Abstract
Toxicity of heavy-metals in soil is a major constraint for the production of carrots (Daucus carota L.). Different plant growth regulators are being used to overcome this problem. It has been found that plant growth regulators induce stress tolerance in plants. In this study, the role of exogenously applied plant growth regulator, gibberellic acid (GA3) was examined in soil grown two carrot cultivars under four different levels of lead (0, 50, 100, and 150 mg/kg) with one level of gibberellic acid (50 ppm). Results showed that Pb stress retarded the plant growth and reduced chlorophyll contents in the leaves of both carrot cultivars. A significant decrease was observed in photosynthetic attributes by Pb addition alone. However, exogenously applied GA3 ameliorated the plant growth and chlorophyll contents in the leaves of both carrot cultivars under Pb stressed conditions. Moreover, GA3 also decreased the uptake of Pb concentration in carrot leaves and roots. In addition, GA3 significantly regulated the phenolic compounds concentration in both carrot cultivars under Pb stress. In this study, cultivar T-29 was found to be more tolerant to Pb stress, however, cultivar Mevarick experienced higher damage regarding plant growth under Pb stress.
1. Introduction
Excessive use and accumulation of toxic and dangerous industrial materials destroy our soil, air and water. Among these hazards and toxic materials, different types of heavy metals which can be found everywhere in nature, cause critical harmful impacts on living beings [1]. In the environment, heavy metal contamination is causing annoying situations because their presence is harmful to savage life, humans, plants and mostly vegetables [2]. Heavy metals enter into different parts of the plant (via foliar adsorption, specific elements deposition in leaves and root uptake), and therefore, contamination of these metals can alter the plant structure [3].
According to the survey report of the Environment Protection Agency, lead (Pb) is found as the most commonly available heavy metal which disturbs the environmental system [4]. It can be found everywhere in the soil due to its anthropogenic and natural behavior; due to its high toxicity, it creates a danger zone for plants, animals and humans [5]. Moreover, different sources of Pb also cut down world widely (i.e., Pb alkyl in gasoline), but in many countries, the emission of Pb cannot be stopped [6]. In China, cadmium (Cd) and Pb concentration in vegetables were observed as 0.003–0.624 mg/kg and 0.003–0.195 mg/kg fresh weight respectively, and concentration of Cd and Pb is found at a maximum that crosses the approved value [7].
For plant growth, essential elements are required, uptake of a large amount of heavy metal in tissues of the plant and its organs does not positively control vegetation and reproductive parts of the plant [8,9]. The toxicity of heavy metals severely affects the efficiency of the plant and retarded the growth and production of plants by damaging their antioxidant system. The toxicity of heavy metal always depends upon the dose and varies from one species to another species [10,11]. However, in all other aspects regarding plant growth, development and yield decreased down due to the high accumulation of toxic metals in the plant tissues and ultimately it disturbs the plant efficiency and many other essential processes [12].
For the reduction of heavy metal contamination and lessen the fruit quality as well as fruit safety risk by preventing the uptake of heavy metal and transportation into different edible parts of plants we can adopt traditional strategies. In traditional methods, a variety of thermal, physical, and chemical treatments are being used to overcome the toxicity of heavy metals in soil [13]. However, these methods are much too costly, therefore, most recent trend in agricultural sciences is to promote and regulate the plant growth by using plant growth regulators. Plant growth regulators (PGRs) are those compounds that promote growth of plants and enhance stress tolerance in plants under different stress conditions [14,15,16]. It has been observed that different types of plant growth regulators like gibberellic acid, jasmonic acid and indole acitic acid enhance the abiotic stress in plants [15,17]. Among different PGRs, gibberellic acid (GA3) acts as a hormones stimulator, promotes various physiological processes and biochemical in plants [18]. GA3 application on plants of carrot at different stages of growth promotes the leaf growth and inhibits the growth of the root. Exogenous application of GA3 causes divergence of plant bolting by instituting a short thickened condensed stem but infrequently started to flower [19].
Carrot (Daucus carrota L.) contains many important minerals, polyacetylene, carotenoids content and vitamins showing that it is a healthy and nutritious crop. It is eaten worldwide and as ranking among vegetables it is among top ten. It contains a lot of beta-carotene and also has a good amount of phosphorus, iron, vitamin B, calcium and folic acid [20]. It is commonly eaten as salad and also has a lot of other uses in cuisine. Carrot production is much higher as compared to other vegetables in the world due to its use both in raw and cooked forms. It is also acknowledged as great worth and consumed to a large extent [20]. Keeping in view the importance of carrots in human life, the present study was carried out to understand the effects of different levels of Pb and GA3 on morpho-physiological and biochemical changes in two cultivars of carrot under soil conditions.
2. Materials and Methods
2.1. Plant Material and Treatment
This study was conducted at the Institute of Horticultural Sciences, University of Agriculture Faisalabad, Pakistan. Two carrot (Daucus carota L.) cultivars “V1 (T-29)” and “V2 (Maverick)” were used in this study. These cultivars are the local cultivars used in Pakistan. However, there is no study published yet which confirms the resistance of these cultivars against abiotic conditions. Therefore, the present study was carried out to evaluate their resistance under lead (Pb) stress, and to check the role of gibberellic acid (GA3)—how it can increase plant growth under Pb stress. Four levels of Pb (0, 50, 100 and 150 mg/kg soil) were selected. Lead chloride chemical was used for the source of Pb and spiked in the soil one month before starting the experiment. For each treatment, four replications were maintained. Pb was spiked in the soil (loamy soil) one month before starting the experiment. A total of 48 pots were filled with amended soil and each pot diameter was 59 cm and height 38 cm. Each pot was filled with 6 kg amended soil. Selected seeds of carrot were sterilized by dipping in ethanol (70%) for 1 min followed by sodium hypochlorite for 5 min and finally rinsed with distilled water five times and directly sown in the pot. Three carrot plants were grown in each pot. After 21 days of seed germination, all the seedlings were sprayed with GA3 (50 ppm). The foliar spray was applied from 9:00 to 10:00 a.m. The plants from control plots were sprayed with distilled water. The required amount of GA3 was prepared in 95% ethanol (C2H5OH) and distilled water. Previously, GA3 at 50 ppm concentration has been shown to increase the growth of the plant in carrot plants [21]. A total of 200 mL solution of GA3 was applied to each pot. During GA3 application, other plants were covered with a polyethene bag. When plant length reached up to maximum height (40–45 days after seed germination), morphological parameters were measured and samples for biochemical analysis were collected and preserved at −80 °C.
2.2. Morphological Observations
After harvesting, root length was measured in centimeters (cm) by using a measuring scale. Roots diameter were measured by using a digital instrument Vernier caliper, at the base of the stem, average width or diameter measured in millimeters (mm). Plant height was measured in cm by using measuring tape. The carrots’ fresh weight was measured by digital balance, plants were selected from each treatment and their average weight computed in grams (g). The weight of the carrot plant in treatment was computed with the assistance of digital balance. When plants attained maximum height and their age was about 42–45 days after seed germination plants were harvested for fresh weight determination. For dry weight, plants were placed under sunlight for 24 h, after that plants were labeled and transferred into a drying oven (DHG-9202 53 L Series Thermal Electrical Thermostate drying oven) at 65 °C for 72 h. Freshly harvested carrots were chosen from each treatment, and the color of the root was recorded and noted according to naked eye vision for both carrot cultivars, i.e., pale red (1), red (2), and deep red (3) for red cultivar T-29 while pale orange (1), orange (2), and deep orange (3) for orange cultivar Maverick. Hardness was checked by cutting, i.e., soft (1) and hard (2). Sweetness sweet (1), less sweet (2) and bitterness yes (1) and no (2) and crunchiness yes (1), no (2) and fewer crunchiness (3) checked by eating and drinking the juice of random carrots (Table 1).

Table 1.
Effects of different treatments of gibberellic acid (50 ppm) and lead (50, 100 mg/kg soil) on morphological traits (hardness, sweetness, bitterness and crunchiness) of carrot cvs. “V1 (T-29)” and “V2 (Maverick)”.
2.3. Total Soluble Solids and pH
Total soluble solids consider a very important quality perimeter carrot plant. Full mature roots of the plant were taken and extracted juice from it, after that double-layered fine cheese mesh cloth was used to remove debris. A drop from carrot pure extract taken from variety 1 plants and variety 2 plants was placed on the prism, closed the lid of refract-o-meter and the value in (°Brix) recorded directly from the screen of the instrument at 20–25 °C room temperature. After harvesting juice from mature roots was extracted and measured the pH by using WTW Inolab at room temperature.
2.4. Heavy Metal Concentration in Carrots
For the estimation of Pb in plant roots samples were kept in an oven for 24 h at 65 °C, then at 550 °C in the muffle furnace for 20 h to prepare ash. For the incubation, ash was incubated at 70 °C for 2 h with 31% HNO3 and 17.5% H2O2, after that the ash was dissolved in distilled water. Atomic absorption spectrophotometer (Avanta Sigma®,Bodo, Norway) was used for the determination of the Pb concentration [22].
2.5. Chlorophyll Contents Determination
A chlorophyll meter (Opti Sciences®, Oklahoma City, OK, USA) was used to measure the chlorophyll contents of carrot leaves. Fully matured and much expanded leaves were selected which provide non-destructive and accurate data for the determination of leaf chlorophyll content. For this instance, 20 readings were taken down from randomly selected plants for the estimation of chlorophyll content.
2.6. Estimation of Antioxidants (TPC, TFC, DPPH)
The Folin–Ciocalteu method was used for measuring the TPC, total phenolic contents were analyzed at 750 nm [23]. For the estimation of total phenolic contents (TPC), plant samples (200 mL), 1 mL Folin–Ciocalteu reagent, water that contains anhydrous Na2CO3 (75 g/L) were taken up to 800 mL and mixed them gently. After that, absorption was taken at 750 nm after a reaction time of 2 h.
Brand-Williams et al. [24] method was followed for the determination of DPPH activity. Seed sample (1 g) was taken down mash properly and added to 10 mL of ethanol and homogenized the mixture of crude extract was mixed with 3.9 mL methanol and 1 mL of DPPH solution in a test tube and estimate the absorbance at 517 nm after the incubation of 30 min. By using the following formula, the content of DPPH was calculated.
DPPH radical activity = [A517 (blank) − A517 (sample) ÷ A517 (blank)] × 100%
According to Chatatikun et al. [25], total flavonoid content was calculated by following chloride colorimetric method. By using the concentration of about 80, 90, 100 μg/mL, a standard solution of quercetin was prepared in 96% ethanol. A 50 μL of each extract (1 mg/mL), 10 μL of 10% of AlCl2 and 150 μL of ethanol (96%) was mixed. Successively, 10 μL of 1 M sodium acetate (C2H3NaO2) was added to the mixture in a 96 well plate. Consequently, ethanol (96%) was used to be as a reagent blank. All reagents were mixed and incubated for 40 min at room temperature, especially in dark. The absorbance level was recorded at 415 nm with a UV spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
2.7. Total Soluble Proteins
For the total proteins, carrot root samples (100 g) were collected and washed with distilled water and chopped properly after that in the blander by using 50 mL distilled water. Then homogenized the mixture and stored in the refrigerator at −20 °C to −80 °C. The freeze-dried sample was taken down to about 0.1 g and 2 mL distilled water was added and vortexed for about 5 min at room temperature. After that falcon tubes were centrifuged (Scilogex®, Rocky Hill, CT, USA) at 5000 rpm for 20 min and the supernatant was collected and then stored at 4 °C for 2 h. Sample of 0.5 mL extracted protein was diluted by 1:3 distilled water and further diluted in 0.5 mL of lowery reagent and again vortex at room temperature. After that, samples were placed in a water bath at 37 °C for 10 min, then incubated by adding 1.5 mL of Folin/Ciocalteu (F/C) reagent at 52 °C for 20 min. Absorbance was measured at 680 nm by UV spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
2.8. Statistical Analysis
By using Statistics 8.1 software, data were analyzed concerning two-way factorial method under complete randomized design (CRD). Multiple pair-wise comparison was done by using Tukey’s test for the better description of mean values with 95% (p ≤ 0.05) confidence interval.
3. Results
3.1. Effect of Gibberellic Acid on Carrot Growth under Pb Stress
Effect of various levels of lead (Pb) and gibberellic acid (GA3) on root length (cm), root diameter (cm), plant height (cm), yield per pot (kg) and plant fresh weight (g) are shown in Table 2. The Pb exposure to plants clearly decreased root length, root diameter, plant height, yield per pot and plant fresh weight as compared to other treatments. However, this decrease was more prominent in plants where Cr was applied as 100 to 150 mg/kg Pb as compared to other treatments. Exogenously application of GA3 significantly enhanced fresh weight under Pb stress conditions. Treatment of GA3 alone had a significant result on root length, but under Pb stress its amelioration effect was greater at the 100 mg/kg Pb as compared to other treatments. Varietal compression presented that among both cultivars, T-29 had maximum root length (Table 2). In the case of root diameter, foliar application of GA3 significantly enhanced root diameter in both cultivars. Control treatment showed maximum diameter in T-29 verity, and GA3 treatment increased root diameter in Maverick (Table 2). Under the application of GA3 under 150 mg/kg Pb cultivar T-29 showed maximum plant height. Pb stress only with various amounts decreased the plant height of both cultivars; however, more reduction occurred in Maverick at 50 mg/kg Pb (Table 2). Application of Pb significantly enhanced fresh weights in plants and maximum weight at 50 mg/kg Pb in T-29 as compared to other treatments (Table 2).

Table 2.
Effects of different treatments of gibberellic acid (50 ppm) and lead (50, 100 mg/kg soil) on root length (cm), root diameter (cm), plant height (cm) and plant fresh weight (g) of two carrot cultivars “V1 (T-29)” and “V2 (Maverick)”.
3.2. Total Soluble Solids and pH
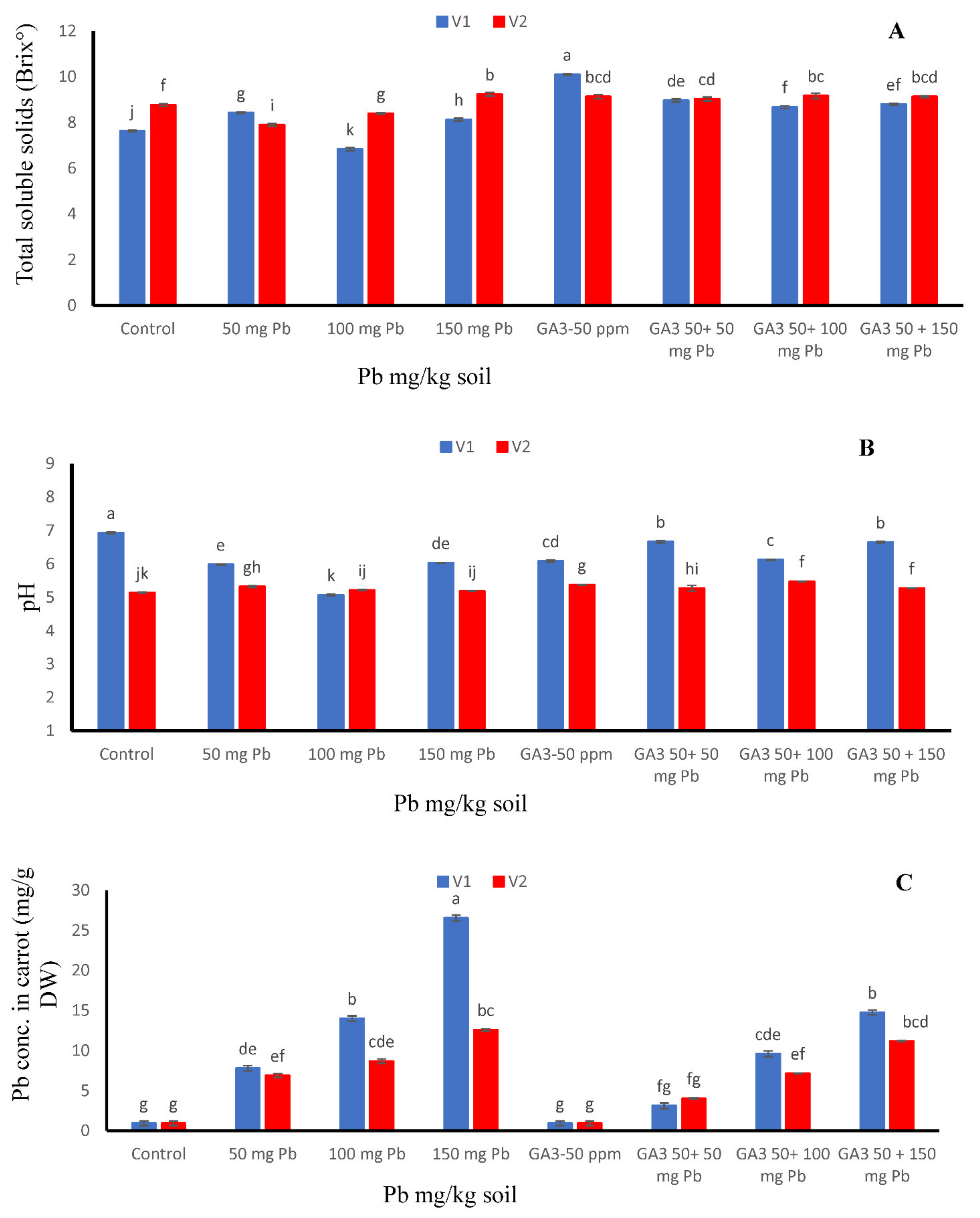
The effects of various amounts of Pb and GA3 on TSS and pH aspects are given in Figure 1. Lead addition alone significantly reduced TSS content (6.833 °Brix) in T-29 cultivar when compared to other treatments at 100 mg/kg Pb (Figure 1A). Foliar application of GA3 alone significantly improved the TSS content (10 °Brix) in T-29 as compared to other treatments at 50 ppm GA3. The higher values of pH were detected at control (6.93) but the application of 100 mg/kg Pb lowest pH value (5.07) in cultivar T-29 as compared to other treatments (Figure 1B).
Figure 1.
Effects of different treatments of gibberellic acid (50 ppm) and lead (0, 50, 100, 150 mg/kg) on (A) total soluble solids, (B) pH and (C) Pb concentration in two carrot cultivars “V1 (T-29)” and “V2 (Maverick)”. Each data values are represented as means and ±SD of four replications. Different lower case letters are representing the significant difference between treatments and the same lower case letters represent no significant difference according to Tukey’s test (p ≤ 0.05).
3.3. Pb Concentration in Carrot Seedlings
As lead (heavy metal) is generally absorbed by edible parts (roots) of carrots, Pb meaningful addition was found in carrot roots of both cultivars (Figure 1C). We evaluated Pb accumulation in roots of both cultivars and determined the effects of exogenous application of GA3 on Pb uptake in roots. After exogenous application of GA3 significantly decreased the Pb content (3.15 mg/kg) in root at 50 mg/kg Pb + 50 ppm GA3 (Figure 1C) as compared to control and other treatments. Pb uptake was maximum at 150 mg/kg Pb level in cultivar T-29 (Figure 1C).
3.4. Chlorophyll Contents
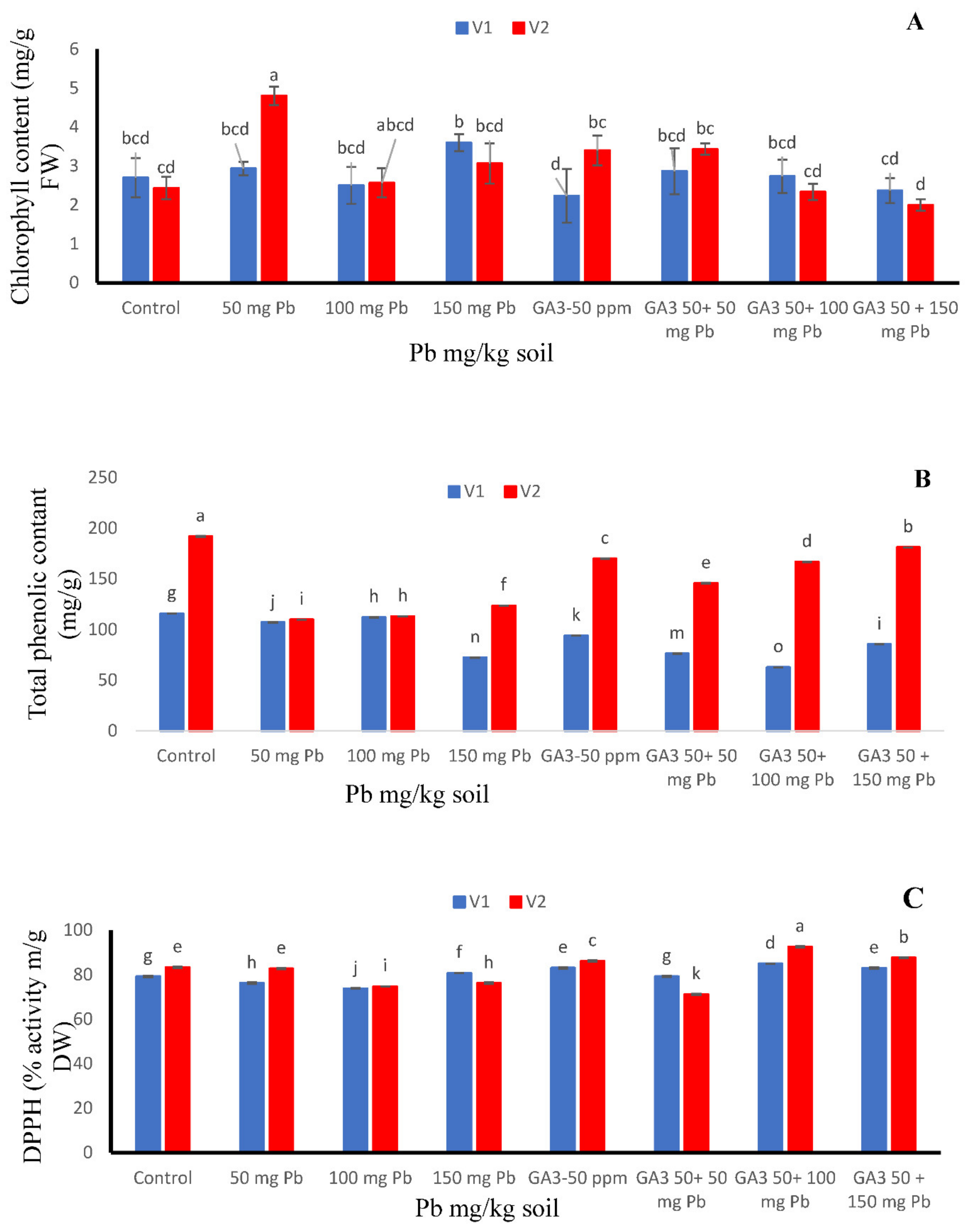
The effects of various amounts of Pb and GA3 on chlorophyll content are presented in Figure 2. Pb addition alone significantly increased the chlorophyll concentration in the Maverick cultivar when compare to both cultivars (Figure 2A). Foliar application of GA3 alone and combined GA3 50 + 50 mg/kg Pb significantly improved leaf chlorophyll content in Maverick as compared to where the combined application was done. The higher values were detected at 50 mg/kg Pb with exogenous application of GA3 in Maverick cultivar as compared to T-29 (Figure 2A).
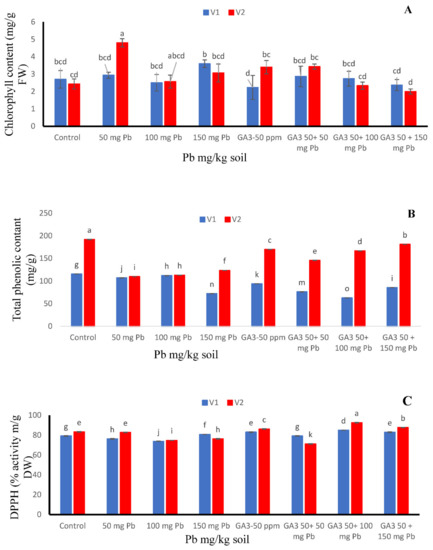
Figure 2.
Effects of different treatments of gibberellic acid (50 ppm) and lead (0, 50, 100, 150 mg/kg) on (A) chlorophyll content, (B) total phenolic contents and (C) DPPH activity in two carrot cultivars “V1 (T-29)” and “V2 (Maverick)”. Each data values are represented as means and ±SD of four replications. Different lower case letters are representing the significant difference between treatments and the same lower case letters represent no significant difference according to Tukey’s test (p ≤ 0.05).
3.5. Total Phenolic Contents
When no stress nor growth regulator was applied (control), both cultivars offered maximum phenolic contents. However, cv. T-29 displayed higher TPC value succeeding to 115.85 mg/g followed by cv. Maverick. Further, T-29 showed better results at Pb 100 mg/kg level while Maverick showed effective results at GA3 50 ppm + Pb 150 mg/kg (Figure 2B).
3.6. DPPH Radical Scavenging Activity
Among both cultivars, Pb and GA3 interacted substantially either applied singly or in combination regarding their DPPH activity (Figure 2C). In Maverick, DPPH activity showed better response under GA3 50 ppm + Pb 100 mg/kg level which represented maximum value as 92.531 μg/mL. However, cv. T-29 showed advanced outcomes at GA3 50 ppm + Pb 100 mg/kg succeeding to 84.948 μg/mL (Figure 2C).
3.7. Total Flavonoid Contents
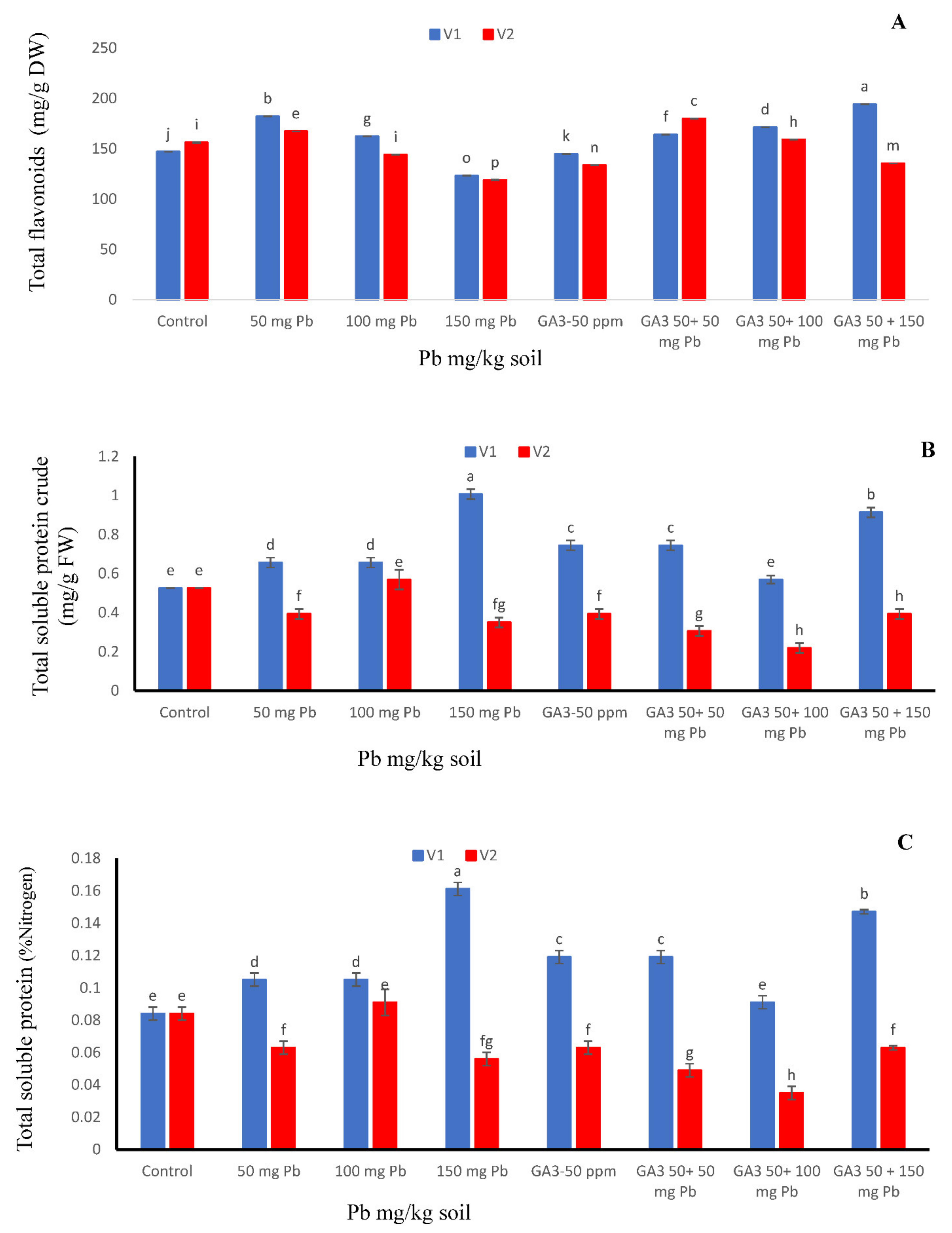
Total flavonoid results are remarkably significant, not only for analysis but also for their interaction. Maximum flavonoid contents were detected in T-29 when the growth regulator was applied under Pb 150 mg/kg level. However, in Maverick, maximum TFC activity was exhibited at GA3 + Pb 50 mg/kg. Increasing concentration of Pb in solution gradually decreased TFC contents, however, combined application showed increasing order with respect to Pb stress. Contrary, both cultivars exhibited minimum flavonoid contents when the Pb stress level was at its peak (Figure 3A).
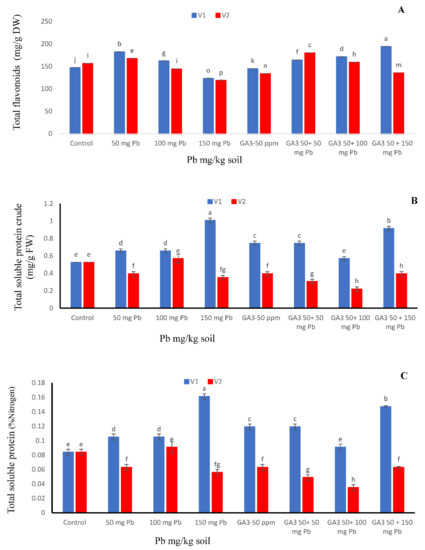
Figure 3.
Effects of different treatments of gibberellic acid (50 ppm) and lead (0, 50, 100, 150 mg/kg) on (A) total flavonoid content, (B) total soluble protein crude, (C) total soluble protein (nitrogen %) and in two carrot cultivars “V1 (T-29)” and “V2 (Maverick)”. Each data values are represented as means and ±SD of four replications. Different lower case letters are representing the significant difference between treatments and the same lower case letters represent no significant difference according to Tukey’s test (p ≤ 0.05).
3.8. Crude Protein Percentage
Protein contents of carrot cultivars “T-29” and “Maverick” was significantly affected by exogenous application of heavy metal, gibberellic acid, and their combinations (Figure 3B). Maximum crude protein contents were observed in cv. T-29 at Pb 150 mg/kg level; whereas, in Maverick, maximum crude protein contents were observed in Pb 100 mg/kg treated plants (Figure 3B). Though, both cultivars assessed minimum crude protein contents (0.53%) in control conditions along with GA3 + Pb 100 mg/kg (Figure 3B).
3.9. Nitrogen Contents (%)
Calculated data particularized the consequence of heavy metal, enlargement enhancer and their combinations on nitrogen content. Maximum nitrogen contents (0.17%) were found in T-29 carrot cultivar that were entirely treated with Pb 150 mg/kg followed by GA3 50 ppm + Pb 150 mg/kg. In contrast, both cultivars showed minimum nitrogen contents (0.08%) when neither GA3 was applied nor Pb stress (Figure 3C).
4. Discussion
Crop yield is majorly affected by the excessive accumulation of heavy metals in soil. This accretion ultimately traps root endodermis by providing a natural barrier for heavy metals. So that, metals successively approach plant upper parts by disrupting certain plant mechanisms [26]. As carrot root is an edible part, so it is badly affected by these poisonous heavy metals. To overcome this disruptive effect, certain growth regulators are being used. These regulators respond differently depending upon crop physiology and morphological characters [27]. Findings are to be compared with the similar study of Santos [28] who concluded that exogenously applied Pb and GA3 give better results concerning plant root length. As heavy metal’s entry into plant parts may disturb many physiological processes therefore the presented results declared that Pb suppressed the plant growth and development. This might be possible that the concentration of macro and micronutrients varied due to Pb contamination in soil. Ultimately, the plant had to face the absence or non-existence of crucial nutrients [29]. Calculated outcomes are surely analogous to the findings that Pb had led to the increment of biomass index as well as the fresh weight of the eggplant seedlings, but this increase severely affected plant growth due to toxicity of heavy metal’s effect [30]. Generally, leaves are assumed to be the crucial sites for photosynthetic reaction resulting in the enhancement of fruit size and crop yield [31]. These findings are in line with the study of Hadi [32] who concluded that plant length and root length was reduced under Pb stress and GA3 significantly enhance plant growth. Results of the present study are just to be contradicted by the conclusions of Lima et al. [33] because plant exhibited more weight under Pb stress but up to a limiting extent. As well as stress level increased, plant slowed down its growth rate. Additionally, high yield under low levels of Pb could be ascribed to its positive role in enhancing biomass along with fresh weight [30]. Quality of tomato can be assessed by using TSS that articulated its nutritional value [34]. Different ratios of TSS content were reported in different tomato studies ranging from 3.67% to 5.01% [35]. Even though it has been reported that various carrot cultivars conquer different TSS content, pH of soil controls numerous parts of toxin and biological processes, mainly included dissolution, complexation, precipitation and acid base reactions of different species of metal and microbial activity [36].
The present findings relating to chlorophyll content offered that maximum chlorophyll content was observed under Pb stress but with its enhancing level, reverse phenomena occurred. Besides this, the dominant effect of Pb decreased the chlorophyll content concerning the outcome of Ali and Al-Qahtani [37] and Gajewska et al. [38]; it was possibly investigated by the intense collaboration of heavy metal to SH group of enzymes of chlorophyll biosynthesis along with lipid peroxidation degradation [39]. Another study concluded that extrinsically applied plant hormones could develop heavy metal lethal resistance [40,41].
Interestingly, gibberellins are more important to lessen the toxic effects of all negative interactions [42,43,44]. Lower Pb stress level could enhance chlorophyll content that could be credited to its affirmative role in developing biomass index but conversely [45]. Certain outcomes were stated relating to B. napus where chlorophyll content was harmfully lowered under Pb accumulation [29,46]. Whereas, chlorophyll disintegration is correspondingly convoyed by the inhibitory restriction of Mg uptake as a result of which Pb substitutes other minerals like Mg [47]. It is concluded that plants conquer higher levels of TPC activity under Pb stress conditions [48]. Whereas phenolics, principally total phenols and flavonoids can be oxidized by peroxidase activity subsequently acting against hydrogen peroxide scavenging, phenolic/ASC/POX systems in contrast to heavy metal detoxification [49]. Heavy metals disturb the structural form of plasma membrane along with protein fraction by assisting their incursion into cells [50]. On the other hand, major enzymes of chlorophyll biosynthesis and α-amino laevulinate dehydrogenase monitor protein regeneration but toxic effect of heavy metal accumulation overturn it [50]. This lethal accumulation also condenses many enzymatic activities belonging to light and dark reactions [51]. In general, plants consume Pb by their roots from the soil and later on the largest fraction of Pb is racked up within roots in an insoluble form [4]. Therefore, a wide of corporal and biochemical malfunctioning may occur concerning plant growth and nutrient uptake [52,53,54].
5. Conclusions
In conclusion, the findings of the present study described that exogenously applied GA3 significantly lowered the Pb-toxicity in both carrot cultivars by decreasing interior Pb concentration in plants. Our findings showed an adverse effect of Pb overcome by foliar application on carrot cultivars by increasing plant growth and chlorophyll contents and stimulating the antioxidant activities and phenolic compounds. Subsequently, T-29 cultivar was presumed to be the best Pb-tolerant cultivar. To study the GA3 ameliorative effects with Pb stressful conditions, a controlled environmental conditions-based study is required. Hence, more studies are required in order to see the GA3 role and its mechanism towards various plant species under Pb stress.
Author Contributions
Data curation, A.A.; Formal analysis, B.A., R.A., A.N. and S.B.; Funding acquisition, M.A.G., M.M.A., B.A., R.A., R.W.K.Q., A.N. and M.A.; Investigation, R.W.K.Q., S.B. and A.A.; Methodology, M.A.G., M.M.A., R.W.K.Q., A.N., S.B. and M.H.A.; Project administration, M.M.A., B.A., A.N., M.A., M.H.S. and A.A.; Resources, M.A.G. and B.A.; Software, R.W.K.Q., S.B., M.H.S. and S.A.; Supervision, S.A.; Visualization, M.H.A. and A.A.; Writing—original draft, R.A., M.A., M.H.S., M.H.A. and S.A.; Writing—review and editing, M.H.S., M.H.A. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to express their deepest gratitude to the University of Tabuk, for the technical and financial support for this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful to Higher Education Commission, Pakistan (HEC) for the facilities and encouragement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Tariq, F.; Wang, X.; Saleem, M.H.; Khan, Z.I.; Ahmad, K.; Saleem Malik, I.; Munir, M.; Mahpara, S.; Mehmood, N.; Ahmad, T.; et al. Risk Assessment of heavy metals in basmati rice: Implications for public health. Sustainability 2021, 13, 8513. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Noor, I.; El-Esawi, M.A.; Hayat, K.; Rizwan, M.; Abbas, Z.; El-Sheikh, M.A.; Alyemeni, M.N. Iron–lysine mediated alleviation of chromium toxicity in spinach (Spinacia oleracea L.) plants in relation to morpho-physiological traits and iron uptake when irrigated with tannery wastewater. Sustainability 2020, 12, 6690. [Google Scholar] [CrossRef]
- Wierzbicka, M.H.; Przedpeska, E.; Ruzik, R.; Ouerdane, L.; Poeć-Pawlak, K.; Jarosz, M.; Szpunar, J.; Szakiel, A. Comparison of the toxicity and distribution of cadmium and lead in plant cells. Protoplasma 2007, 231, 99–111. [Google Scholar] [CrossRef]
- Lăcătuşu, R.; Lăcătuşu, A.R. Vegetable and fruits quality within heavy metals polluted areas in Romania. Carpathian J. Earth Environ. Sci. 2008, 3, 115–129. [Google Scholar]
- Adriano, D.C. Arsenic. In Trace Elements in Terrestrial Environments; Springer: New York, NY, USA, 2001; pp. 219–261. [Google Scholar]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M. Determination of heavy metals in fish and vegetables in Bangladesh and health implications. Human Ecol. Risk Assess. 2015, 21, 986–1006. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef]
- Tian, T.; Ali, B.; Qin, Y.; Malik, Z.; Gill, R.A.; Ali, S.; Zhou, W. Alleviation of lead toxicity by 5-aminolevulinic acid is related to elevated growth, photosynthesis, and suppressed ultrastructural damages in oilseed rape. BioMed. Res. Int. 2014, 2014, 530642. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Zang, L.; Ali, B.; Farooq, M.A.; Cui, P.; Yang, S.; Zhou, W.J. Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 2015, 120, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Qu, C.; Chen, Z.; Hu, C. Antioxidant enzyme systems and the ascorbate-glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 2015, 138, 526–536. [Google Scholar] [CrossRef]
- Liu, J.; Qian, M.; Cai, G.; Yang, J.; Zhu, Q. Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J. Hazard. Mater. 2007, 143, 443–447. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Q.; Wang, M.; Gao, X.; Chen, J.; Shen, C. Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotoxicol. Environ. Saf. 2020, 190, 110090. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.; Xia, X.; Li, X.; Shi, K.; Yu, J.; Zhou, Y. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 2014, 16, 462–473. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Pogrzeba, M.; Rusinowski, S.; Krzyżak, J.; Jia, G. Exogenous jasmonic acid decreased Cu accumulation by alfalfa and improved its photosynthetic pigments and antioxidant system. Ecotoxicol. Environ. Saf. 2020, 190, 110176. [Google Scholar] [CrossRef] [PubMed]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 20, 838. [Google Scholar] [CrossRef]
- Jung, H.; Jo, S.H.; Jung, W.Y.; Park, H.J.; Lee, A.; Moon, J.S.; Seong, S.Y.; Kim, J.K.; Kim, Y.S.; Cho, H.S. Gibberellin Promotes Bolting and Flowering via the Floral Integrators RsFT and RsSOC1-1 under Marginal Vernalization in Radish. Plants 2020, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Akand, M.H.; Hossain, M.I.; Sarkar, M.D.; Ullah, A. Effect of Nitrogen on the Growth and Yield of Carrot (Daucus carota L.). Agriculturists 2013, 11, 76–81. [Google Scholar] [CrossRef]
- Abbas, E.D. Effect of GA3 on growth and some physiological characterizes in carrot plant (Daucus carota L.). Ibn AL-Haitham J. Pure Appl. Sci. 2011, 24, 33–39. [Google Scholar]
- Uwah, E.I.; Gimba, M.S.; Gwaski, P.A. Determination of Zn, Mn, Fe and Cu in Spinach and Lettuce Cultivated in Potiskum, Yobe State, Nigeria. J. Agric. Econ. Dev. 2012, 1, 69–74. [Google Scholar]
- Liebert, M.; Licht, U.; Böhm, V.; Bitsch, R. Antioxidant properties and total phenolics content of green and black tea under different brewing conditions. Z. Lebensm. Und-Forsch. A 1999, 208, 217–220. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Phytochemical screening and free radical scavenging activities of orange baby carrot and carrot (Daucus carota Linn.) root crude extracts. J. Chem. Pharma. Res. 2013, 5, 97–102. [Google Scholar]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar]
- Ding, C.F.; Zhang, T.L.; Wang, X.X.; Zhou, F.; Yang, Y.R.; Yin, Y. Effects of soil type and genotype on lead concentration in rootstalk vegetables and the selection of cultivars for food safety. J. Environ. Manag. 2013, 122, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Nunez, J.J.; Davis, R.M. Influence of gibberellic acid on carrot growth and severity of Alternaria leaf blight. Plant Dis. 2000, 84, 555–558. [Google Scholar] [CrossRef]
- Ali, B.; Mwamba, T.M.; Gill, R.A.; Yang, C.; Ali, S.; Daud, M.K.; Wu, Y.; Zhou, W.J. Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regul. 2014, 74, 261–273. [Google Scholar] [CrossRef]
- Skrebsky, E.C.; Tabald, L.A.; Pereira, B.; Rauber, R.; Maldaner, J.; Cargnelutti, D.; Gonçalves, J.F.; Castro, G.Y.; Shetinger, M.R.C.; Nicoloso, F.T. Effect of cadmium on growth, micronutrient concentration, and d-aminolevulinic acid dehydratase and acid phosphatase activities in plants of Pfaffia glomerata. Braz. J. Plant Physiol. 2008, 20, 285–294. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Khan, M.I.R.; Masood, A.; Khan, N.A. Role of gibberellins in regulation of source–sink relations under optimal and limiting environmental conditions. Curr. Sci. 2011, 7, 998–1007. [Google Scholar]
- Hadi, F.; Bano, A.; Fuller, M.P. The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): The role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 2010, 80, 457–462. [Google Scholar] [CrossRef]
- Lima, F.D.S.; do Nascimento, C.W.; da Silva, F.B.; de Carvalho, V.G.; Ribeiro Filho, M.R. Lead concentration and allocation in vegetable crops grown in a soil contaminated by battery residues. Hortic. Bras. 2009, 27, 362–365. [Google Scholar] [CrossRef]
- Ghani, M.A.; Abbas, M.M.; Amjad, M.; Ziaf, K.; Ali, B.; Shaheen, T.; Awan, F.S.; Khan, A.N. Production and characterization of tomato derived from Interspecific hybridization between cultivated tomato and its wild relatives. J. Hortic. Sci. Biotechnol. 2020, 95, 506–520. [Google Scholar] [CrossRef]
- Rokaya, P.R.; Baral, D.R.; Gautam, D.M.; Shrestha, A.K.; Paudyal, K.P. Effect of Pre-harvest application of gibberellic acid on fruit quality and shelf life of mandarin. J. Plant Sci. 2016, 7, 1033–1039. [Google Scholar] [CrossRef]
- Fairbrother, A.; Wenstel, R.; Sappington, K.; Wood, W. Framework for metals risk assessment. Ecotoxicol. Environ. Saf. 2007, 68, 145–227. [Google Scholar] [CrossRef]
- Ali, M.H.; Al-Qahtani, K.M. Assessment of some heavy metals in vegetables, cereals and fruits in Saudi Arabian markets. Egy. J. Aquat. Res. 2012, 38, 31–37. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M.; Słaba, M.; Mazur, J. Effect of nickel on antioxidative enzyme activities, praline and chlorophyll contents in wheat shoots. Biol. Plant 2006, 50, 653–659. [Google Scholar] [CrossRef]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pterisensi formis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Zhu, X.F.; Jiang, T.; Wang, Z.W.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J. Hazard. Mater. 2012, 239, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 98–403. [Google Scholar] [CrossRef]
- Al-Hakimi, A.M.A. Modification of cadmium toxicity in pea seedlings by kinetin. Plant Soil Environ. 2007, 53, 129. [Google Scholar] [CrossRef]
- El-Monem, A.; Sharaf, M.M.; Farghal, I.I.; Sofy, M. Role of gibberellic acid in abolishing the detrimental effects of Cd and Pb on broad bean and lupin plants. J. Agric. Biol. Sci. 2009, 5, 6–13. [Google Scholar]
- Masood, A.; Khan, M.I.R.; Fatma, M.; Asgher, M.; Khan, N.A. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 2016, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fargašová, A. Phytotoxic effects of Cd, Zn, Pb, Cu and Fe on Sinapis alba L. seedlings and their accumulation in roots and shoots. Biol. Plant. 2001, 44, 471–473. [Google Scholar] [CrossRef]
- Ali, B.; Song, W.J.; Hu, W.Z.; Luo, X.N.; Gill, R.A.; Wang, J.; Zhou, W.J. Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastructural changes in oilseed rape. Ecotoxicol. Environ. Saf. 2014, 102, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Kanwal, S.; Uddin, F.; Azmat, R. Phytotoxicity of Pb II. Changes in chlorophyll absorption spectrum due to toxic metal Pb stress on Phaseolus mungo and Lens culinaris. Pak. J. Biol. Sci. 2006, 9, 2062–2068. [Google Scholar] [CrossRef]
- Kaimoyo, E.; Farag, M.A.; Sumner, L.W.; Wasmann, C.; Cuello, J.L.; VanEtten, H. Sublethal levels of electric current elicit the biosynthesis of plant secondary metabolites. J. Biotechnol. Progr. 2008, 24, 377–384. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Govarthanan, M.; Kamala-Kannan, S.; Kim, S.A.; Seo, Y.S.; Park, J.H.; Oh, B.T. Synergistic effect of chelators and Herbaspirillum sp. GW103 on lead phytoextraction and its induced oxidative stress in Zea mays. Arch. Microbiol. 2016, 198, 737–742. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.X.; Gao, B.; Hu, Y.; Xu, H. Lead-resistant strain KQBT-3 inoculants of Tricholoma lobayensis Heim that enhance remediation of lead-contaminated soil. Environ. Technol. 2015, 36, 2451–2458. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar] [CrossRef]
- Lamhamdi, M.; Bakrim, A.; Aarab, A.; Lafont, R.; Sayah, F. Effects of lead phytotoxicity on wheat seed germination and seedling growth. Comptes Rendus Biol. 2011, 334, 118–126. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).