Fire’s Effects on Grassland Restoration and Biodiversity Conservation
Abstract
:1. Introduction
2. Materials and Methods
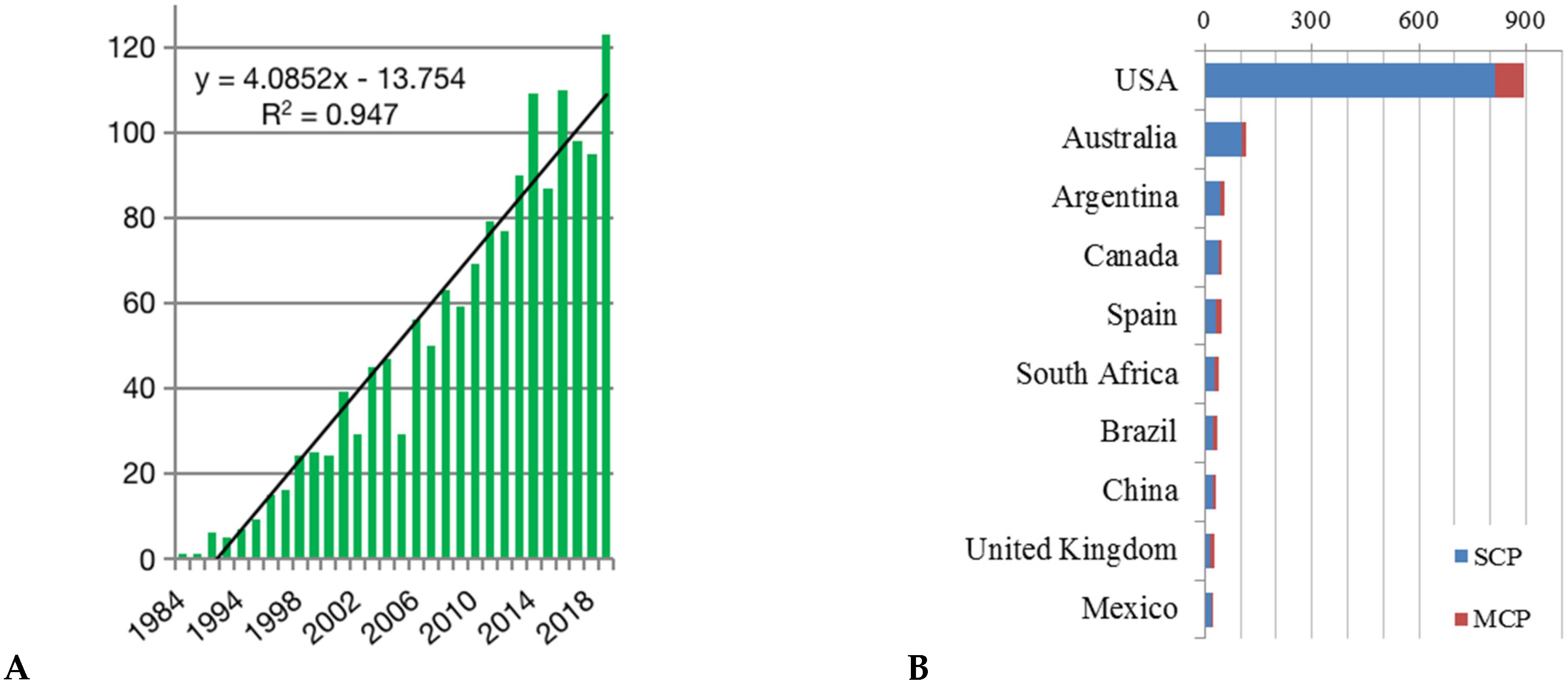
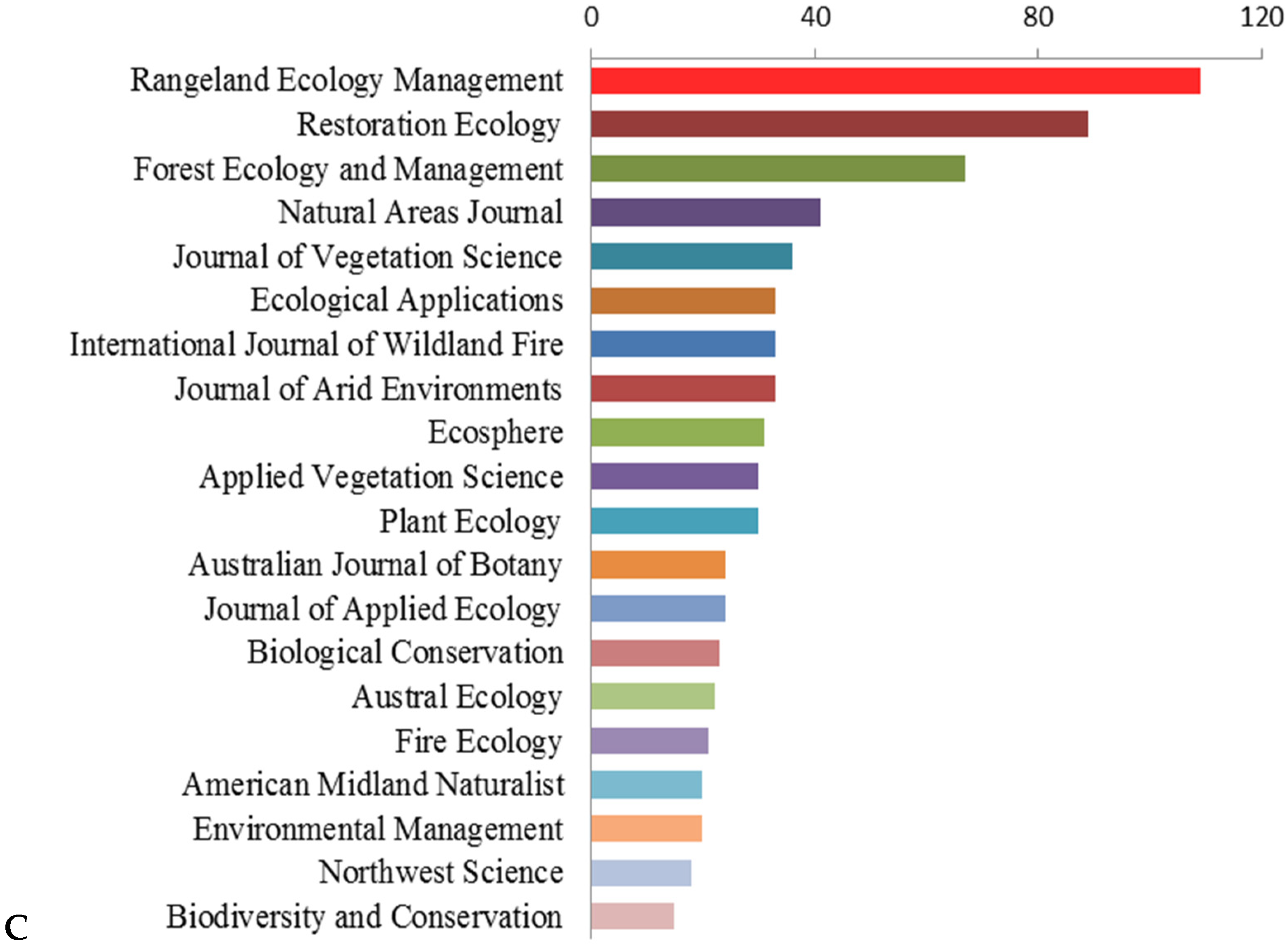
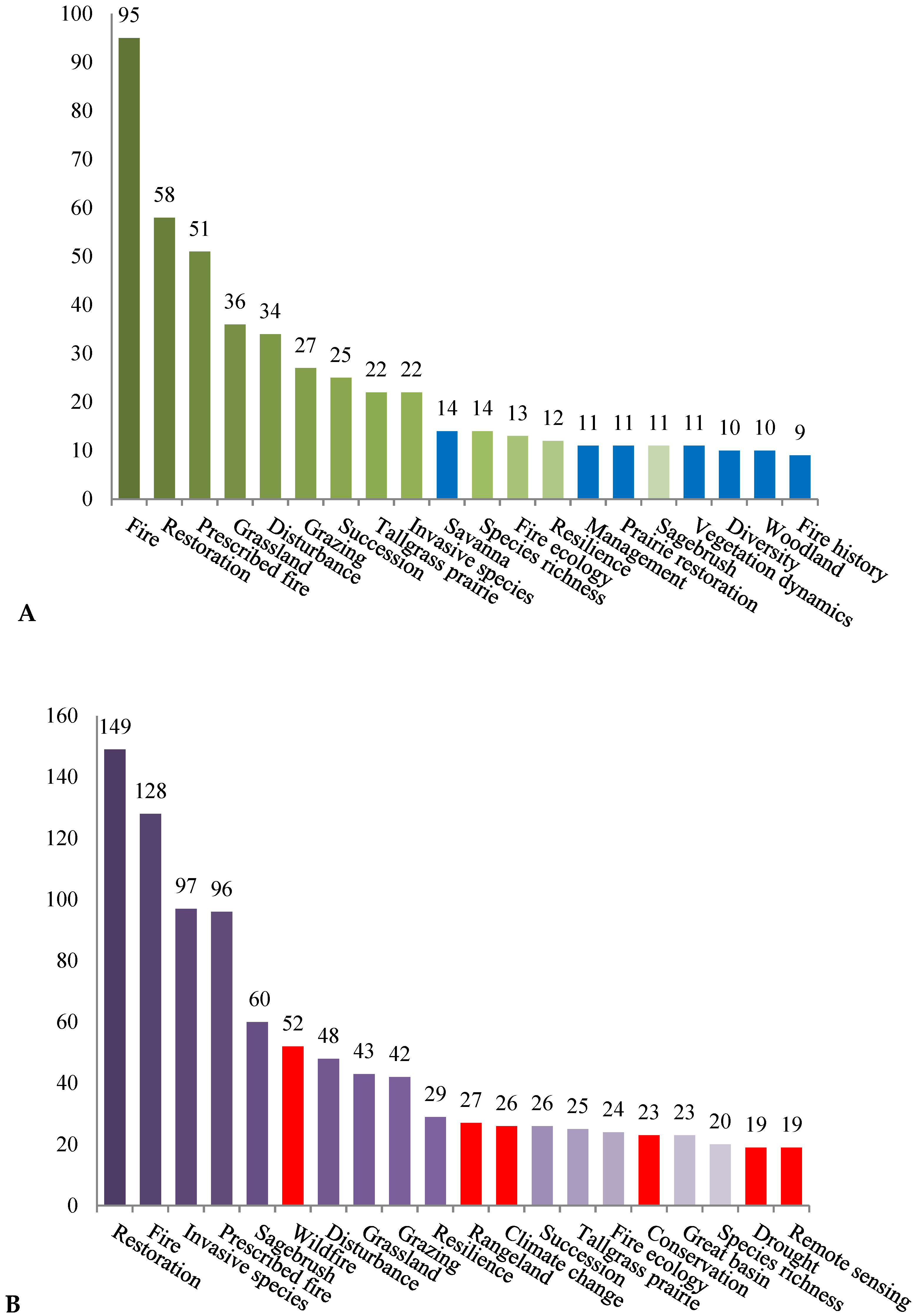
3. Results
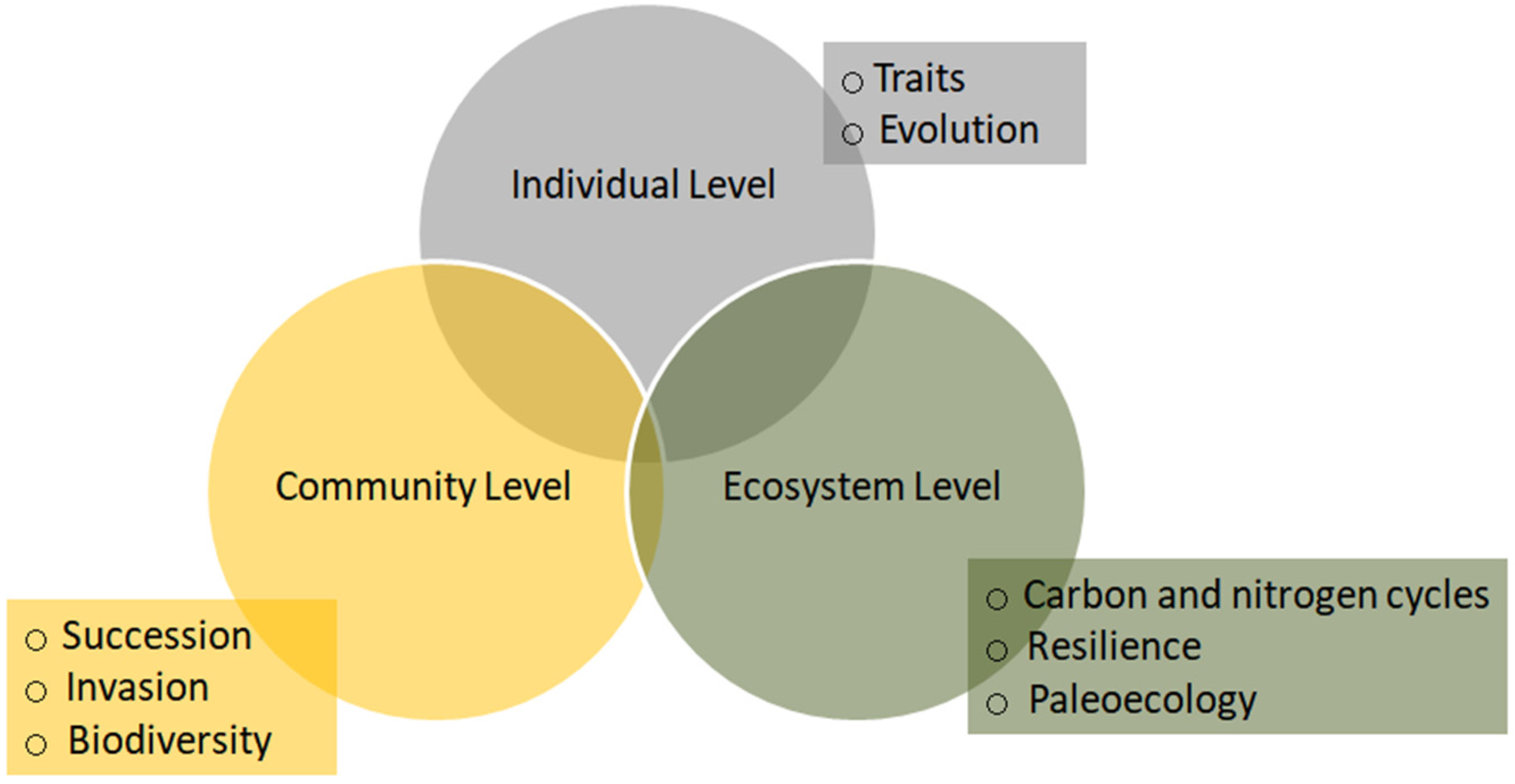
4. Major Progresses
4.1. Individual-Level Effects
4.1.1. Species Traits
4.1.2. Evolution
4.2. Community-Level Effects
4.2.1. Community Succession
4.2.2. Invasion
4.2.3. Biodiversity
4.3. Ecosystem-Level Effects
4.3.1. Carbon and Nitrogen Cycles
4.3.2. Resilience
4.3.3. Paleoecology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buisson, E.; Le Stradic, S.; Silveira, F.A.O.; Durigan, G.; Overbeck, G.E.; Fidelis, A.; Fernandes, G.W.; Bond, W.J.; Hermann, J.M.; Mahy, G.; et al. Resilience and restoration of tropical and subtropical grasslands, savannas, and grassy woodlands. Biol. Rev. 2019, 94, 590–609. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.J.; Birks, H.J.B. What is natural? The need for a long-term perspective in biodiversity conservation. Science 2006, 314, 1261–1265. [Google Scholar] [CrossRef] [Green Version]
- Bowman, D.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- White, R.P.; Murray, S.; Rohweder, M. Pilot Analysis of Global Ecosystems: Grassland Ecosystems; World Resources Institute: Washington, DC, USA, 2000; Volume 4, p. 275. [Google Scholar]
- Valko, O.; Torok, P.; Deak, B.; Tothmeresz, B. Review: Prospects and limitations of prescribed burning as a management tool in European grasslands. Basic Appl. Ecol. 2014, 15, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.L.; Nippert, J.B.; Blair, J.M.; Briggs, J.M.; Blackmore, P.; Ratajczak, Z. Fire frequency, state change and hysteresis in tallgrass prairie. Ecol. Lett. 2021, 24, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Pennington, R.T.; Hughes, C.E. The remarkable congruence of New and old world savanna origins. New Phytol. 2014, 204, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Leys, B.A.; Marlon, J.R.; Umbanhowar, C.; Vanniere, B. Global fire history of grassland biomes. Ecol. Evol. 2018, 8, 8831–8852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannusch, H.J.; Rogers, W.E.; Lodge, A.G.; Starns, H.D.; Tolleson, D.R. Semi-arid savanna herbaceous production and diversity responses to interactive effects of drought, nitrogen deposition, and fire. J. Veg. Sci. 2020, 31, 255–265. [Google Scholar] [CrossRef]
- Kelly, L.T.; Brotons, L. Using fire to promote biodiversity. Science 2017, 355, 1264–1265. [Google Scholar] [CrossRef]
- Ellsworth, L.M.; Kauffman, J.B.; Reis, S.A.; Sapsis, D.; Moseley, K. Repeated fire altered succession and increased fire behavior in basin big sagebrush–native perennial grasslands. Ecosphere 2020, 11, e03124. [Google Scholar] [CrossRef]
- Bahia, R.; Zalba, S. Changes in grassland bird communities and breeding success after a fire in the Argentinian Pampas. Biodivers. Conserv. 2019, 28, 3767–3786. [Google Scholar] [CrossRef]
- Lamont, B.B.; He, T.H.; Yan, Z.G. Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination. Biol. Rev. 2019, 94, 903–928. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, Z.; Nippert, J.B.; Briggs, J.M.; Blair, J.M. Fire dynamics distinguish grasslands, shrublands and woodlands as alternative attractors in the Central Great Plains of North America. J. Ecol. 2014, 102, 1374–1385. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, Z.; D’Odorico, P.; Nippert, J.B.; Collins, S.L.; Brunsell, N.A.; Ravi, S. Changes in spatial variance during a grassland to shrubland state transition. J. Ecol. 2017, 105, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Rossiter, N.A.; Setterfield, S.A.; Douglas, M.M.; Hutley, L.B. Testing the grass-fire cycle: Alien grass invasion in the tropical savannas of northern Australia. Divers. Distrib. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Chambers, J.C.; Bradley, B.A.; Brown, C.S.; D’Antonio, C.; Germino, M.J.; Grace, J.B.; Hardegree, S.P.; Miller, R.F.; Pyke, D.A. Resilience to Stress and Disturbance, and Resistance to Bromus tectorum L. Invasion in Cold Desert Shrublands of Western North America. Ecosystems 2014, 17, 360–375. [Google Scholar] [CrossRef]
- Mouillot, F.; Ratte, J.P.; Joffre, R.; Moreno, J.M.; Rambal, S. Some determinants of the spatio-temporal fire cycle in a Mediterranean landscape (Corsica, France). Landsc. Ecol. 2003, 18, 665–674. [Google Scholar] [CrossRef]
- Koerner, S.E.; Burkepile, D.E.; Fynn, R.W.; Burns, C.E.; Eby, S.; Govender, N.; Hagenah, N.; Matchett, K.J.; Thompson, D.I.; Wilcox, K.R. Plant community response to loss of large herbivores differs between North American and South African savanna grasslands. Ecology 2014, 95, 808–816. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Bassett, T.; Grman, E.; Brudvig, L.A. Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. J. Appl. Ecol. 2017, 54, 1070–1079. [Google Scholar] [CrossRef] [Green Version]
- Karp, A.T.; Behrensmeyer, A.K.; Freeman, K.H. Grassland fire ecology has roots in the late Miocene. Proc. Natl. Acad. Sci. USA 2018, 115, 12130–12135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, N.A.; Camac, J.S.; Morgan, J.W. Effects of drought and fire on resprouting capacity of 52 temperate Australian perennial native grasses. New Phytol. 2019, 221, 1424–1433. [Google Scholar] [CrossRef]
- Pausas, J.G.; Paula, S. Grasses and fire: The importance of hiding buds. New Phytol. 2020, 226, 957–959. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, M.L.; Norman, L.M.; Buckley, S.; Wallace, C.S.; Coe, M.A. Multi-index time series monitoring of drought and fire effects on desert grasslands. Remote Sens. Environ. 2016, 183, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Ansley, R.J.; Castellano, M.J. Strategies for savanna restoration in the southern Great Plains: Effects of fire and herbicides. Restor. Ecol. 2006, 14, 420–428. [Google Scholar] [CrossRef]
- Wright, B.R.; Clarke, P.J. Resprouting responses of Acacia shrubs in the Western Desert of Australia—Fire severity, interval and season influence survival. Int. J. Wildland Fire 2007, 16, 317–323. [Google Scholar] [CrossRef]
- Archibald, S.; Hempson, G.P.; Lehmann, C. A unified framework for plant life-history strategies shaped by fire and herbivory. New Phytol. 2019, 224, 1490–1503. [Google Scholar] [CrossRef]
- Benson, E.J.; Hartnett, D.C. The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecol. 2006, 187, 163–177. [Google Scholar] [CrossRef]
- Lamont, B.B.; Enright, N.J.; He, T. Fitness and evolution of resprouters in relation to fire. Plant Ecol. 2011, 212, 1945–1957. [Google Scholar] [CrossRef]
- Gashaw, M.; Michelsen, A. Influence of heat shock on seed germination of plants from regularly burnt savanna woodlands and grasslands in Ethiopia. Plant Ecol. 2002, 159, 83–93. [Google Scholar] [CrossRef]
- Morgan, J. Importance of canopy gaps for recruitment of some forbs in Themeda triandra-dominated grasslands in south-eastern Australia. Aust. J. Bot. 1998, 46, 609–627. [Google Scholar] [CrossRef]
- Grigulis, K.; Lavorel, S.; Krainer, U.; Legay, N.; Baxendale, C.; Dumont, M.; Kastl, E.; Arnoldi, C.; Bardgett, R.D.; Poly, F. Relative contributions of plant traits and soil microbial properties to mountain grassland ecosystem services. J. Ecol. 2013, 101, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Lamont, B.B.; He, T. Fire-proneness as a prerequisite for the evolution of fire-adapted traits. Trends Plant Sci. 2017, 22, 278–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesk, P.A.; Westoby, M. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 2004, 92, 310–320. [Google Scholar] [CrossRef]
- Burrows, G.E.; Alden, R.; Robinson, W.A. The lens in focus–lens structure in seeds of 51 Australian Acacia species and its implications for imbibition and germination. Aust. J. Bot. 2018, 66, 398–413. [Google Scholar] [CrossRef]
- Lamont, B.B.; Downes, K.S. Fire-stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecol. 2011, 212, 2111–2125. [Google Scholar] [CrossRef]
- Lawes, M.J.; Keith, D.A.; Bradstock, R.A. Advances in understanding the influence of fire on the ecology and evolution of plants: A tribute to Peter, J. Clarke. Plant Ecol. 2016, 217, 597–605. [Google Scholar] [CrossRef] [Green Version]
- López-Villalta, J.S. Trait-driven vs. syndrome-driven diversification in the Mediterranean woody flora. Ecol. Mediterr. 2014, 40, 27–33. [Google Scholar] [CrossRef]
- El-ahmir, S.M.; Lim, S.L.; Lamont, B.B.; He, T. Seed size, fecundity and postfire regeneration strategy are interdependent in Hakea. PLoS ONE. 2015, 10, e0129027. [Google Scholar]
- Causley, C.L.; Fowler, W.M.; Lamont, B.B.; He, T. Fitness benefits of serotiny in fire-and drought-prone environments. Plant Ecol. 2016, 217, 773–779. [Google Scholar] [CrossRef]
- Gomez-Gonzalez, S.; Torres-Diaz, C.; Bustos-Schindler, C.; Gianoli, E. Anthropogenic fire drives the evolution of seed traits. Proc. Natl. Acad. Sci. USA 2011, 108, 18743–18747. [Google Scholar] [CrossRef] [Green Version]
- Howe, H.F. Succession and fire season in experimental prairie plantings. Ecology 1995, 76, 1917–1925. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Proctor, J. Vegetation and soil and plant chemistry on ultramafic rocks in the tropical Far East. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 105–124. [Google Scholar] [CrossRef] [Green Version]
- Lesica, P.; Cooper, S.V.; Kudray, G. Recovery of big sagebrush following fire in southwest Montana. Rangel. Ecol. Manag. 2007, 60, 261–269. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Risler, J.; Kean, L. Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral. Ecol. 2004, 29, 156–176. [Google Scholar] [CrossRef]
- Nieman, W.A.; van Wilgen, B.W.; Leslie, A.J. A review of fire management practices in African savanna-protected areas. Koedoe 2021, 63, 1–13. [Google Scholar] [CrossRef]
- Prichard, S.J.; Stevens-Rumann, C.S.; Hessburg, P.F. Tamm review: Shifting global fire regimes: Lessons from reburns and research needs. For. Ecol. Manag. 2017, 396, 217–233. [Google Scholar] [CrossRef]
- Pyke, D.A.; Brooks, M.L.; D’Antonio, C. Fire as a restoration tool: A decision framework for predicting the control or enhancement of plants using fire. Restor. Ecol. 2010, 18, 274–284. [Google Scholar] [CrossRef]
- Emery, S.M.; Gross, K.L. Effects of timing of prescribed fire on the demography of an invasive plant, spotted knapweed Centaurea maculosa. J. Appl. Ecol. 2005, 42, 60–69. [Google Scholar] [CrossRef]
- Chambers, J.C.; Miller, R.F.; Board, D.I.; Pyke, D.A.; Roundy, B.A.; Grace, J.B.; Schupp, E.W.; Tausch, R.J. Resilience and Resistance of Sagebrush Ecosystems: Implications for State and Transition Models and Management Treatments. Rangel. Ecol. Manag. 2014, 67, 440–454. [Google Scholar] [CrossRef] [Green Version]
- Bates, J.D.; Davies, K.W.; Sharp, R.N. Shrub-Steppe Early Succession Following Juniper Cutting and Prescribed Fire. Environ. Manage. 2011, 47, 468–481. [Google Scholar] [CrossRef]
- Drewa, P.B.; Platt, W.J.; Moser, E.B. Fire effects on resprouting of shrubs in headwaters of southeastern longleaf pine savannas. Ecology 2002, 83, 755–767. [Google Scholar] [CrossRef]
- Mack, M.C.; D’Antonio, C.M.; Ley, R.E. Alteration of ecosystem nitrogen dynamics by exotic plants: A case study of C4 grasses in Hawaii. Ecol. Appl. 2001, 11, 1323–1335. [Google Scholar]
- Davies, K.W.; Boyd, C.S.; Beck, J.L.; Bates, J.D.; Svejcar, T.J.; Gregg, M.A. Saving the sagebrush sea: An ecosystem conservation plan for big sagebrush plant communities. Biol. Conserv. 2011, 144, 2573–2584. [Google Scholar] [CrossRef] [Green Version]
- D’Antonio, C.M.; Hughes, R.F.; Tunison, J.T. Long-term impacts of invasive grasses and subsequent fire in seasonally dry Hawaiian woodlands. Ecol. Appl. 2011, 21, 1617–1628. [Google Scholar] [CrossRef]
- Davies, K.W.; Sheley, R.L. Promoting native vegetation and diversity in exotic annual grass infestations. Restor. Ecol. 2011, 19, 159–165. [Google Scholar] [CrossRef]
- Wilson, J.B.; Peet, R.K.; Dengler, J.; Pärtel, M. Plant species richness: The world records. J. Veg. Sci. 2012, 23, 796–802. [Google Scholar] [CrossRef]
- Dengler, J.; Janišová, M.; Török, P.; Wellstein, C. Biodiversity of Palaearctic grasslands: A synthesis. Agric. Ecosyst. Environ. 2014, 182, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Horváth, Z.; Ptacnik, R.; Vad, C.F.; Chase, J.M. Habitat loss over six decades accelerates regional and local biodiversity loss via changing landscape connectance. Ecol. Lett. 2019, 22, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Xu, M.; Zhao, Y.; Gao, L.; Wang, S. Moss-dominated biological soil crusts improve stability of soil organic carbon on the Loess Plateau, China. Plant Soil Environ. 2019, 65, 104–109. [Google Scholar] [CrossRef]
- Casas, G.; Darski, B.; Ferreira, P.M.; Kindel, A.; Müller, S.C. Habitat structure influences the diversity, richness and composition of bird assemblages in successional Atlantic rain forests. Trop. Conserv. Sci. 2016, 9, 503–524. [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B. Herbivory, serotiny and seedling defence in Western Australian Proteaceae. Oecologia 2001, 126, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Wood, J.T.; MacGregor, C.; Michael, D.R.; Cunningham, R.B.; Crane, M.; Montague-Drake, R.; Brown, D.; Muntz, R.; Driscoll, D.A. How predictable are reptile responses to wildfire? Oikos 2008, 117, 1086–1097. [Google Scholar] [CrossRef]
- Allred, B.W.; Fuhlendorf, S.D.; Hamilton, R.G. The role of herbivores in Great Plains conservation: Comparative ecology of bison and cattle. Ecosphere 2011, 2, 17. [Google Scholar] [CrossRef]
- Garcia-Carmona, M.; Arcenegui, V.; Garcia-Orenes, F.; Mataix-Solera, J. The role of mosses in soil stability, fertility and microbiology six years after a post-fire salvage logging management. J. Environ. Manage. 2020, 262, 110287. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.F.; Ratchford, J.; Roundy, B.A.; Tausch, R.J.; Hulet, A.; Chambers, J. Response of Conifer-Encroached Shrublands in the Great Basin to Prescribed Fire and Mechanical Treatments. Rangel. Ecol. Manag. 2014, 67, 468–481. [Google Scholar] [CrossRef] [Green Version]
- Johansen, J.R.; Stclair, L.L.; Webb, B.L.; Nebeker, G.T. Recovery patterns of cryptogamic soil crusts in desert rangelands following fire disturbance. Bryologist 1984, 87, 238–243. [Google Scholar] [CrossRef]
- Condon, L.A.; Pyke, D.A. Resiliency of biological soil crusts and vascular plants varies among morphogroups with disturbance intensity. Plant Soil. 2018, 433, 271–287. [Google Scholar] [CrossRef]
- Slate, M.L.; Durham, R.A.; Pearson, D.E. Strategies for restoring the structure and function of lichen-moss biocrust communities. Restor. Ecol. 2020, 28, S160–S167. [Google Scholar] [CrossRef]
- Yarnell, R.W.; Scott, D.M.; Chimimba, C.T.; Metcalfe, D.J. Untangling the roles of fire, grazing and rainfall on small mammal communities in grassland ecosystems. Oecologia 2007, 154, 387–402. [Google Scholar] [CrossRef]
- Panzer, R. Compatibility of prescribed burning with the conservation of insects in small, isolated prairie reserves. Conserv. Biol. 2002, 16, 1296–1307. [Google Scholar] [CrossRef] [Green Version]
- Coppedge, B.R.; Fuhlendorf, S.D.; Harrell, W.C.; Engle, D.M. Avian community response to vegetation and structural features in grasslands managed with fire and grazing. Biol. Conserv. 2008, 141, 1196–1203. [Google Scholar] [CrossRef]
- Hovick, T.J.; Elmore, R.D.; Fuhlendorf, S.D.; Engle, D.M.; Hamilton, R.G. Spatial heterogeneity increases diversity and stability in grassland bird communities. Ecol. Appl. 2015, 25, 662–672. [Google Scholar] [CrossRef]
- Davis, M.A.; Peterson, D.W.; Reich, P.B.; Crozier, M.; Query, T.; Mitchell, E.; Huntington, J.; Bazakas, P. Restoring savanna using fire: Impact on the breeding bird community. Restor. Ecol. 2000, 8, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Joern, A.; Laws, A.N. Ecological Mechanisms Underlying Arthropod Species Diversity in Grasslands. Annu. Rev. Entomol. 2013, 58, 19. [Google Scholar] [CrossRef] [Green Version]
- Chiarani, E.; Bettio, M.; Fontana, C.S. Temporal changes in bird communities in areas with different histories of fire disturbance in highland grasslands of Brazil. PLoS ONE 2020, 15, 18. [Google Scholar] [CrossRef]
- Betancourt, J.L. Energy flow and the “grassification” of desert shrublands. Proc. Natl. Acad. Sci. USA 2015, 112, 9504–9505. [Google Scholar] [CrossRef] [Green Version]
- Mulqueeny, C.M.; Goodman, P.S.; O’Connor, T.G. Landscape-level differences in fire regime between block and patch-mosaic burning strategies in Mkuzi Game Reserve, South Africa. Afr. J. Range. For. Sci. 2010, 27, 143–150. [Google Scholar] [CrossRef]
- Van der Werf, G.R.; Randerson, J.T.; Giglio, L.; Leeuwen, T.T.v.; Chen, Y.; Rogers, B.M.; Mu, M.; Van Marle, M.J.; Morton, D.C.; Collatz, G.J. Global fire emissions estimates during 1997–2016. Earth Syst. Sci. Data. 2017, 9, 697–720. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Rojas, M.; Erickson, T.E.; Martini, D.; Dixon, K.W.; Merritt, D.J. Soil physicochemical and microbiological indicators of short, medium and long term post-fire recovery in semi-arid ecosystems. Ecol. Indic. 2016, 63, 14–22. [Google Scholar] [CrossRef]
- Pellegrini, A.F.; Hoffmann, W.A.; Franco, A.C. Carbon accumulation and nitrogen pool recovery during transitions from savanna to forest in central Brazil. Ecology 2014, 95, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Romanyà, J.; Casals, P.; Vallejo, V.R. Short-term effects of fire on soil nitrogen availability in Mediterranean grasslands and shrublands growing in old fields. For. Ecol. Manag. 2001, 147, 39–53. [Google Scholar] [CrossRef]
- Blank, R.R.; Allen, F.L.; Young, J.A. Influence of simulated burning of soil-litter from low sagebrush, squirreltail, cheatgrass, and medusahead on water-soluble anions and cations. Int. J. Wildland Fire 1996, 6, 137–143. [Google Scholar] [CrossRef]
- Stubbs, M.M.; Pyke, D.A. Available nitrogen: A time-based study of manipulated resource islands. Plant Soil. 2005, 270, 123–133. [Google Scholar] [CrossRef]
- Perry, L.G.; Blumenthal, D.M.; Monaco, T.A.; Paschke, M.W.; Redente, E.F. Immobilizing nitrogen to control plant invasion. Oecologia 2010, 163, 13–24. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Ahlstrom, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 553, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Suding, K.N. A leak in the loop. Nature 2013, 503, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Q.; Hui, D.F.; Luo, Y.Q. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: A meta-analysis. Ecol. Appl. 2001, 11, 1349–1365. [Google Scholar] [CrossRef]
- Allen, C.R.; Gunderson, L.; Johnson, A.R. The use of discontinuities and functional groups to assess relative resilience in complex systems. Ecosystems 2005, 8, 958–966. [Google Scholar] [CrossRef]
- Davies, G.M.; Bakker, J.D.; Dettweiler-Robinson, E.; Dunwiddie, P.W.; Hall, S.A.; Downs, J.; Evans, J. Trajectories of change in sagebrush steppe vegetation communities in relation to multiple wildfires. Ecol. Appl. 2012, 22, 1562–1577. [Google Scholar] [CrossRef]
- Bestelmeyer, B.T.; Tugel, A.J.; Peacock Jr, G.L.; Robinett, D.G.; Shaver, P.L.; Brown, J.R.; Herrick, J.E.; Sanchez, H.; Havstad, K.M. State-and-transition models for heterogeneous landscapes: A strategy for development and application. Rangel. Ecol. Manag. 2009, 62, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.M.; Latimer, A.M.; Silander, J.A. Climatic controls on ecosystem resilience: Postfire regeneration in the Cape Floristic Region of South Africa. Proc. Natl. Acad. Sci. USA 2015, 112, 9058–9063. [Google Scholar] [CrossRef] [Green Version]
- Feurdean, A.; Ruprecht, E.; Molnar, Z.; Hutchinson, S.M.; Hickler, T. Biodiversity-rich European grasslands: Ancient, forgotten ecosystems. Biol. Conserv. 2018, 228, 224–232. [Google Scholar] [CrossRef]
- Strömberg, C.A.E. Evolution of grasses and grassland ecosystems. Annu. Rev. Earth. Planet. Sci. 2011, 39, 517–544. [Google Scholar] [CrossRef]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Feurdean, A.; Marinova, E.; Nielsen, A.B.; Liakka, J.; Veres, D.; Hutchinson, S.M.; Braun, M.; Timar-Gabor, A.; Astalos, C.; Mosburgger, V. Origin of the forest steppe and exceptional grassland diversity in Transylvania (central-eastern Europe). J. Biogeogr. 2015, 42, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Bond, W.J.; Midgley, G.F.; Woodward, F.I. The importance of low atmospheric CO2 and fire in promoting the spread of grasslands and savannas. Glob. Chang. Biol. 2003, 9, 973–982. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Liu, G. Fire’s Effects on Grassland Restoration and Biodiversity Conservation. Sustainability 2021, 13, 12016. https://doi.org/10.3390/su132112016
Yan H, Liu G. Fire’s Effects on Grassland Restoration and Biodiversity Conservation. Sustainability. 2021; 13(21):12016. https://doi.org/10.3390/su132112016
Chicago/Turabian StyleYan, Hui, and Guixiang Liu. 2021. "Fire’s Effects on Grassland Restoration and Biodiversity Conservation" Sustainability 13, no. 21: 12016. https://doi.org/10.3390/su132112016
APA StyleYan, H., & Liu, G. (2021). Fire’s Effects on Grassland Restoration and Biodiversity Conservation. Sustainability, 13(21), 12016. https://doi.org/10.3390/su132112016




