorption cumulative | 9.787 | 9.679 |
| BJH Vp (cm3 g−1) | ||
| (i) BJH sorption | 0.03412 | 0.02682 |
| (ii) BJH desorption | 0.03448 | 0.02783 |
| Rp (Å) | ||
| (i) BJH sorption | 18.93 | 21.16 |
| (ii) BJH desorption | 94.24 | 18.73 |
| Model | Parameters | Compost | Peat | ||||
|---|---|---|---|---|---|---|---|
| Zn | Pb | Cd | Zn | Pb | Cd | ||
| qexp (mg g−1) | 11.278 | 9.392 | 10.527 | 3.061 | 8.177 | 4.466 | |
| Equilibrium time (min) | 120 | 30 | 60 | 210 | 210 | 210 | |
| PFO | qe(1) (mg g−1) | 10.933 | 9.354 | 10.425 | 2.922 | 8.132 | 4.479 |
| k1 (min−1) | 0.057 | 3.123 | 0.108 | 0.061 | 0.022 | 0.012 | |
| R2 | 0.970 | 0.998 | 0.981 | 0.853 | 0.931 | 0.923 | |
| SSE | 6.177 | 0.194 | 3.409 | 2.468 | 8.820 | 2.918 | |
| PSO | qe(2) (mg g−1) | 11.660 | 9.392 | 10.655 | 3.135 | 9.101 | 4.941 |
| k2 (mg g−1 min−1) | 0.008 | 0.383 | 0.029 | 0.029 | 0.003 | 0.004 | |
| R2 | 0.991 | 0.999 | 0.987 | 0.877 | 0.958 | 0.935 | |
| SSE | 1.793 | 0.087 | 2.223 | 2.037 | 4.997 | 2.203 | |
| Elovich | α (mg g−1 min−1) | 21.669 | 1600.052 | 212.278 | 400.801 | 2.239 | 0.391 |
| β (mg g−1) | 0.751 | 1.366 | 1.020 | 4.448 | 0.789 | 1.248 | |
| R2 | 0.961 | 0.921 | 0.945 | 0.961 | 0.949 | 0.918 | |
| SSE | 7.411 | 9.064 | 8.315 | 0.392 | 5.567 | 2.564 | |
| M&W | kM&W S (cm min−1 cm−1) | 0.038 | 3.041 | 0.070 | 0.001 | 0.012 | 0.001 |
| R2 | 0.938 | 0.994 | 0.926 | 0.544 | 0.913 | 0.655 | |
| SSE | 16.907 | 0.698 | 18.888 | 46.698 | 14.921 | 48.066 | |
| W&M | kW&M (mg g−1 min−0.5) | 0.554 | 0.447 | 0.529 | 0.151 | 0.396 | 0.198 |
| R2 | 0.620 | 0.556 | 0.578 | 0.632 | 0.704 | 0.726 | |
| SSE | 266.014 | 282.744 | 307.417 | 19.379 | 89.302 | 19.622 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, J.Z.; Lupion, R.M.; Raimondi, I.M.; Pejon, O.J.; Rodrigues, V.G.S. Sorption Efficiency of Potentially Toxic Elements onto Low-Cost Materials: Peat and Compost. Sustainability 2021, 13, 12847. https://doi.org/10.3390/su132212847
Lima JZ, Lupion RM, Raimondi IM, Pejon OJ, Rodrigues VGS. Sorption Efficiency of Potentially Toxic Elements onto Low-Cost Materials: Peat and Compost. Sustainability. 2021; 13(22):12847. https://doi.org/10.3390/su132212847
Chicago/Turabian StyleLima, Jacqueline Zanin, Renan Marques Lupion, Isabela Monici Raimondi, Osni José Pejon, and Valéria Guimarães Silvestre Rodrigues. 2021. "Sorption Efficiency of Potentially Toxic Elements onto Low-Cost Materials: Peat and Compost" Sustainability 13, no. 22: 12847. https://doi.org/10.3390/su132212847
APA StyleLima, J. Z., Lupion, R. M., Raimondi, I. M., Pejon, O. J., & Rodrigues, V. G. S. (2021). Sorption Efficiency of Potentially Toxic Elements onto Low-Cost Materials: Peat and Compost. Sustainability, 13(22), 12847. https://doi.org/10.3390/su132212847








