Circular Carbon Economy (CCE): A Way to Invest CO2 and Protect the Environment, a Review
Abstract
:1. Introduction
2. Greenhouse Effect
2.1. Greenhouse Gases
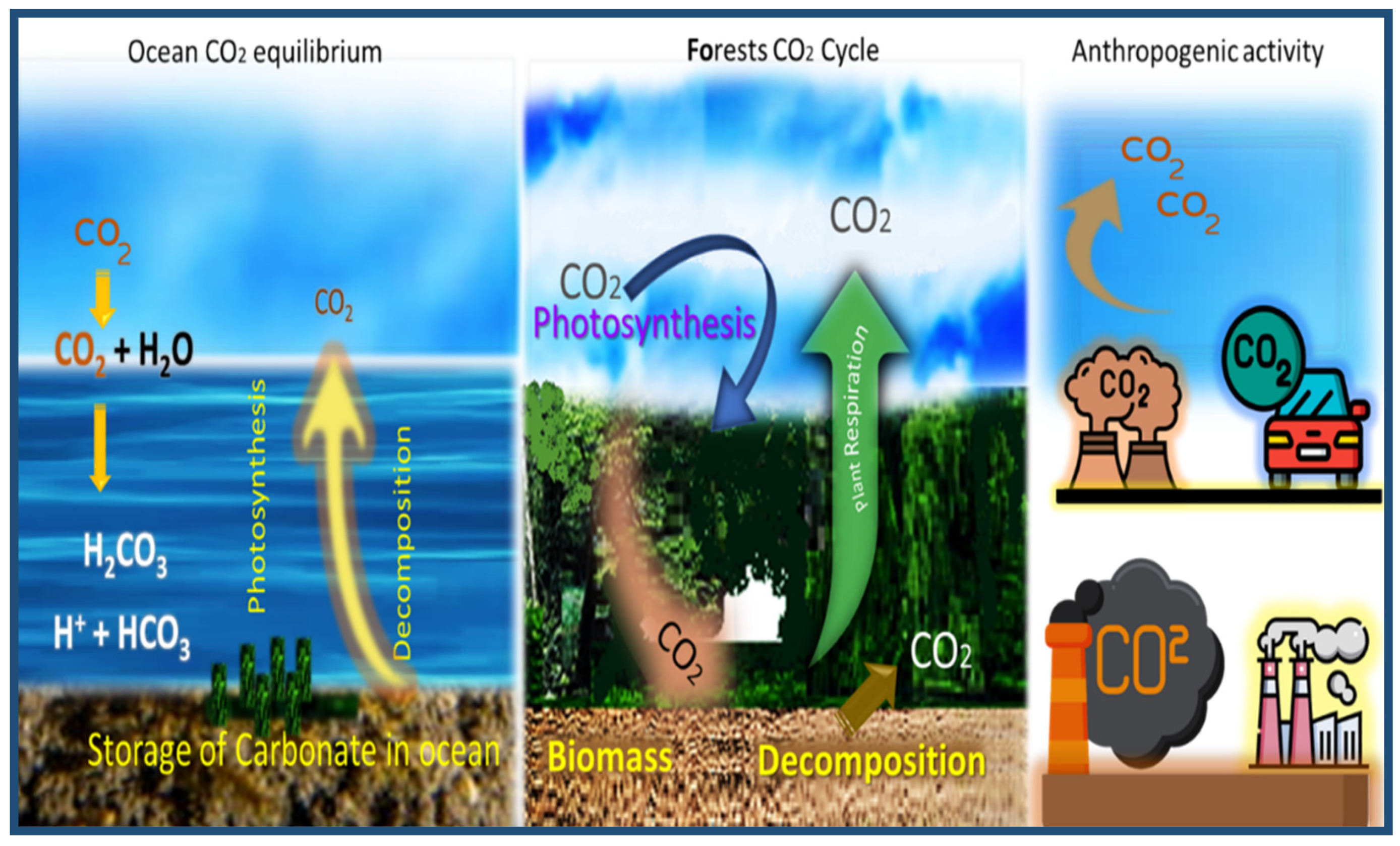
2.2. Carbon Cycle
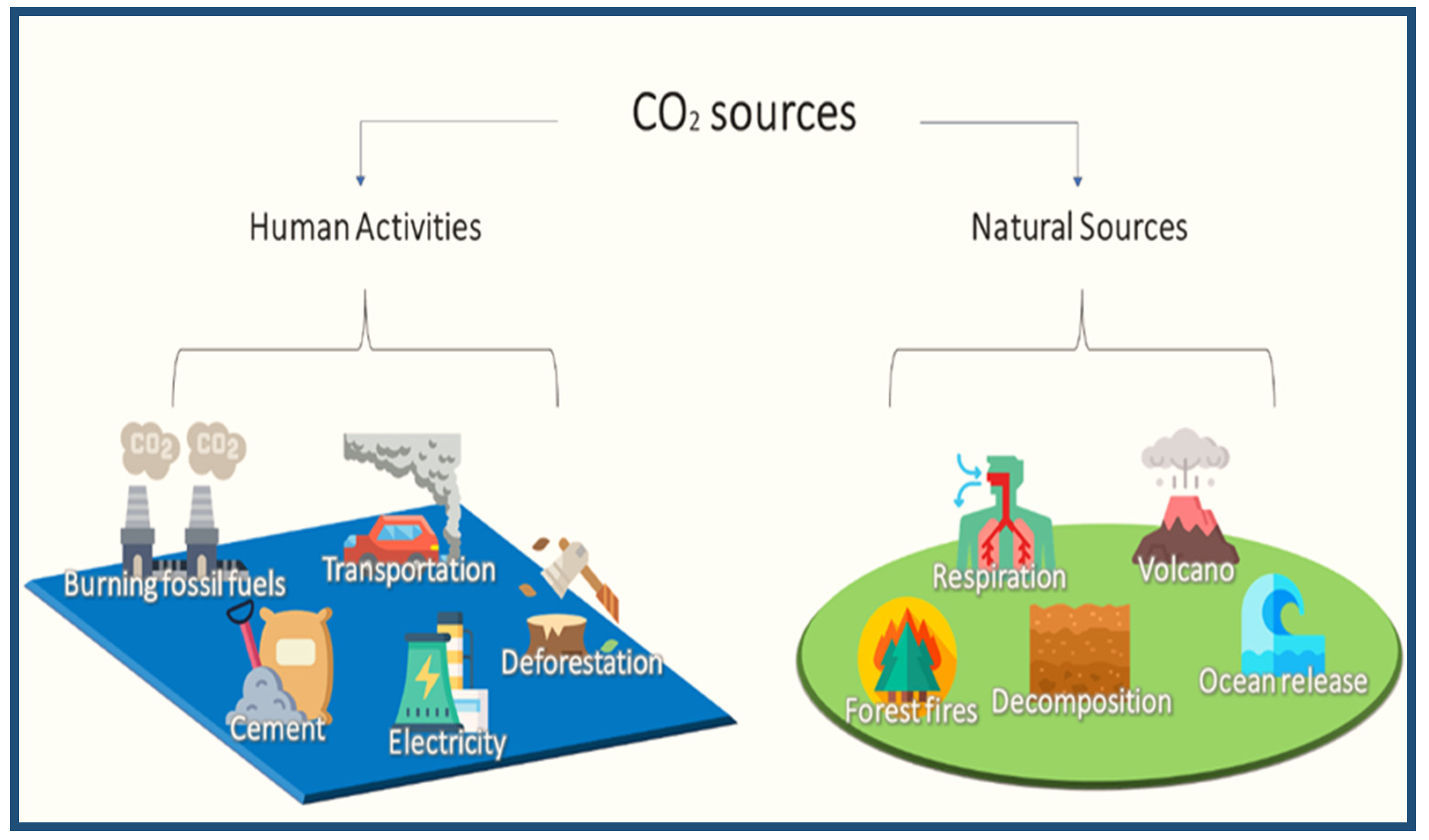
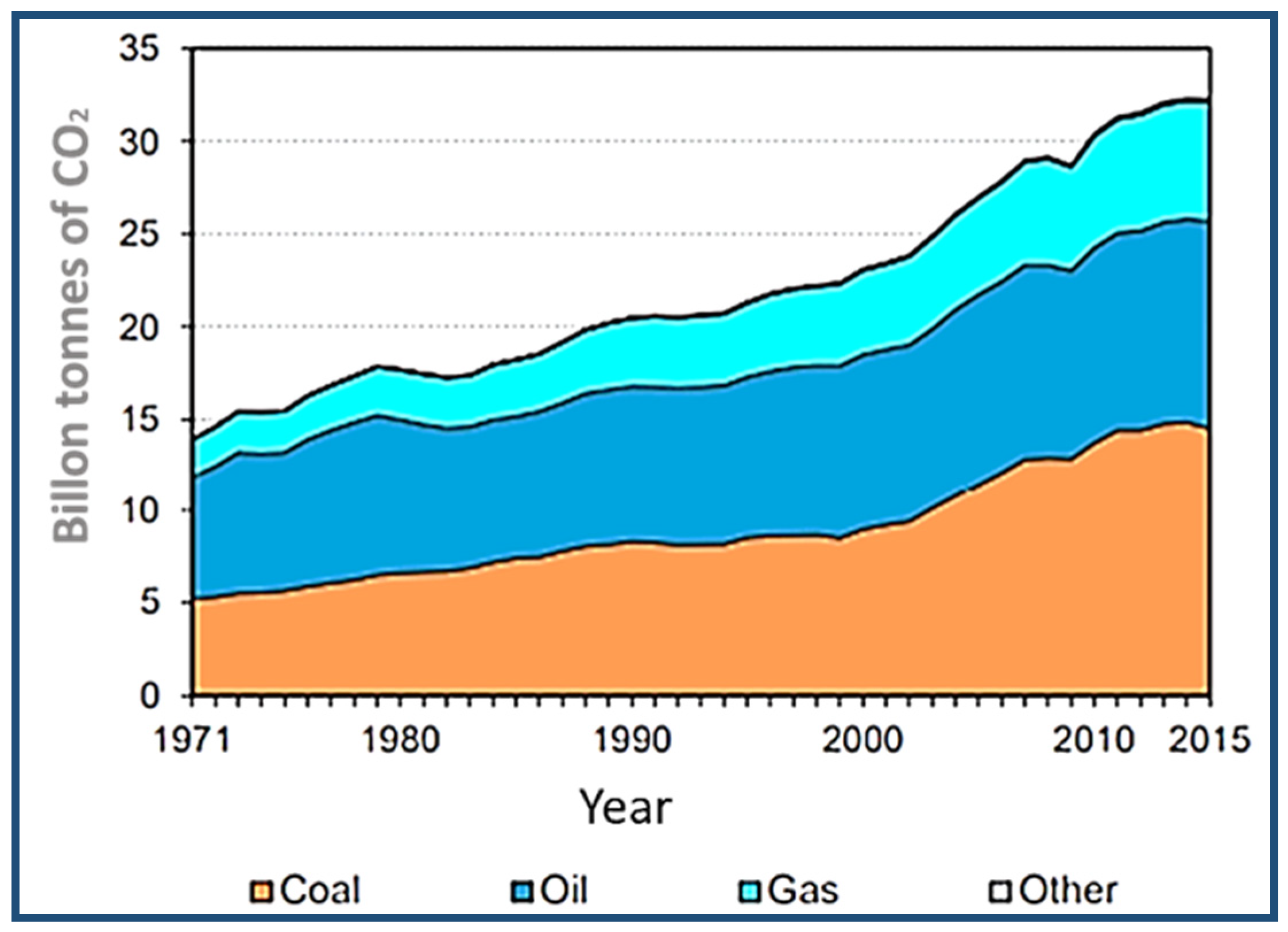
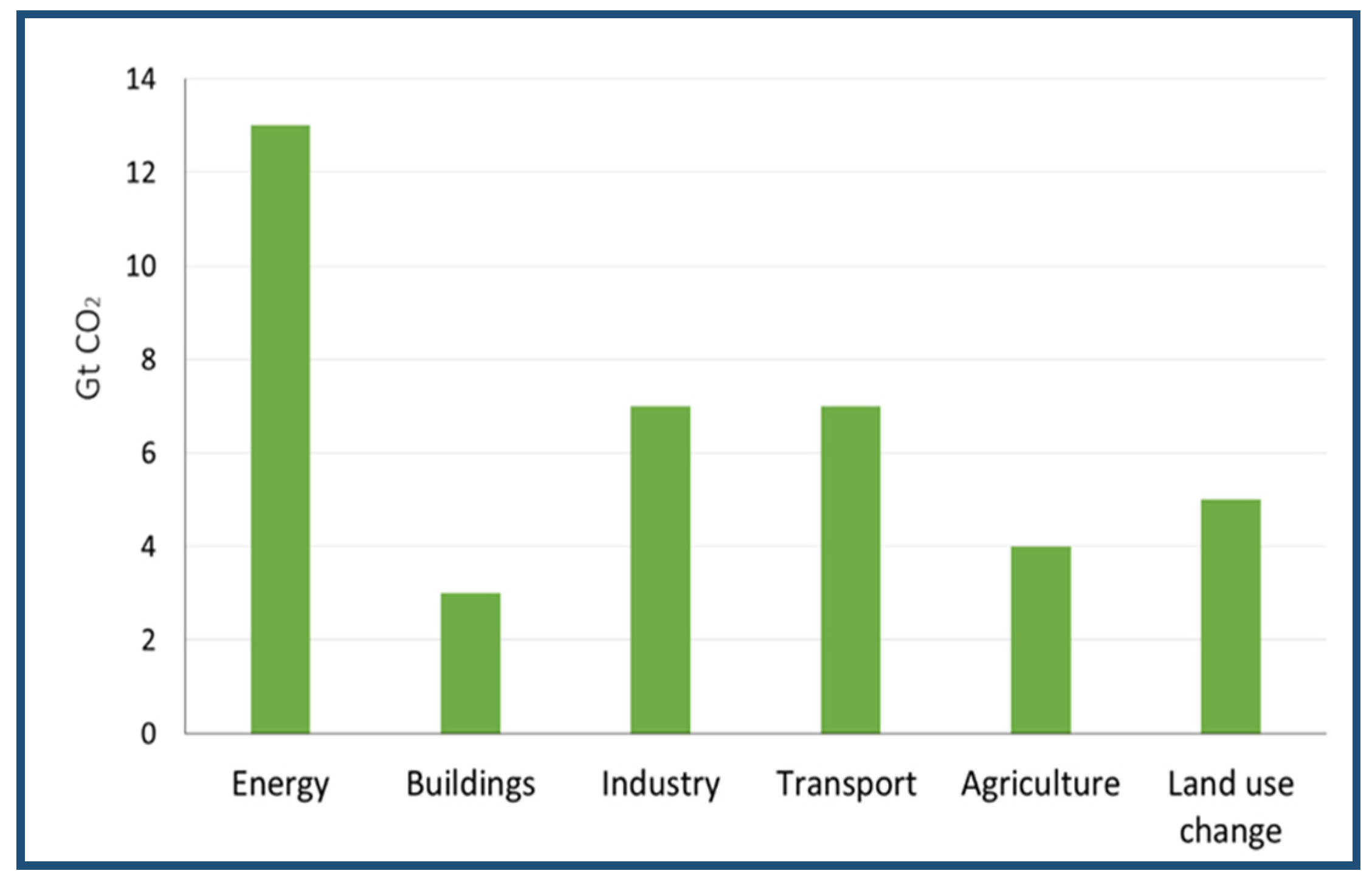
2.3. CO2 Sources
3. The Circular Carbon Economy (CCE)
4. Carbon Capture, Utilisation, and Storage (CCUS)
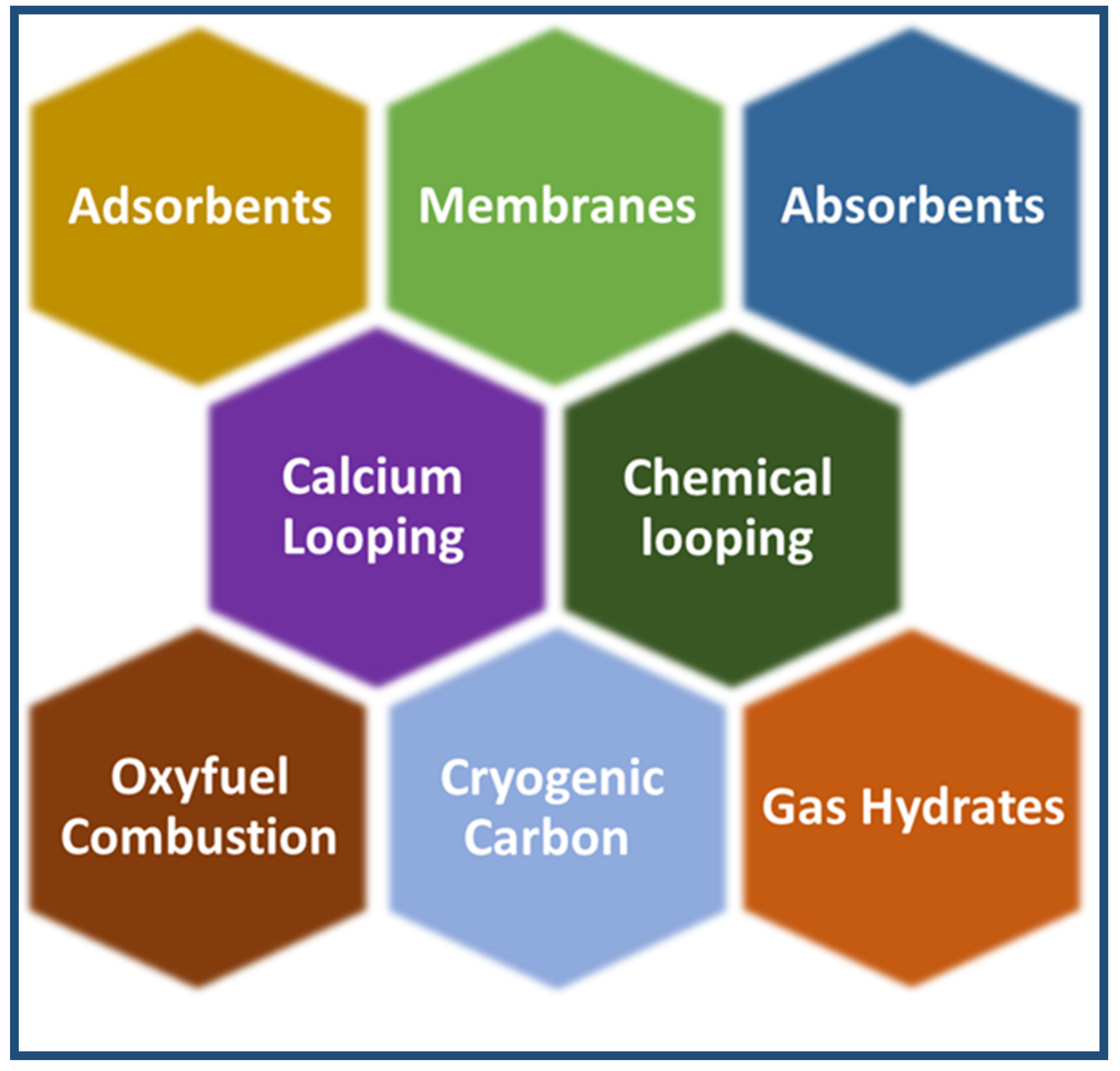
4.1. CO2 Capture
4.2. CO2 Transport
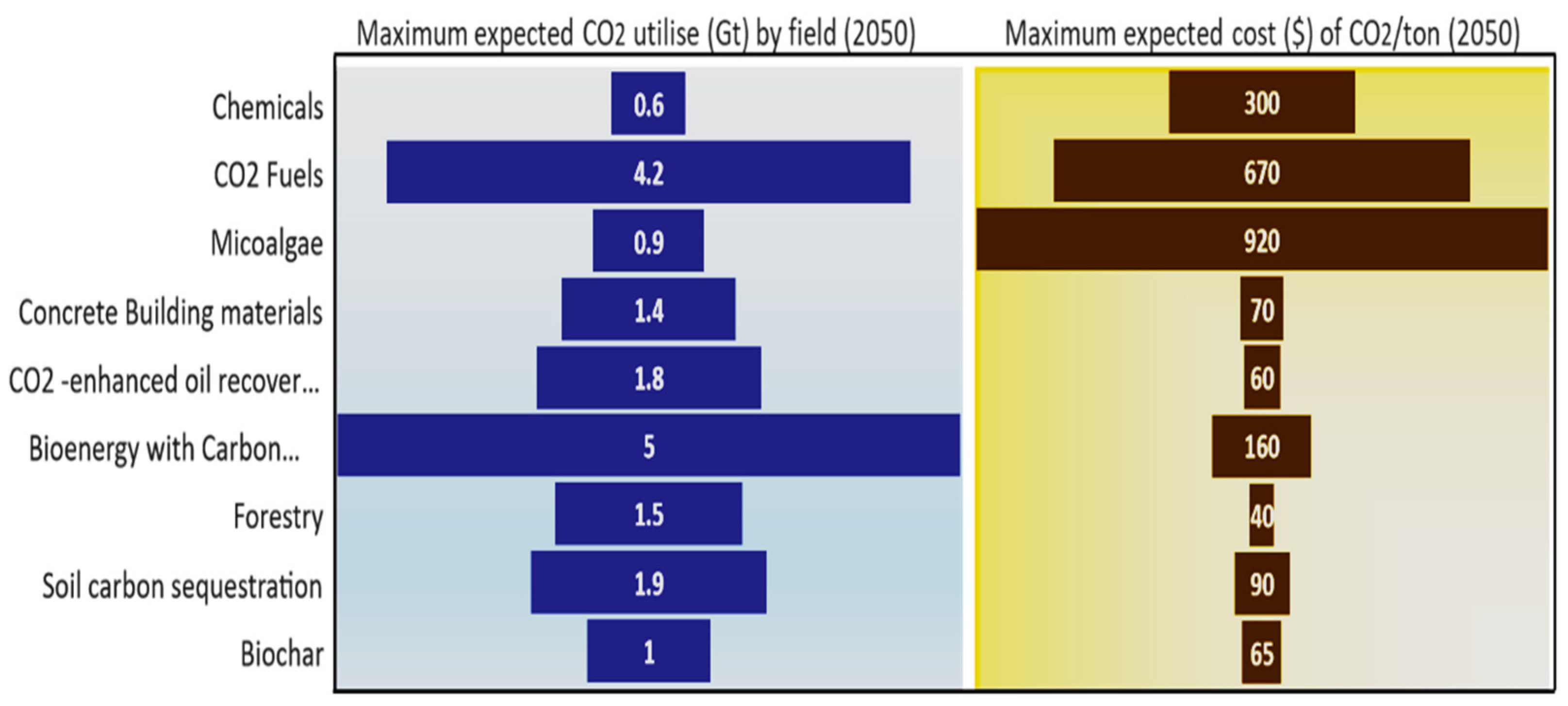
4.3. CO2 Utilisation
4.3.1. Carbon Dioxide—Enhanced Oil Recovery (CO2-EOR)
4.3.2. Synthetic Fuel
4.3.3. Chemical Industry
4.3.4. Concrete Building Materials
4.3.5. Microalgae
4.3.6. Bioenergy Carbon Capture and Storage (BECCS) Trees
4.4. CO2 Storage
4.4.1. CO2 Storage in a Geological Formation
4.4.2. CO2 Mineralisation
4.4.3. Ocean Storage of CO2
5. Current and Future Technology
5.1. Fossil Fuel Alternatives
5.2. Processing Building Materials
5.2.1. Steel Production and Use
5.2.2. Cement and Concrete Production and Use
5.2.3. Asphalt Production and Paving
5.2.4. Heavy Transport
5.2.5. Construction Process
5.3. Hydrogen Technologies and Fuel Cells
- (I)
- Hydrogen is used as a secondary value of energy. It uses the excess electrical energy generated from renewable energy sources to produce hydrogen via electrolysis, which also works in storing renewable energy.
- (II)
- The employment of fuel cells as significant energy providers, for transport and fixed energy sources.
- (III)
- Natural gas networks may be converted to hydrogen-based networks, dramatically reducing carbon dioxide emissions into the atmosphere [106].
5.4. Planting Trees and Mangrove Forests
5.5. Electric Transport
6. The Application That Involves CO2 and Its Economic Impact
| Pathway | Utilisation Potential (Mt of CO2 per Year) | The Cost of CO2 Utilisation (USD) per Year | Ref. |
|---|---|---|---|
| Total industries | 8500 | 3,544,500 million | [117] |
| Chemicals | 180 | 75,060 million | [119] |
| Fuels | 2000 | 834 billion | [119] |
| (EOR) | 70–80 | 29,190–33,360 million | [115] |
| Food | 8 | 3336 million | [122] |
| Carbonated drinks | 2.9 | 12,093.3 million | [123] |
| Pathway | Utilisation Potential CO2 per a Year | The Cost of CO2 Utilisation (USD) | Ref. |
|---|---|---|---|
| Urea | 130 Mt | 54,210 million | [115] |
| Methanol | 10.0 Mt | 4170 million | [123] |
| Cyclic carbonates | 0.04 Mt | 16,680 thousand | [124] |
| Dimethyl ether | 5.00 Mt | 2085 million | [123] |
| Ethylene carbonate | 0.04 Mt | 16,680 thousand | [124] |
| Di-methyl carbonate | 0.04 Mt | 16,680 thousand | [124] |
| Copolymers | 0.04 Mt | 16,680 thousand | [124] |
| Polymers | 1.50 Mt | 6255 million | [123] |
| Fine chemicals | 0.04 Mt | 16,680 thousand | [124] |
| Salicylic acid | 0.03 Mt | 12,510 thousand | [124] |
| Formaldehyde | 0.90 Mt | 3753 million | [123] |
| Formic acid | 5.00 Mt | 2085 million | [123] |
7. Socioeconomic Impact
8. The Future of CCUS
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grim, R.G.; Huang, Z.; Guarnieri, M.T.; Ferrell, J.R.; Tao, L.; Schaidle, J.A. Transforming the carbon economy: Challenges and opportunities in the convergence of low-cost electricity and reductive CO2 utilisation. Energy Environ. Sci. 2020, 13, 472–494. [Google Scholar] [CrossRef]
- Rizos, V.; Egenhofer, C.; Elkerbout, M. Circular Economy for Climate Neutrality: Setting the Priorities for the EU. CEPS Policy Brief 2019. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3493573 (accessed on 1 September 2021).
- DiChristopher, T. EPA Chief Scott Pruitt Says Carbon Dioxide Is Not a Primary Contributor to Global Warming. CNBC 2017. Available online: https://www.cnbc.com/2017/03/09/epa-chief-scott-pruitt.html (accessed on 1 September 2021).
- Res, C.; April, P.; Idso, S.B. CO2-induced global warming: A skeptic’s view of potential climate change. Clim. Res. 1998, 10, 69–82. [Google Scholar]
- Covey, K.; Soper, F.; Pangala, S.; Bernardino, A.; Pagliaro, Z.; Basso, L.; Cassol, H.; Fearnside, P.; Navarrete, D.; Novoa, S.; et al. Carbon and beyond: The biogeochemistry of climate in a rapidly changing Amazon. Front. For. Glob. Chang. 2021, 4, 11. [Google Scholar]
- Maghrabi, A.; Almutairi, M.; Aldosari, A.; Altilasi, M.; Alshehri, A. Cosmic rays detection in Saudi Arabia: Review of the facilities and preliminarily results. J. King Saud Univ. Sci. 2021, 33, 101495. [Google Scholar] [CrossRef]
- Seppälä, A.; Matthes, K.; Randall, C.E.; Mironova, I.A. What is the solar influence on climate? Overview of activities during CAWSES-II. Eur. J. Nucl. Med. Mol. Imaging 2014, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gray, L.J.; Beer, J.; Geller, M.; Haigh, J.D.; Lockwood, M.; Matthes, K.; Cubasch, U.; Fleitmann, D.; Harrison, G.; Hood, L.; et al. Solar influences on climate. Rev. Geophys. 2010, 48. [Google Scholar] [CrossRef]
- Junior, C.H.L.S.; Pessôa, A.C.M.; Carvalho, N.S.; Reis, J.B.C.; Anderson, L.O.; Aragão, L.E.O.C. The Brazilian Amazon deforestation rate in 2020 is the greatest of the decade. Nat. Ecol. Evol. 2020, 5, 144–145. [Google Scholar] [CrossRef]
- Cramer, W.; Bondeau, A.; Schaphoff, S.; Lucht, W.; Smith, B.; Sitch, S. Tropical forests and the global carbon cycle: Impacts of atmospheric carbon dioxide, climate change and rate of deforestation. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Gomes, V.H.F.; Vieira, I.C.G.; Salomão, R.P.; Ter Steege, H. Amazonian tree species threatened by deforestation and climate change. Nat. Clim. Chang. 2019, 9, 547–553. [Google Scholar] [CrossRef]
- Nwilo, P.C.; Olayinka, D.N.; Okolie, C.J.; Emmanuel, E.I.; Orji, M.J.; Daramola, O.E. Impacts of land cover changes on deserti fi cation in northern Nigeria and implications on the Lake Chad Basin. J. Arid Environ. 2020, 181, 104190. [Google Scholar] [CrossRef]
- Seven Messages about the Circular Economy and Climate Change. World Resour. Forum 2019. Available online: https://www.ovam.be/ (accessed on 1 September 2021).
- Williams, E. Achieving Climate Goals by Closing the Loop in a Circular Carbon Economy; KAPSARC: Riyadh, Saudi Arabia, 2019; pp. 1–13. [Google Scholar]
- Djuričin, S.; Pataki, D.E.; Xu, X. A comparison of tracer methods for quantifying CO2 sources in an urban region. J. Geophys. Res. Space Phys. 2010, 115, 1–13. [Google Scholar] [CrossRef]
- Schmelz, W.J.; Hochman, G.; Miller, K.G. Total cost of carbon capture and storage implemented at a regional scale: Northeastern and midwestern United States: Total Cost of Carbon Capture and Storage. Interface Focus 2020, 10, 20190065. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; Rosa, L.; Mazzotti, M. Life cycle assessment of carbon dioxide removal technologies: A critical review. Energy Environ. Sci. 2021, 14, 1701–1721. [Google Scholar] [CrossRef]
- Zhong, W.; Haigh, J.D. The greenhouse effect and carbon dioxide. Weather 2013, 68, 100–105. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Fasullo, J.T. Tracking Earth’s energy: From El Niño to Global Warming. Surv. Geophys. 2012, 33, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Trenberth, K.E.; Fasullo, J.; Kiehl, J. Earth’s global energy budget. Bull. Am. Meteorol. Soc. 2009, 90, 311–324. [Google Scholar] [CrossRef]
- Morgan, M.R. Climate change 2001. Weather 2004, 59, 217–223. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Renewables, V. Inventory of U.S. greenhouse gas emissions and sinks: 1990–2009. Fed. Regist. 2011, 76, 10026. [Google Scholar]
- WMO; GTW. The State of Greenhouse Gases in the Atmosphere. Greenh. Gas Bull. 2020, 16, 5–8. [Google Scholar]
- Graber, J.; Amthor, J.; Dahlman, R.; Drell, D.; Weatherwax, S. Carbon Cycling and Biosequestration Integrating Biology and Climate through Systems Science Report from the March 2008 Workshop. Available online: https://doi.org/10.2172/948438 (accessed on 1 September 2021).
- Sunquist, E.T.; Broecker, W.S. The carbon cycle and atmospheric CO2. Eos Trans. Am. Geophys. Union 1986, 67, 191. [Google Scholar] [CrossRef]
- Seinberh, M. History of CO2 greenhouse gas mitigation technologies. Energy Convers. Manag. 1992, 33, 311–315. [Google Scholar]
- Ebhota, W.S.; Tabakov, P.Y. Development of domestic technology for sustainable renewable energy in a zero-carbon emission -driven economy. Int. J. Environ. Sci. Technol. 2020, 18, 1253–1268. [Google Scholar] [CrossRef]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular economy: The concept and its limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. Completing the Picture: How the Circular Economy Tackles Climate Change; Ellen MacArthur Foundation: Cowes, UK, 2019; pp. 1–62. [Google Scholar]
- Ma, S.; Hu, S.; Chen, D.; Zhu, B. A case study of a phosphorus chemical firm’s application of resource efficiency and eco-efficiency in industrial metabolism under circular economy. J. Clean. Prod. 2015, 87, 839–849. [Google Scholar] [CrossRef]
- Arabia, S. G20 Drives Joint Efforts to Safeguard the Planet. 2020. Available online: https://www.g20.org/ (accessed on 1 September 2021).
- Arabia, S. G20 Statement on the Circular Carbon Economy; G20 Engagement Groups: New York, NY, USA, 2020; p. 20. [Google Scholar]
- Gas, I.; March, U. G20 Saudi Arabia the Role of Gas Technologies in the Circular Carbon Economy; International Gas Union: Vevey, Switzerland, 2020. [Google Scholar]
- Annual Report 2018: Growing through Transformation. 2018. Available online: https://www.sabic.com/assets/zh/Images/SABIC-AR-English-2018_tcm11-18629.pdf (accessed on 1 September 2021).
- Wich, T.; Lueke, W.; Deerberg, G.; Oles, M. Carbon2Chem®-CCU as a step toward a circular economy. Front. Energy Res. 2020, 7, 162. [Google Scholar] [CrossRef] [Green Version]
- Farabi-Asl, H.; Itaoka, K.; Chapman, A.; Kato, E.; Kurosawa, A. Key factors for achieving emission reduction goals cognizant of CCS. Int. J. Greenh. Gas Control. 2020, 99, 103097. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2020, 761, 143203. [Google Scholar] [CrossRef]
- Netto, A.L.A.; Câmara, G.; Rocha, E.; Silva, A.L.; Andrade, J.C.S.; Peyerl, D.; Rocha, P. A first look at social factors driving CCS perception in Brazil: A case study in the Recôncavo Basin. Int. J. Greenh. Gas Control 2020, 98, 103053. [Google Scholar] [CrossRef]
- Mathieu, P. The IPCC special report on carbon dioxide capture and storage. ECOS 2006. In Proceedings of the 19th International Conference on Efficiency, Cost, Optimisation, Simulation and Environmental Impact of Energy Systems, Aghia Pelagia, Greece, 12–14 July 2006. [Google Scholar]
- CCSA; TUC. The Economic Benefits of Carbon Capture and Storage in the UK. Trades Union Congr. 2014. Available online: https://www.tuc.org.uk/sites/default/files/carboncapturebenefits_1.pdf (accessed on 1 September 2021).
- Olfe-Kräutlein, B. Advancing CCU technologies pursuant to the SDGs: A challenge for policy Making. Front. Energy Res. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Sahebdelfar, S. Catalytic conversions of CO2 to help mitigate climate change: Recent process developments. Process Saf. Environ. Prot. 2021, 145, 172–194. [Google Scholar] [CrossRef]
- Styring, P.; Jansen, D.; de Coninck, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; Centre for Low Carbon Futures: New York, NY, USA, 2011. [Google Scholar]
- Goel, M. Carbon capture and storage technology for sustainable energy. OPEC Bull. 2010, 39, 20–23. [Google Scholar]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilisation and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Global CO2 Initiative. Global Roadmap for Implementing CO2 Utilization; University of Michigan: Ann Arbor, MI, USA, 2016. [Google Scholar]
- Hasan, M.M.F.; First, E.L.; Boukouvala, F.; Floudas, C.A. A multi-scale framework for CO2 capture, utilisation, and sequestration: CCUS and CCU. Comput. Chem. Eng. 2015, 81, 2–21. [Google Scholar] [CrossRef] [Green Version]
- Creamer, A.E.; Gao, B. Carbon Dioxide Capture: An Effective Way to Combat Global Warming; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Rosli, A.; Ahmad, A.L.; Lim, J.K.; Low, S.C. Advances in liquid absorbents for CO2 capture: A review. J. Phys. Sci. 2017, 28, 121–144. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Khdary, N.H.; Ghanem, M.A.; Merajuddine, M.G.; Bin Manie, F.M. Incorporation of Cu, Fe, Ag, and Au nanoparticles in mercapto-silica (MOS) and their CO2 adsorption capacities. J. CO2 Util. 2014, 5, 17–23. [Google Scholar] [CrossRef]
- Baxter, L.; Baxter, A.; Burt, S. Cryogenic CO2 capture as a cost-effective CO2 capture process. Int. J. Hydrog. Energy 2011, 36, 10355–10365. [Google Scholar]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane processes for direct carbon dioxide capture from air: Possibilities and limitations. Front. Chem. Eng. 2021, 3, 17. [Google Scholar] [CrossRef]
- Khdary, N.H.; Abdelsalam, M.E. Polymer-silica nanocomposite membranes for CO2 capturing. Arab. J. Chem. 2020, 13, 557–567. [Google Scholar] [CrossRef]
- Zhou, X.; Zang, X.; Long, Z.; Liang, D. Multiscale analysis of the hydrate based carbon capture from gas mixtures containing carbon dioxide. Sci. Rep. 2021, 11, 9197. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Galvita, V.V.; Poelman, H.; Marin, G.B. Advanced chemical looping materials for CO2 utilisation: A review. Materials 2018, 11, 1187. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Carbon Management: Implications for R&D in the Chemical Sciences and Technology; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Eide, L.I.; Batum, M.; Dixon, T.; Elamin, Z.; Graue, A.; Hagen, S.; Hovorka, S.; Nazarian, B.; Nøkleby, P.H.; Olsen, G.I.; et al. Enabling large-scale carbon capture, utilisation, and storage (CCUS) using offshore carbon dioxide (CO2) infrastructure developments—A review. Energies 2019, 12, 1945. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Tsau, J.-S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Adlen, E.; Hepburn, C. Carbon Capture methods compared: Costs, scalability, permanence, cleanness related posts. Energy Post 2019, 10, 1–8. Available online: https://energypost.eu/10-carbon-capture-methods-compared-costs-scalability-permanence-cleanness/ (accessed on 1 September 2021).
- Ahmad, O. Khowaiter, the Circular Carbon Economy; Saudi Aramco: Dhahran, Saudi Arabia, 2020. [Google Scholar]
- Farajzadeh, R.; Eftekhari, A.A.; Dafnomilis, G.; Lake, L.; Bruining, J. On the sustainability of CO2 storage through CO2—Enhanced oil recovery. Appl. Energy 2020, 261, 114467. [Google Scholar] [CrossRef]
- Alshammari, Y.M. Achieving climate targets via the circular carbon economy: The case of Saudi Arabia. C J. Carbon Res. 2020, 6, 54. [Google Scholar] [CrossRef]
- Sorcar, S.; Hwang, Y.; Lee, J.; Kim, H.; Grimes, K.M.; Grimes, C.A.; Jung, J.-W.; Cho, C.-H.; Majima, T.; Hoffmann, M.R.; et al. CO2, water, and sunlight to hydrocarbon fuels: A sustained sunlight to fuel (Joule-to-Joule) photoconversion efficiency of 1%. Energy Environ. Sci. 2019, 12, 2685–2696. [Google Scholar] [CrossRef] [Green Version]
- Mikhelkis, L.; Govindarajan, V. Techno-economic and partial environmental analysis of carbon capture and storage (CCS) and carbon capture, utilisation, and storage (CCU/S): Case study from proposed waste-fed district-heating incinerator in Sweden. Sustainability 2020, 12, 5922. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Bocin-Dumitriu, A.; Tzimas, E. CO2 utilisation pathways: Techno-economic assessment and market opportunities. Energy Procedia 2014, 63, 7968–7975. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilisation. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Zhang, N.; Duan, H.; Miller, T.R.; Tam, V.W.; Liu, G.; Zuo, J. Mitigation of carbon dioxide by accelerated sequestration in concrete debris. Renew. Sustain. Energy Rev. 2020, 117, 109495. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Yew, G.Y.; Koyande, A.K.; Chew, K.W.; Vo, D.V.N.; Show, P.L. Green technology for the industrial production of biofuels and bioproducts from microalgae: A review. Environ. Chem. Lett. 2020, 18, 1967–1985. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.; Felix, V.; JoselynMonica, M.; Malolan, R. A conceptual review on microalgae biorefinery through thermochemical and biological pathways: Bio-circular approach on carbon capture and wastewater treatment. Bioresour. Technol. Rep. 2020, 11, 100477. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, Z.; Huang, Y.; Huang, L.; Hu, L.; Xu, D.; Zhou, J.; Cen, K. Improving growth rate of microalgae in a 1191m2 raceway pond to fix CO2 from flue gas in a coal-fired power plant. Bioresour. Technol. 2015, 190, 235–241. [Google Scholar] [CrossRef]
- Langholtz, M.; Busch, I.; Kasturi, A.; Hilliard, M.R.; McFarlane, J.; Tsouris, C.; Mukherjee, S.; Omitaomu, O.A.; Kotikot, S.M.; Allen-Dumas, M.R.; et al. The economic accessibility of CO2 sequestration through bioenergy with carbon capture and storage (BECCS) in the US. Land 2020, 9, 299. [Google Scholar] [CrossRef]
- Santori, G.; Charalambous, C.; Ferrari, M.; Brandani, S. Adsorption arti fi cial tree for atmospheric carbon dioxide capture, puri fi cation and compression. Energy 2018, 162, 1158–1168. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Xu, T.; Wang, F.; Diao, Y.; Li, X.; Ma, X.; Tian, H. A study on the CO2-enhanced water recovery efficiency and reservoir Pressure Control Strategies. Geofluids 2019. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Dai, Z.; Xu, T.; Wang, F.; Jia, S. Effects of Dip-Angle on the CO2-Enhanced Water Recovery Efficiency and Reservoir Pressure Control Strategies. 2021. Available online: https://doi.org/10.5194/egusphere-egu2020-13028 (accessed on 1 September 2021).
- Araújo, O.D.Q.F.; De Medeiros, J.L. Carbon capture and storage technologies: Present scenario and drivers of innovation. Curr. Opin. Chem. Eng. 2017, 17, 22–34. [Google Scholar] [CrossRef]
- Pattnaik, R.; Sethi, K.; Das, D. Soil carbon sequestration through various agronomic practices: A way to mitigate climate change impacts. J. Pharmacogn. Phytochem. 2020, 9, 755–761. [Google Scholar]
- IEAGHG. Case Studies of CO2 Storage in Depleted Oil and Gas Fields; IEAGHG: Cheltenham, UK, 2017. [Google Scholar]
- Hannis, S.; Lu, J.; Chadwick, A.; Hovorka, S.; Kirk, K.; Romanak, K.; Pearce, J. CO2 storage in depleted or depleting oil and gas fields: What can we learn from existing projects? Energy Procedia 2017, 114, 5680–5690. [Google Scholar] [CrossRef]
- Kolenković, I.; Saftić, B. Geological storage of carbon dioxide. Rudarsko Geolosko Naftni Zbornik 2014, 28, 9–22. [Google Scholar]
- Maldal, T.; Tappel, I. CO2 underground storage for Snøhvit gas field development. Energy 2004, 29, 1403–1411. [Google Scholar] [CrossRef]
- Mathieson, A.; Midgely, J.; Wright, I.; Saoula, N.; Ringrose, P. In Salah CO2 storage JIP: CO2 sequestration monitoring and verification technologies applied at Krechba, Algeria. Energy Procedia 2011, 4, 3596–3603. [Google Scholar] [CrossRef] [Green Version]
- Global CCS Institute. Geological Storage of CO2: Safe, Permanent, and Abundant; Global CCS Institute: Washington, DC, USA, 2018. [Google Scholar]
- Lackner, K.S.; Brennan, S. Envisioning carbon capture and storage: Expanded possibilities due to air capture, leakage insurance, and C-14 monitoring. Clim. Chang. 2009, 96, 357–378. [Google Scholar] [CrossRef]
- Lackner, K.S.; Brennan, S.; Matter, J.M.; Park, A.H.A.; Wright, A.; Van der Zwaan, B. The urgency of the development of CO2 capture from ambient air. Proc. Natl. Acad. Sci. USA 2012, 109, 13156–13162. [Google Scholar] [CrossRef] [Green Version]
- IEAGHG. Steve Goldthorpe, Carbonation and Enhanced; Report:2013/TR6; IEAGHG: Cheltenham, UK, 2013. [Google Scholar]
- Rackley, S.A. Ocean storage. Carbon Capture Storage 2010. [Google Scholar] [CrossRef]
- Heinrich, J. Legal Implications of CO2 Ocean Storage; Laboratory for Energy and the Environment: Cambridge, UK, 2002; p. 17. [Google Scholar]
- Zhang, Y.; Lu, X.; Ji, X. Carbon dioxide capture. Deep Eutectic Solvents Synth. Prop. Appl. 2019, 297–319. [Google Scholar] [CrossRef]
- Preparing Your Business for the Zero-Carbon Circular Economy. Available online: http://www.lowcarbonlivingcrc.com.au/sites/all/files/publications_file_attachments/lclguide_buildingsmes_web.pdf (accessed on 1 September 2021).
- Karlsson, I.; Rootzén, J.; Johnsson, F. Reaching net-zero carbon emissions in construction supply chains—Analysis of a Swedish road construction project. Renew. Sustain. Energy Rev. 2019, 120, 109651. [Google Scholar] [CrossRef]
- Chen, H.M. Additional resources and final remarks. Libr. Technol. Rep. 2017, 53, 28–30. [Google Scholar]
- IEA. Global Energy Review 2020: The Impacts of the COVID-19 Crisis on Global Energy Demand and CO2 Emissions; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Mostafaeipour, A.; Dehshiri, S.H.; Dehshiri, S.H.; Jahangiri, M.; Techato, K. A thorough analysis of potential geothermal project locations in afghanistan. Sustainability 2020, 12, 8397. [Google Scholar] [CrossRef]
- Kihlström, V.; Elbe, J. Constructing markets for solar energy—a review of literature about market barriers and government responses. Sustainability 2021, 13, 3273. [Google Scholar] [CrossRef]
- Darwish, A.S.; Al-Dabbagh, R. Wind energy state of the art: Present and future technology advancements. Renew. Energy Environ. Sustain. 2020, 5, 7. [Google Scholar] [CrossRef]
- Brown, A.; Waldheim, L.; Landalv, I.; Saddler, J.; Ebadian, M.; McMillan, J.D.; Bonomi, A.; Klein, B. Advanced Biofuels—Potential for Cost Reduction. IEA Bioenergy 2020. Available online: https://www.ieabioenergy.com/wp-content/uploads/2020/02/T41_CostReductionBiofuels-11_02_19-final.pdf (accessed on 1 September 2021).
- Pacheco-Torgal, F.; Labrincha, J.A. The future of construction materials research and the seventh un Millennium Development Goal: A few insights. Constr. Build. Mater. 2013, 40, 729–737. [Google Scholar] [CrossRef]
- Iida, S.; Sakata, K. Hydrogen technologies and developments in Japan. Clean Energy 2019, 3, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef]
- Waring, B.; Neumann, M.; Prentice, I.C.; Adams, M.; Smith, P.; Siegert, M. What role can forests play in tackling climate change? Grantham Inst. Discuss. Pap. 2020, 6, 1–8. [Google Scholar]
- Eid, E.M.; Arshad, M.; Shaltout, K.H.; El-Sheikh, M.A.; Alfarhan, A.H.; Picó, Y.; Barcelo, D. Effect of the conversion of mangroves into shrimp farms on carbon stock in the sediment along the southern Red Sea coast, Saudi Arabia. Environ. Res. 2019, 176, 108536. [Google Scholar] [CrossRef] [PubMed]
- Jakovac, C.C.; Latawiec, A.E.; Lacerda, E.; Lucas, I.L.; Korys, K.A.; Iribarrem, A.; Malaguti, G.A.; Turner, R.K.; Luisetti, T.; Strassburg, B.B.N. Costs and Carbon Benefits of Mangrove Conservation and Restoration: A Global Analysis. Ecol. Econ. 2020, 176, 106758. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Ahmed, M.T.; Alrumman, S.A.; Ahmed, D.A.; Eid, E.M. Evaluation of the carbon sequestration capacity of arid mangroves along nutrient availability and salinity gradients along the Red Sea coastline of Saudi Arabia. Oceanologia 2019, 62, 56–69. [Google Scholar] [CrossRef]
- Bonsu, N.O. Towards a circular and low-carbon economy: Insights from the transitioning to electric vehicles and net zero economy. J. Clean. Prod. 2020, 256, 120659. [Google Scholar] [CrossRef]
- Kätelhön, A.; Meys, R.; Deutz, S.; Suh, S.; Bardow, A. Climate change mitigation potential of carbon capture and utilisation in the chemical industry. Proc. Natl. Acad. Sci. USA 2019, 116, 11187–11194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, S.; Bringezu, S. Use of carbon dioxide as raw material to close the carbon cycle for the German chemical and polymer industries. J. Clean. Prod. 2020, 271, 122775. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. Putting CO2 to use. Energy Rep. 2019. Available online: https://www.oecd-ilibrary.org/energy/putting-co2-to-use_dfeabbf4-en (accessed on 1 September 2021).
- Zimmermann, A.W.; Wunderlich, J.; Müller, L.; Buchner, G.A.; Marxen, A.; Michailos, S.; Armstrong, K.; Naims, H.; Mccord, S.; Styring, P.; et al. Techno-economic assessment guidelines for CO2 utilisation. Front. Energy Res. 2020, 8, 1–23. [Google Scholar] [CrossRef] [Green Version]
- IEA. Exploring Clean Energy Pathways: The Role of CO2 Storage; IEA: Paris, France, 2019. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Meunier, N.; Chauvy, R.; Mouhoubi, S.; Thomas, D.; De Weireld, G. Alternative production of methanol from industrial CO2. Renew. Energy 2020, 146, 1192–1203. [Google Scholar] [CrossRef]
- Song, C. CO2 Conversion and Utilisation: An Overview. ACS Symp. Ser. 2016. Available online: https://pubs.acs.org/doi/pdf/10.1021/bk-2002-0809.ch001 (accessed on 1 September 2021).
- Gulzar, A.; Ansari, M.B.; He, F.; Gai, S.; Yang, P. Carbon dioxide utilisation: A paradigm shift with CO2 economy. Chem. Eng. J. Adv. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Esposito, E.; Dellamuzia, L.; Moretti, U.; Fuoco, A.; Giorno, L.; Jansen, J.C. Simultaneous production of biomethane and food grade CO2 from biogas: An industrial case study. Energy Environ. Sci. 2018, 12, 281–289. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Alper, E.; Orhan, O.Y. CO2 utilisation: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Long, D.J.; Tang, L. The impact of socio-economic institutional change on agricultural carbon dioxide emission reduction in China. PLoS ONE 2021, 16, e0251816. [Google Scholar] [CrossRef]
- Caldeira, K.; Brown, P.T. Reduced emissions through climate damage to the economy. Proc. Natl. Acad. Sci. USA 2018, 116, 714–716. [Google Scholar] [CrossRef] [Green Version]
- Righetti, T.; Richardson, J.; Koski, K.; Taylor, S. The Carbon Storage Future of Public Lands. Pace Environ. Law Rev. 2020. Available online: https://digitalcommons.pace.edu/cgi/viewcontent.cgi?article=1847&context=pelr (accessed on 1 September 2021).
- Otto, I.M.; Donges, J.F.; Cremades, R.; Bhowmik, A.; Hewitt, R.; Lucht, W.; Rockström, J.; Allerberger, F.; McCaffrey, M.; Doe, S.S.P.; et al. Social tipping dynamics for stabilising Earth’s climate by 2050. Proc. Natl. Acad. Sci. USA 2020, 117, 2354–2365. [Google Scholar] [CrossRef] [Green Version]
- Mitchell-Larson, E.; Heidug, W. Achieving Net-Zero in the G20: A Novel Supply-Side Climate Policy to Value Carbon Sinks; Climate Change, Sustainable Energy and Environment: Berlin, Germany, 2020; Available online: https://www.g20-insights.org/wp-content/uploads/2020/11/achieving-net-zero-in-the-g20-a-novel-supply-side-climate-policy-to-value-carbon-sinks-1606065435.pdf (accessed on 1 September 2021).
- Tapia, J.F.D.; Lee, J.Y.; Ooi, R.E.; Foo, D.C.; Tan, R.R. Review article A review of optimisation and decision-making models for the planning of CO2 capture, utilisation and storage (CCUS) systems. Sustain. Prod. Consum. 2017, 13, 1–15. [Google Scholar]
- Popa, A. Why capturing and using or storing carbon dioxide is vital for the world’s energy future. Proc. Inst. Civ. Eng. Civ. Eng. 2021, 174, 8. [Google Scholar]
- Tsiropuolus, I.; Nijs, W.; Tarvydas, D.; Ruiz Castello, P. Towards Net-Zero Emissions in the EU Energy System by 2050; Technical Report EUR 29981 EN; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Bhagat, C.; Dudhagara, P.; Tank, S. Trends, application and future prospectives of microbial carbonic anhydrase mediated carbonation process for CCUS. J. Appl. Microbiol. 2017, 124, 316–335. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An overview of solar photovoltaic panels’ end-of-life material recycling. Energy Strateg. Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]








| Greenhouse Gas | Main Activity Source | Average Lifetime in the Atmosphere | 100-Years GWP |
|---|---|---|---|
| CO2 | Fossil fuels, cement production, land-use change | Variable * | 1 |
| CH4 | Fossil fuels, rice paddies, waste dumps, livestock | 12 | 28 |
| N2O | Fertilizers, combustion industrial processes | 121 | 265 |
| CHF3 | Electronics, refrigerants | 222 | 12,400 |
| CF3CH2F | Refrigerants | 13 | 1300 |
| CH3CHF2 | Industrial processes | 1.5 | 138 |
| CF4 | Aluminium production | 50,000 | 6630 |
| C2F6 | Aluminium production | 10,000 | 11,100 |
| SF6 | Electrical insulation | 3200 | 23,500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsarhan, L.M.; Alayyar, A.S.; Alqahtani, N.B.; Khdary, N.H. Circular Carbon Economy (CCE): A Way to Invest CO2 and Protect the Environment, a Review. Sustainability 2021, 13, 11625. https://doi.org/10.3390/su132111625
Alsarhan LM, Alayyar AS, Alqahtani NB, Khdary NH. Circular Carbon Economy (CCE): A Way to Invest CO2 and Protect the Environment, a Review. Sustainability. 2021; 13(21):11625. https://doi.org/10.3390/su132111625
Chicago/Turabian StyleAlsarhan, Latifah M., Alhanouf S. Alayyar, Naif B. Alqahtani, and Nezar H. Khdary. 2021. "Circular Carbon Economy (CCE): A Way to Invest CO2 and Protect the Environment, a Review" Sustainability 13, no. 21: 11625. https://doi.org/10.3390/su132111625
APA StyleAlsarhan, L. M., Alayyar, A. S., Alqahtani, N. B., & Khdary, N. H. (2021). Circular Carbon Economy (CCE): A Way to Invest CO2 and Protect the Environment, a Review. Sustainability, 13(21), 11625. https://doi.org/10.3390/su132111625







