Abstract
The term corallivorous gastropod refers to a group of snails that feeds on coral and inhabits coral communities worldwide. Outbreaks of these species cause serious damage to coral communities. There are various reasons behind the outbreaks; however, further clarifications are needed. It may be possible to predict outbreaks by measuring the number of floating larvae of corallivorous gastropods in seawater. Drupella fragum is the most damaging species in Japan, so we produced antibodies against D. fragum larvae in order to easily detect this species in the field. Antibody specificity analysis in aquarium-hatched corallivorous gastropods showed a higher specificity against D. fragum compared to D. cornus. A field study using the antibody showed that many D. fragum larvae were detected from June to November at all stations. The larvae at the Shirigai station were collected in June and July in large numbers compared to the other stations. Large groups of D. fragum were collected around the sampling point in Shirigai in September 2016. Our results imply that there is a possibility that outbreaks could be predicted using this antibody.
1. Introduction
The Ashizuri-Uwakai coast in Southwest Shikoku is a rugged rias coastline. It is affected by the Kuroshio current, a warm current that enables coral communities with many diverse organisms to develop in this area. This area is also popular for fishing and leisure activities such as scuba diving; therefore, the corals are important for supporting biodiversity and local resources. Accordingly, 22 marine park districts were established in Ashizuri-Uwakai, where various conservation activities are carried out.
The reduction of damage to the coral community by various coral predators is the most important conservation issue. There are many kinds of creatures that eat corals, such as corallivorous gastropods and crown-of-thorns starfish [1]. Therefore, both species have been the target of extermination activities in various regions. The corallivorous gastropods belonging to the genus Drupella eat the live corals by radula [2] and have been known to interfere with the recruitment and growth of juvenile corals [3,4,5]. The gastropods usually form groups on the coral consisting of 1-10 individuals and are known to contribute to the species diversity of the coral by eating fast-growing corals such as Acroporidae [6,7]. However, gastropod outbreaks have occurred, which caused damage to coral communities in Thailand [8], Hong Kong [9], the Indian Ocean [10,11,12], the Red Sea [11], and Australia [13]. In Japan, outbreaks of D. fragum were rampant. The outbreak of Drupella spp. in Japan along the Kuroshio Current was confirmed for the first time in Miyakejima [14] and reported in Okinawa [15], Miyazaki [16,17], Kochi [18], and Ehime [19].
Extermination activities have been carried out continuously in Ainan-town, Ehime, from 1991 to the present. Drupella fragum accounted for 88% of the gastropods exterminated in 1991 [19]. Since the Drupella spp. inhabits the gaps of coral branches, they are difficult to find, and intensive labor is required for extermination. Extermination activity is performed continuously when outbreaks occur, and it is thought that Drupella fragum comprises a large percentage of the exterminated individuals.
The gastropods of Drupella spp. tend to preferentially prey on Acropora spp. This may be due to the attractant substance released by a group of Acropora spp. [7].
According to monitoring studies by Ainan Sango Wo Mamoru Kyougikai (the council of the protection in Ainan) in 2017, it was clarified that there are many spots where the Acroporidae corals make up most of the coral coverage in Ainan. Consequently, it can be predicted that when outbreaks of Drupella spp. gastropods occur in the future they will cause serious damage to coral communities in Ainan. It is known that outbreaks of corallivorous organisms can cause serious damage to coral reefs by causing coral populations to disappear rapidly [20]. The factor that causes outbreaks of corallivorous gastropods has not been established, although a change in salt nutrients [14], a decrease in predators due to overfishing, and changes in water temperature and salinity [9] are suspected to be the culprits. We theorized that it would be possible to predict outbreaks by investigating the floating snail larvae in seawater. We focused on D. fragum, which damages corals and has had many outbreaks in Japan. After copulation, D. fragum deposits its egg capsule containing several hundred eggs on the branches and back of dead coral skeletons [21]. The veliger larvae hatched from the eggs swim near the water surface [22]. The floating period is supposed to be 13-19 days [23]; however, almost all snail larvae look similar in form, so identification based on morphology is difficult. In recent years, species identification methods based on DNA sequences have become widespread in a variety of biological fields. However, in investigating the dynamics of D. fragum in the wide area of the ocean, it is necessary to cooperate not only with research institutes but also with various organizations, such as fishing cooperatives and governments. For example, the coral conservation activities in Southwest Shikoku are carried out by municipalities, fishing cooperatives, and volunteers. In order to cooperate with these organizations, the experiment must be low-cost and utilize a simple method that does not require special equipment. In addition, rapid results need to be obtained and immediately reflected in conservation activities.
The simple method for species identification of larvae is immunostaining. The immunostaining method has been developed and used to determine when to set up equipment to collect natural seedlings of scallops Mizuhopecten yessoensis, which is an important species in fisheries [24,25]. Immunostaining is an extremely effective method for rapidly identifying the number of larvae; therefore, in this study, a polyclonal antibody against D. fragum was produced to estimate the number of D. fragum larvae accurately, and its specificity was verified. Furthermore, an antibody against D. fragum was tested for utilization by immunostaining against snail larvae collected in the sea.
2. Materials and Methods
2.1. Collection of D. fragum and D. cornus Larvae in Aquarium
Fifteen adult D. fragum were collected in Nishidomari, Otsuki-town, Kochi, on 13 June 2016 (Figure 1). Drupella fragum were reared in an aquarium measuring 21×21×21 cm with a 100 µm mesh net on the end of the drain pipe. Drupella fragum laid egg capsules on the aquarium wall from 4 July 2016 to 1 August 2016. The veliger larvae which hatched from the eggs were trapped by a net attached to the drainpipe of the aquarium. All the larvae were 1-day post-hatch (dph). The collected larvae were washed with filtered seawater and divided into two groups. One group of larvae was fixed in 10% formalin in filtered seawater at 4 °C and the other group was stored at −30 °C until use.
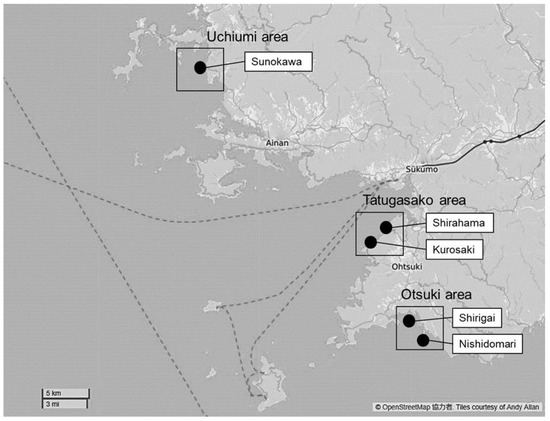
Figure 1.
Location of the research site in Southwest Shikoku. Corals inhabited all stations. Drupella fragum and D. cornus adults were collected at Nishidomari for antigens. The snail larvae were sampled at all stations. Each station was classified as follows: Uchiumi area as Sunokawa, Tatugasako area as Shirahama, and Kurosaki and Otuki area as Shirigai and Nishidomari.
Fifteen adult D. cornus were collected in Nishidomari, Otsuki-town, Kochi, on 25 November 2016. Drupella cornus were reared, and the larvae were collected following the same method as D. fragum.
2.2. Preparation of Antigen and Production of Antibody
Drupella fragum were washed with ice-cold PBS and homogenized with a potter-type homogenizer and ultrasonic cell disruptor (Misonix) in PBS. Thereafter, the samples were centrifuged, and supernatants were collected for antigen testing. The concentration of the supernatant was measured by Protein Assay Rapid Kit Wako II (Wako) with BSA standard and diluted to 1 mg/mL with PBS. The diluted supernatants were stored at −30 ˚C. The production of antibodies was requested from IBL (Immuno-Biological Laboratories Co., Ltd., Fujioka-Shi, Japan).
2.3. Immunohistochemistry
After fixation, D. fragum and D. cornus larvae were immunostained by the following method: the larvae were washed three times in PBS, including 0.1% Polyoxyethylene Sorbitan Monolaurate (Tween 20) for 5 m. Then, the larvae were blocked in 2% Normal Goat Serum in PBS (2% NGS/PBS) at room temperature for 30 m on a shaker. Afterward, the larvae were incubated for 2 h at room temperature on a shaker with the primary antibody solutions diluted in 2% NGS/PBS and were washed 3 times in PBS for 10 m. The primary antibody was detected with alkaline phosphatase-conjugated anti-rabbit antibody (Invitrogen) and NBT/BCIP solution (Sigma).
2.4. Measurement of the Floating D. fragum Larvae in the Field
Sampling of the larvae in the field was performed once a month for one year at five spots chosen from areas in Southwest Shikoku inhabited by coral: Sunokawa, Shirahama, Kurosaki, Shirigai, and Nishidomari (Figure 1) in 2016.
The larvae were collected using a 100 µm mesh plankton net pulled 50 m horizontally on the water’s surface. The collected planktons were fixed in 10% formalin seawater. The snail larvae were sorted under a stereomicroscope and were immunostained using the methods above. After immunostaining, the snail larvae that showed strong signals were considered the number of D. fragum.
2.5. Distribution of Coral and Corallivorous Gastropods at the Sampling Stations
The coral coverage and the habitat status of D. fragum were monitored in January 2016. The monitoring was conducted by snorkeling at each station for about 15 m. The coral coverage was determined in 10% increments of the coral inhabiting the stations. Drupella fragum was observed at each station. If a group of D. fragum had formed, the number of individuals in the group was estimated.
3. Results
3.1. Confirmation of the Antibody Specificity
The immunostaining results of the antibody in this study are shown in Table 1. The immunostained D. fragum larvae and D. cornus showed differences in staining patterns. The staining strength was classified into strong signal, weak signal, and no signal (Figure 2). At all antibody concentrations, more than 90% of D. fragum larvae showed strong signals. The larvae of D. cornus showed strong signals at all antibody concentrations; however, the ratio of individuals showing strong signals was lower than the weak signals.

Table 1.
Antibody specificity analyses against D. fragum larvae and D. cornus larvae.
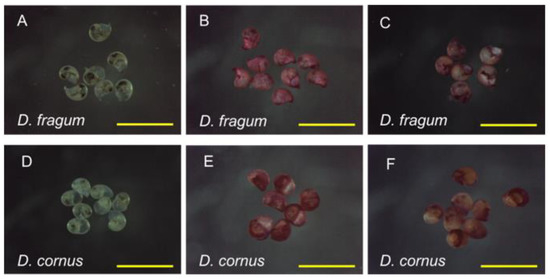
Figure 2.
Classification based on signal strength. The larvae showing strong signals were stained red in their whole body. The larvae showing weak signals were stained light pink or brown in their whole body or stained only around the mouth. The larvae that did not stain were categorized into no signal. (A) Drupella fragum before immunostaining. (B) Strong signal D. fragum larvae. (C) Weak signal D. fragum larvae. (D) Drupella cornus before immunostaining. (E) Strong signal D. cornus larvae. (F) Weak signal D. cornus larvae. Scale bar 500 µm.
The larvae of D. fragum and D. cornus were immunostained with the antibody at various concentrations. For all immunostained reactions, the number of larvae was more than 100 individuals. Depending on the signal strength, it was classified into three categories: Strong signal (Figure 2B,E), Weak signal (Figure 2C,F), and No signal.
3.2. The Change in the Number of Floating Larvae of Snail and D. fragum in the Field
Samples collected at each station were immunostained, and the larvae that showed strong signals were counted as D. fragum. The larvae collected in Shirigai in July are shown in Figure 3. The change in the number of the floating larvae of all snails and D. fragum in the Otsuki area in 2016 is shown in Figure 4. The average number of snails at Nishidomari and Shirigai was low in the period from January to May, and no snails were detected in Shirigai in February, April, and May (Figure 4A). The number of snails increased from June to July in both stations and decreased in August. After September, the average number of snails in Nishidomari was more than 74.89/m3 ± 14.54. The number of snails in Shirigai increased from September to October and then decreased to 20.12/m3 ± 1.65 in December.
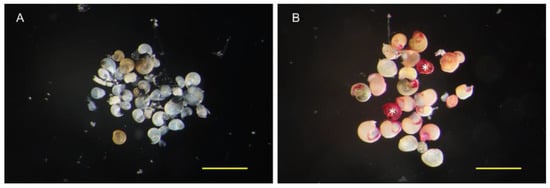
Figure 3.
Immunostaining of the larvae collected in the field. The larvae collected in Shirigai in July are shown in this figure. The larvae before immunostaining (A) and after immunostaining (B) are shown. The larvae classified into the strong signal group are marked with asterisks (B). Scale bar 500 µm.
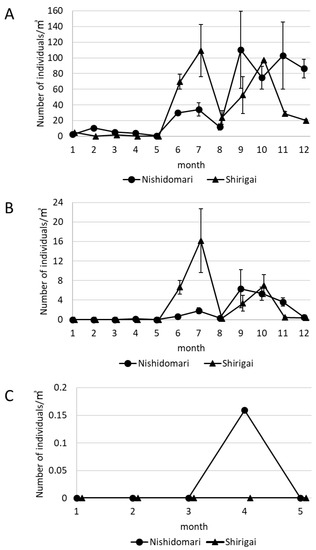
Figure 4.
The change in the number of the total snail larvae and D. fragum larvae in the Otsuki area in 2016. (A) The change in the number of the total snail larvae in the Otsuki area in 2016. (B) The change in the number of D. fragum larvae in the Otsuki area in 2016. (C) The change in the number of D. fragum larvae in the Otsuki area from January to May 2016. The larvae were collected three times in one sampling from June to December (A–C). Error bars indicate standard error (n = 3).
Drupella fragum larvae observed from January to May were 0.16/m3 in Nishidomari in April (Figure 4B,C). The number of D. fragum increased from June to July in both stations, and D. fragum larvae collected in Shirigai in July were the highest (16.20/m3 ± 6.57). The number of D. fragum in both stations decreased in August. Drupella fragum larvae in Nishidomari increased to 6.33/m3 ± 3.86 in September and then gradually decreased. Drupella fragum larvae in Shirigai increased from September to October and decreased after November.
The change in the number of the floating snail larvae and D. fragum larvae in the Tatugasako area in 2016 is shown in Figure 5. The number of snails is approximately 20/m3 in Kurosaki until June, and the number of snails is approximately 20/m3 in Shirahama until May (Figure 5A). The number of snails increased in Kurosaki in July, in Shirahama from June to July, and the number of snails in both stations decreased in August. In Kurosaki, the number of snails increased from September to October, and the average number of snails in October was 49.20/m3 ± 5.81. Furthermore, the larvae in Kurosaki decreased from November. In Shirahama, the snail larvae collected in September were 77.49/m3 ± 31.01 and gradually decreased from October.
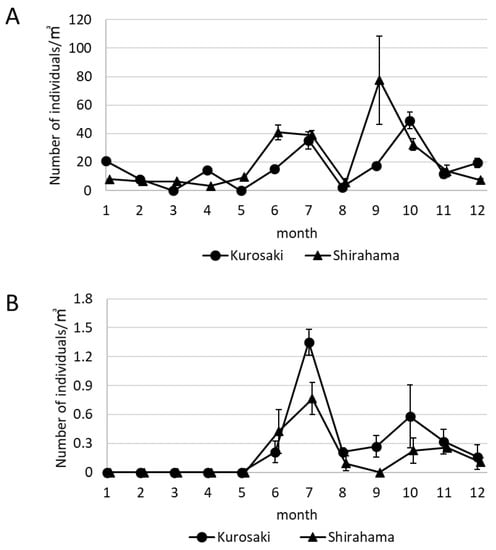
Figure 5.
The change in the number of the total snail larvae and D. fragum larvae in the Tatugasako area in 2016. (A) The change of the number of the total snail larvae in the Tatugasako area in 2016. (B) The change of the number of D. fragum larvae in the Tatugasako area in 2016. The larvae were collected three times in one sampling from June to December (A,B). Error bars indicate standard error (n = 3).
Drupella fragum larvae were not observed in either station from January to May (Figure 5B). The number of D. fragum in both stations increased from June to July, the average number of D. fragum in July in Kurosaki was 1.30/m3 ± 0.13, and the average number of D. fragum in July in Shirahama was 0.77/m3 ± 0.16. Drupella fragum larvae in both stations decreased in August. The number of D. fragum in Kurosaki increased from September to October, gradually decreased from November, and the average number of D. fragum in December was 0.11/m3 ± 0.04. Drupella fragum larvae decreased to 0/m3 in September, and the number of individuals from October was less than 0.30/m3.
The change in the number of floating snail larvae and D. fragum larvae in Sunokawa in the Uchiumi area in 2016 are shown in Figure 6. The average number of snails was low from January to April (Figure 6A). The number of snails increased from May to June and decreased to 1.75/m3 ± 0.15 in July. The number of snails increased from August to September, and the average number of snails in September was 44.32/m3 ± 10.96. The snail larvae gradually decreased after October.
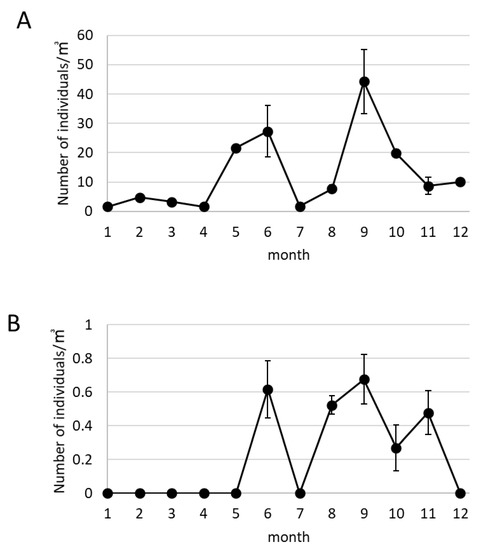
Figure 6.
The change of the number of the total snail larvae and D. fragum larvae in the Uchiumi area in 2016. (A) The change in the number of the total floating snail larvae in the Tatugasako area in 2016. (B) The change in the number of D. fragum larvae in the Uchiumi area in 2016. The larvae were collected three times in one sampling from June to December (A,B). Error bars indicate standard error (n = 3).
The D. fragum larvae did not appear from January to May (Figure 6B). Drupella fragum larvae collected was 0.62/m3 ± 0.17 in June and 0/m3 in July. From August to November, the number of D. fragum remained between 0.27/m3 ± 0.14 and 0.68/m3 ± 0.15, and 0/m3 in December.
3.3. The Distribution of Coral and D. fragum at the Sampling Stations
The coral coverage in Nishidomari was approximately 20%, comprised mostly of genus Acropora. The coral coverage in Shirigai was approximately 20%, and the species of the coral includes not only Acroporidae, but also corals of other groups such as Poritidae and Merulinidae. The coral coverage in Kurosaki was approximately 20%, and Pavona decussate occupied most of the coverage. The corals of Acroporidae were hardly observed. The coral coverage in Shirahama was approximately 30%, and P. decussate made up most of the percentage. The corals of Acroporidae were hardly observed. The coral coverage in Sunokawa was 40%, and most corals were of the genus Acropora.
Groups of D. fragum consisting only of 1–10 individuals were found in Nishidomari, Shirigai, and Sunokawa. In Kurosaki and Shirahama, groups of D. fragum were not found.
4. Discussion
Antibody specificity analysis showed that more than 90% of the D. fragum larvae showed strong signals at all antibody concentrations. Among them, antibodies with concentrations of 1/5000 and 1/10,000 strongly stained D. fragum larvae, with a probability higher than 98%. Among these two concentrations of antibodies, the cross-reactivity against D. cornus of the antibody diluted 1/10,000 was low (5.1%). Therefore, the antibody with the concentration of 1/10,000 can detect D. fragum larvae with the highest probability among the three concentrations of antibodies. It was proven that the antibody diluted to 1/10,000 has high specificity against D. fragum larvae. In addition, D. cornus has been reported in outbreaks in coral communities worldwide [10,13]; however, it has not yet been reported in non-coral reef areas such as Miyazaki and Southwestern Shikoku. Hence, outbreaks of D. fragum are the utmost concern at present. Therefore, even though there were a few cross-reactions, we believe that the antibody in this study is sufficient for predicting outbreaks of D. fragum larvae in this area.
There was a seasonal change in the number of larvae, including D. fragum, collected in the field. The number of snail larvae was low in spring and winter when the water temperature was low and increased in summer when the water temperature was high. Particularly, many snail larvae appeared when the water temperature increased sharply from June to July. The appearance of D. fragum larvae was similar to other snail larvae. The egg-laying season of D. fragum in Ainan-town, Ehime, was reported to be from September to December by visual inspection in the field [19]. The D. fragum larvae floating in the sea should be observed from October to January in Ainan because the larvae take one month to hatch from eggs [23]. However, in our observation, D. fragum larvae were observed at all stations within the year from June. In other words, the season for laying eggs of D. fragum appears to be longer than reported. It was thought that the previous research reports were not able to find egg capsules because D. fragum lays egg capsules in the gaps of branches and the back of dead corals [2]. Furthermore, D. fragum larvae in this area appeared mainly from June to December, and only a few D. fragum larvae appeared in April and only in Nishidomari. This result shows that D. fragum lay eggs in March. Drupella fragum is known to breed annually in Okinawa, which is more southward than Shikoku [20]. These facts suggest that Drupella fragum lays eggs throughout the year if the seawater temperature is high enough. However, in this study, D. fragum larvae, which seems to have hatched in March when the seawater temperature was low, was observed. This phenomenon was observed only at one place and was not continuously observed in subsequent investigations. In other words, as in Okinawa, it does not necessarily mean that Drupella fragum lays eggs year-round in Shikoku; it is thought that the spawning occurred suddenly. For example, D. fragum, which inhabits coves where the water temperature can suddenly rise, lay eggs, and the D. fragum larvae hatched in this cove are dispersed and appear at various stations. In the future, we plan to expand not only the stations for sampling floating larvae but also the area surrounding Shikoku and utilize them for the measurement of D. fragum larvae. Furthermore, it is necessary to confirm whether Drupella fragum has the ability to lay eggs at low water temperature by observation of the gonads.
The number of D. fragum larvae differed depending on the station. The number of larvae in Nishidomari and Shirigai was higher than the number of larvae in other stations. At these two stations, the groups of adult D. fragum were observed visually. However, even in the areas where larvae were observed, D. fragum adults were not observed. The reason for this may be the limitations of the method in confirming the inhabitation of D. fragum adults by visual investigation. However, we also believe that a major reason for this result was the recruitment from other areas with a dense population of D. fragum adults. In the future, in order to clarify the sources of D. fragum larvae, it is necessary to clarify the close relationship between larvae and adults in various places by investigating ocean currents and conducting DNA analyses of adults and larvae sampled in various places.
Large groups of D. fragum were confirmed around the sampling point in Shirigai in September 2016. The amount is presumed to be large, as one diver collected about 1485 individuals with an extermination activity of about 1 hour. This number is comparable to the baseline level of an outbreak. Extermination of crown-of-thorns starfish was carried out several years ago at Shirigai station because its coral community was damaged by crown-of-thorns starfish. Extermination efforts focused mostly on crown-of-thorns starfish, as the number of corallivorous gastropods in recent years in Shirigai was low. A D. fragum outbreak in Shirigai resulted in damage to the corals that had survived the feeding damage by the crown-of-thorns starfish. The concentration of D. fragum larvae in July was the highest of the experimental period at all stations. Considering the growth rate of snails, it is not possible that the D. fragum larvae hatched in July developed into the adult snails that were exterminated in September. However, it is certain that sexually mature D. fragum, which may have contributed to the increase in the number of D. fragum larvae in July, exist in Shirigai. The increase in the number of D. fragum may have been overlooked due to the focus on exterminating crown-of-thorns starfish. However, since it is possible that the larvae may have been recruited from other stations, it is necessary to closely investigate the relationship between the collected samples and the increase in the number of D. fragum larvae and adults living near the sampling stations.
5. Conclusions
In this study, a tool for rapid and low-cost monitoring of D. fragum larvae has been developed. The results of this research provide an effective method for the conservation of coral communities. Although further investigation is necessary to analyze the relationship between the number of D. fragum larvae and subsequent outbreaks, this antibody can predict the number of mature individuals capable of producing the next generation. In the future, we will clarify the reproductive cycle of D. fragum by comparing the number of larvae with the developmental stage of the gonads of adult individuals to determine the relationship between the number of larvae and outbreaks. Furthermore, we expect to detect the beginning of future outbreaks successfully.
Author Contributions
Conceptualization, T.K., T.I., C.M. and T.M.; Data curation, T.M.; Formal analysis, C.M.; Investigation, T.K., T.I., Y.S. and C.M.; Methodology, T.I.; Project administration, T.M.; Supervision, T.M.; Writing—original draft, T.K.; Writing—review and editing, T.I. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Fritzie T. Celino-Brady, Department of Human Nutrition, Food, and Animal Sciences, University of Hawaii, USA, for critically reading an early draft of the manuscript. We would like to express our sincere thanks to Junko Fukada and the staff of Kuroshio Biological Research Foundation for allowing us to use the facility and for the valuable advice on our research. The authors are also thankful for all the Otsuki Park volunteers, Ainan Sango Wo Mamoru Kyougikai (The council of the protection corals in Ainan), and the Ainan town office, who provided information on coral habitat and exterminated corallivorous gastropods as samples. We are also grateful to the students and lab members of the Laboratory of Fish Reproductive Physiology for their support and technical assistance. Finally, we would like to thank the two anonymous referees and the Guest Editors of this Special Issue for their useful suggestions to improve the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schuhmacher, H. Impact of some corallivorous snails on stony corals in the Red Sea. In Proceedings of the 7th International Coral Reef Symposium, Mangilao, GU, USA, 21–26 June 1993; Volume 2, pp. 840–846. [Google Scholar]
- Turner, S.J. The biology and population outbreaks of the corallivorous gastropod Drupella on Indo-Pacific reefs. Oceanogr. Mar. Biol. Ann. Rev. 1994, 32, 461–530. [Google Scholar]
- Miller, M.W. Corallivorous snail removal: Evaluation of impact on Acropora palmata. Coral Reefs 2001, 19, 293–295. [Google Scholar] [CrossRef]
- Schuhmacher, H.; van Treeck, P.; Eisinger, M.; Paster, M. Transplantation of coral fragments from ship groundings on electro-chemically formed reef structures. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; Volume 2, pp. 983–990. [Google Scholar]
- Baums, I.B.; Miller, M.W.; Szmant, A.M. Ecology of a corallivorous gastropod, Coralliophila abbreviata, on two scleractinian hosts. I: Population structure of snails and corals. Mar. Biol. 2003, 142, 1083–1091. [Google Scholar] [CrossRef]
- Fujioka, Y.; Yamazato, K. Host selection of some Okinawan coral associated gastropods belonging to the genera Drupella, Coralliophila and Quoyula. Galaxea 1983, 2, 59–73. [Google Scholar]
- Fuad, A.; Malki, H.; Saber, A. Prey Selection and Feeding Rates of Drupella cornus (Gastropoda: Muricidae) on Corals from the Jordanian Coast of the Gulf of Aqaba, Red Sea. Jordan J. Biol. Sci. 2011, 4, 191–198. [Google Scholar]
- Scott, C.M.; Mehrotra, R.; Hein, M.Y.; Moerland, M.S.; Hoeksema, B.W. Population dynamics of corallivores (Drupella and Acan-thaster) on coral reefs of Koh Tao, a diving destination in the Gulf of Thailand. Raffles Bull. Zool. 2017, 65, 68–79. [Google Scholar]
- Lam, K.; Shin, P.K.S.; Hodgson, P. Severe bioerosion caused by an outbreak of corallivorous Drupella and Diadema at Hoi Ha Wan Marine Park, Hong Kong. Coral Reefs 2007, 26, 893. [Google Scholar] [CrossRef]
- Turner, S.J. Spatial variability in the abundance of the corallivorous gastropod Drupella cornus. Coral Reefs 1994, 13, 41–48. [Google Scholar] [CrossRef]
- Johnson, M.S.; Cumming, R.L. Genetic distinctness of three widespread and morphologically variable species of Drupella (Gas-tropoda, Muricidae). Coral Reefs 1995, 14, 71–78. [Google Scholar] [CrossRef]
- Cumming, R.L. Predation on reef-building corals: Multiscale variation in the density of three corallivorous gastropods, Drupella spp. Coral Reefs 1999, 18, 147–157. [Google Scholar] [CrossRef]
- Black, R.; Johnson, M.S. Growth rates in outbreak populations of the corallivorous gastropod Drupella cornus (Röding 1798) at Ningaloo Reef, Western Australia. Coral Reefs 1994, 13, 145–150. [Google Scholar] [CrossRef]
- Moyer, J.T.; Emerson, W.K.; Ross, M. Massive destruction of scleractinian corals by the muricid gastropod Drupella in Japan and the Philippines. Nautilus 1982, 96, 69–82. [Google Scholar]
- Fujioka, Y. Marine Fauna and Muricacean Gastropods of Miyake Island. Chiribotan 1984, 14, 81–86. [Google Scholar]
- Takayama, S.; Shirasaki, S. Record of coral predator Drupella fragum (BLAINVILLE) in Nichinan Coast, Miyazaki Prefecture (in Japanese). Nanki Seibutu 1990, 32, 121–122. [Google Scholar]
- Koido, T.; Oku, Y.; Fukuda, M.; Nakano, A.; Fukami, H. Drupella outbreak in a large coral community off the coast of Cape Toi, Miyazaki, Japan. Galax- J. Coral Reef Stud. 2017, 19, 31–32. [Google Scholar] [CrossRef][Green Version]
- Nomura, K. Himeshiroreisigaidamasi no daihassei (in Japanese). DIVER 1991, 12, 14–16. [Google Scholar]
- Ishikawa, K.; Suga, H.; Ishikawa, H.; Morikawa, K. On the Scleractinian Coral Feeding Gastropods in the Uwa Sea, the South-west Shikoku: On the Ecology of the Scleractinian Coral Feeding Gastropods(in Japanese). Annu. Bull. Fac. Humanit. Matsuyama Shinonome Coll 1993, 1, 19–25. [Google Scholar]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V.C. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Chang. Biol. 2006, 12, 2220–2234. [Google Scholar] [CrossRef]
- Shimoike, K. Gametogenesis of the corallivorous gastropod (Drupella cornus and D. fragum) and their habitat in Akajima Island (in Japanese). Midoriishi 1995, 6, 12–16. [Google Scholar]
- Shu, Q.S.; Tai, C.T.; Yuichi, P.K.; Chin, S.L.N.; Daisuke, T.; Lutfi, A.R.; Koh, S.T.; Loke, M.C. Egg capsules and veligers of the corallivorous muricid gastropod Drupella rugosa (Born, 1778). Invertebr. Reprod. Dev. 2017, 63, 164–171. [Google Scholar]
- Awakuni, T. Reproduction and Growth of Coral Predators Drupella Fragum and Drupella cornus (Gastropoda: Muricide) [Un-Published]; University of the Ryukyus: Nishihara Town, Japan, 1989. [Google Scholar]
- Shimizu, Y.; Iwai, T.; Takabatake, S.; Kawasaki, T.; Yamashita, M. Production of highly specific polyclonal antibodies to simplify the identification of Japanese scallop Mizuhopecten yessoensis larvae. J. Fish. Technol. 2014, 7, 31–36. [Google Scholar]
- Shimizu, Y.; Kawasaki, T.; Takabatake, S.; Iwai, T.; Yamashita, M. Simplification of the Immunostaining Procedures in Japanese Scallop Mizuhopecten Yessoensis Larvae toward the Spread of Immunostaining Method for Investigating the Larval Distribu-Tion in the Field (Technical Report); Scientific Reports of Hokkaido Fisheries Research Institutes; Hokkaido National Fisheries Research Institute: Sapporo, Japan, 2015; Volume 87, pp. 93–96. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).