Patterns of Structural and Functional Bacterioplankton Metacommunity along a River under Anthropogenic Pressure
Abstract
:1. Introduction
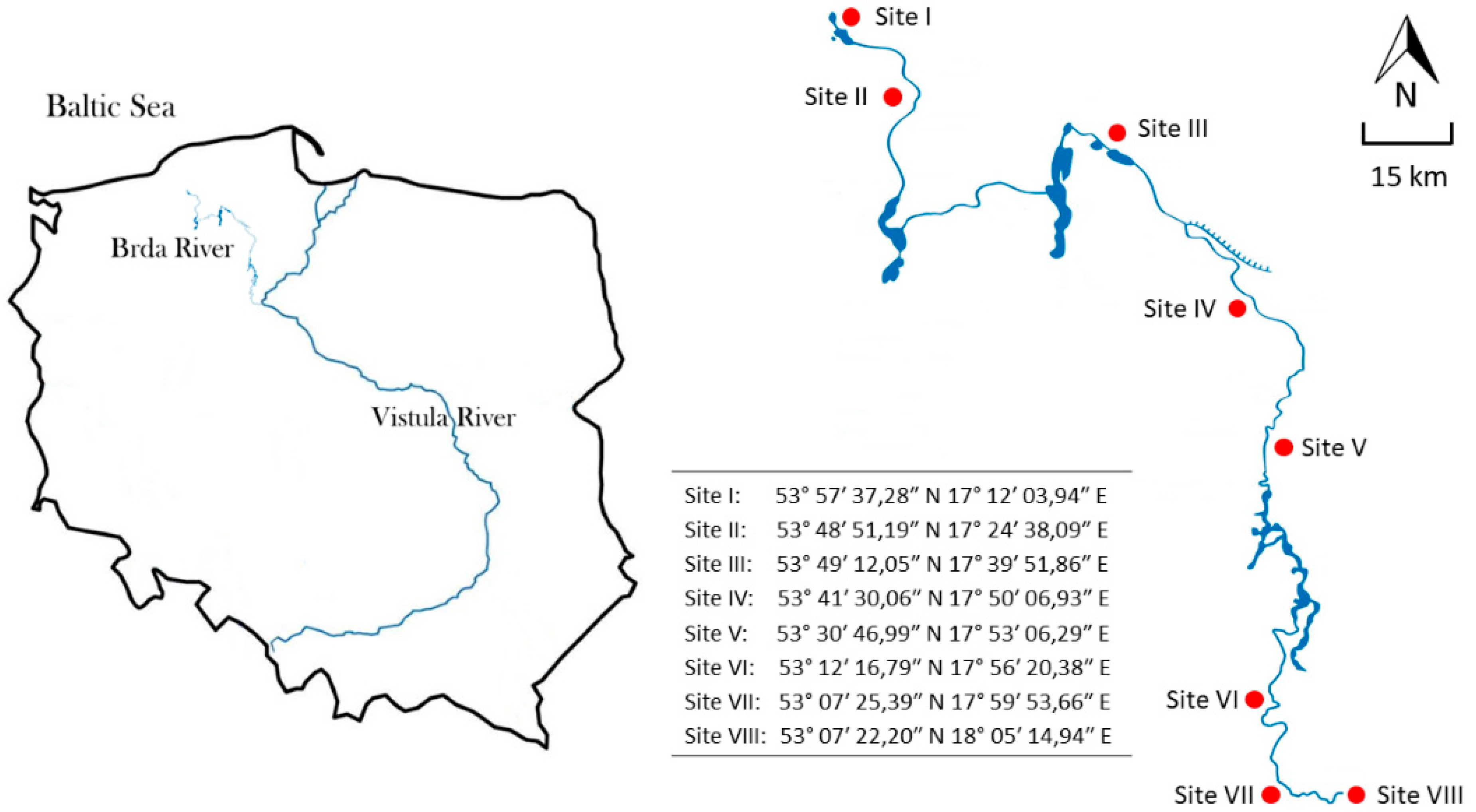
2. Materials and Methods
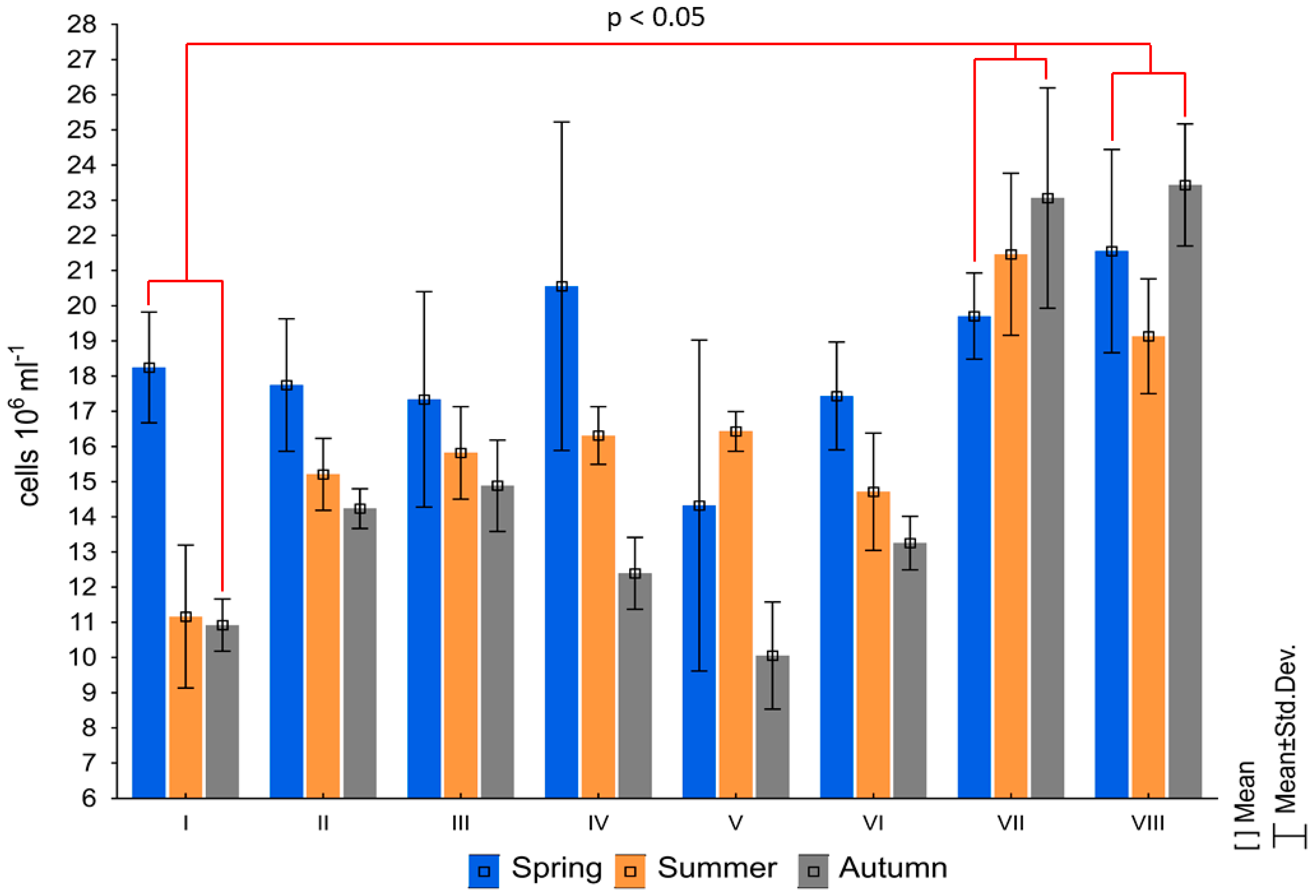
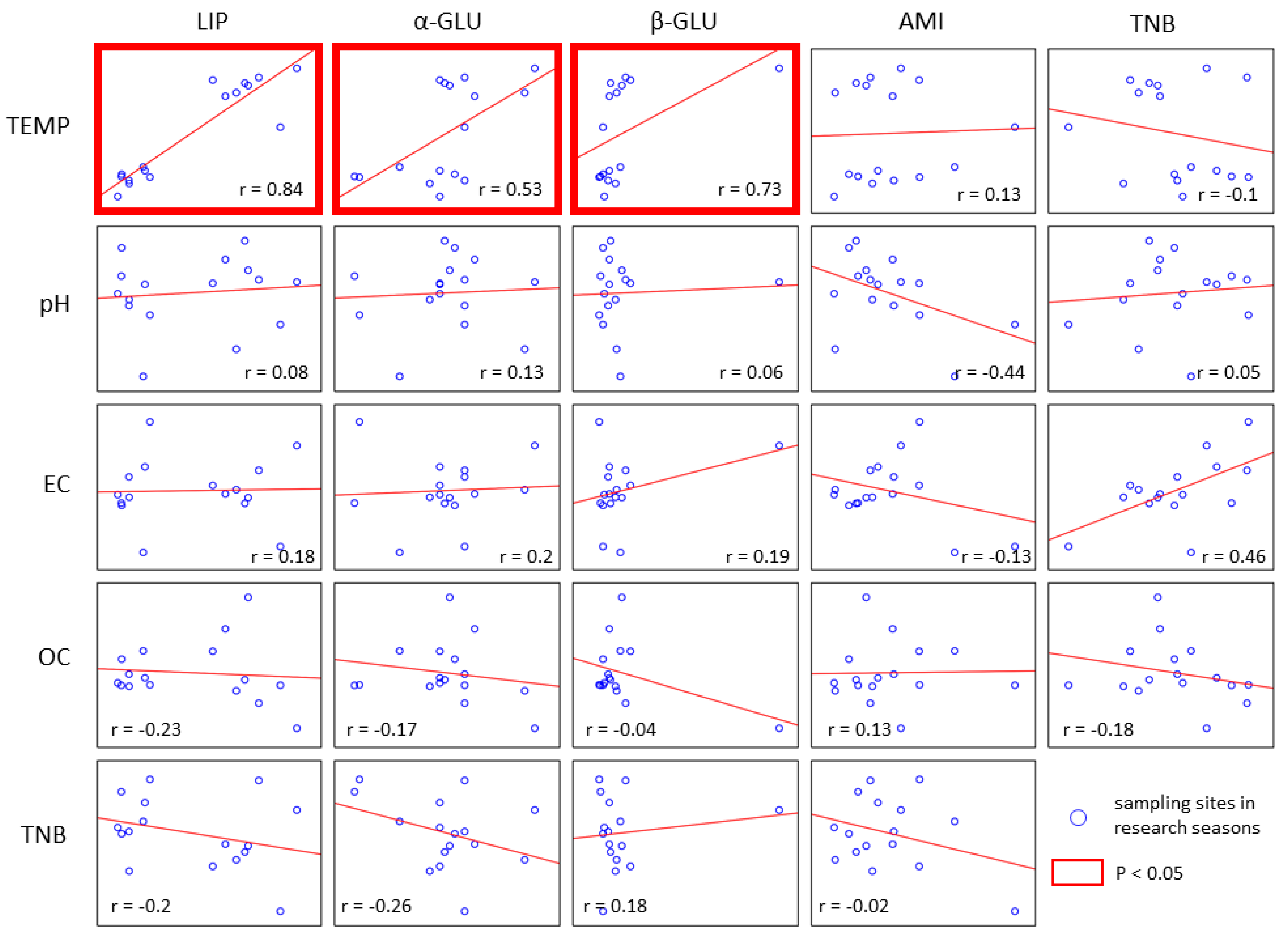
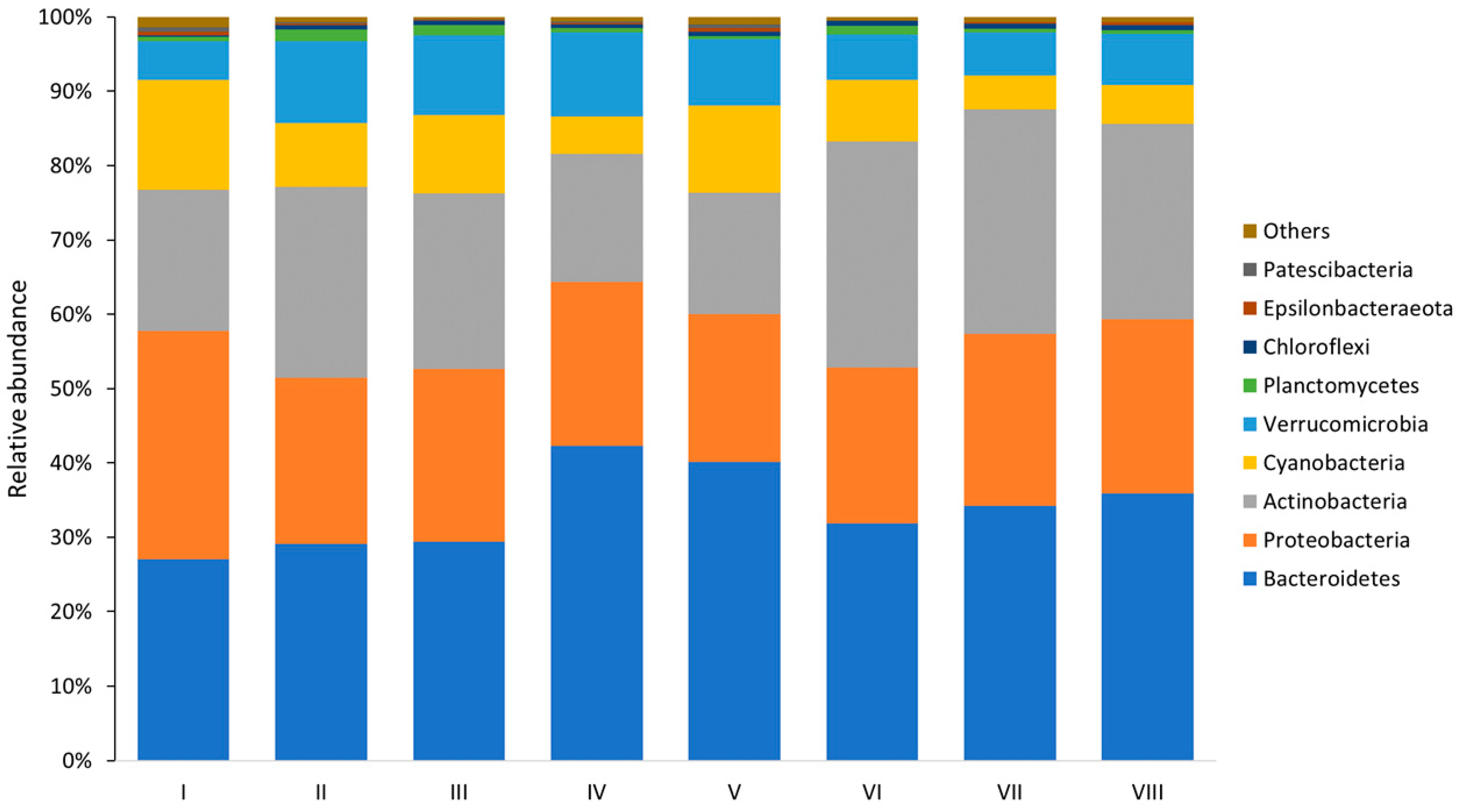
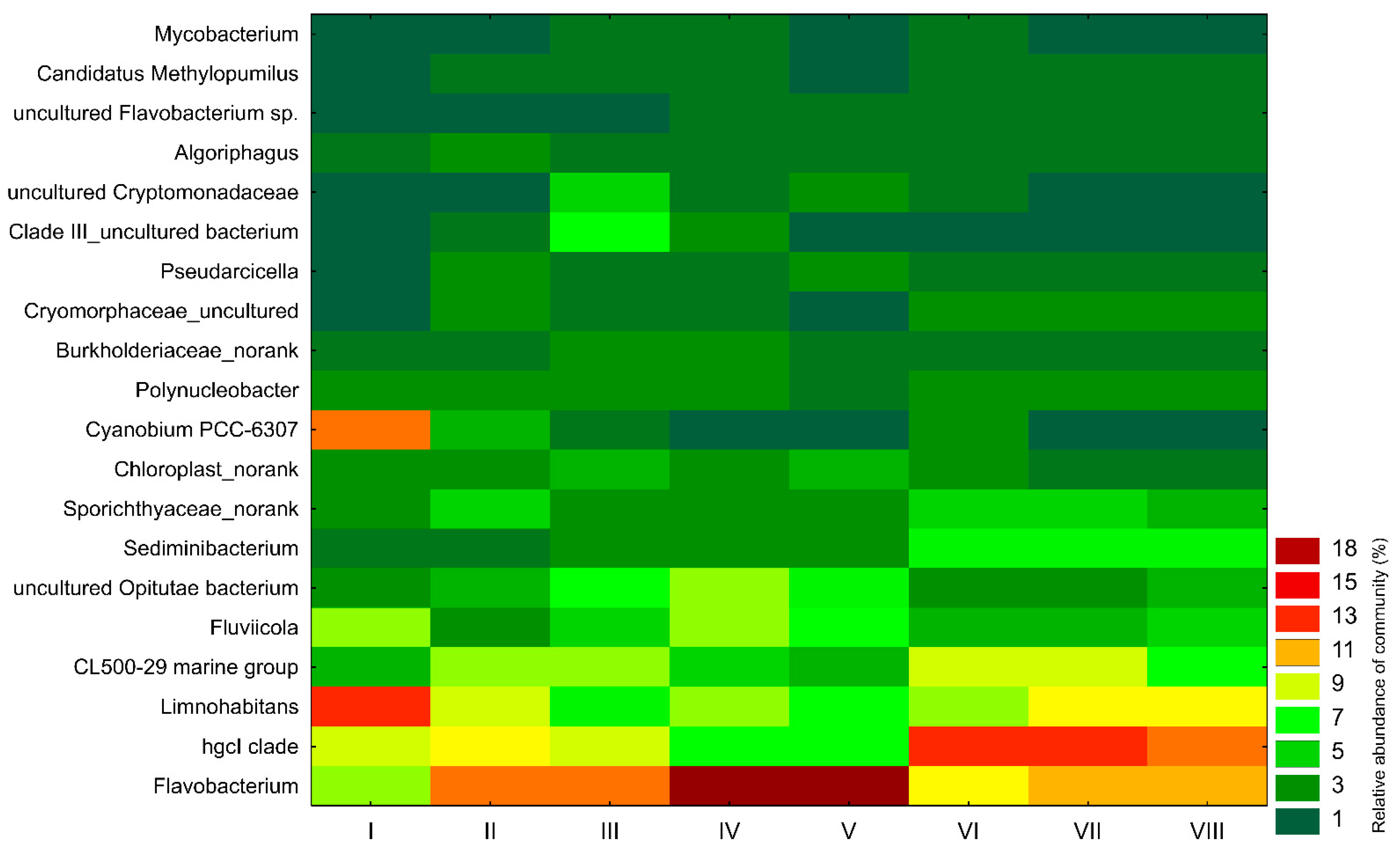
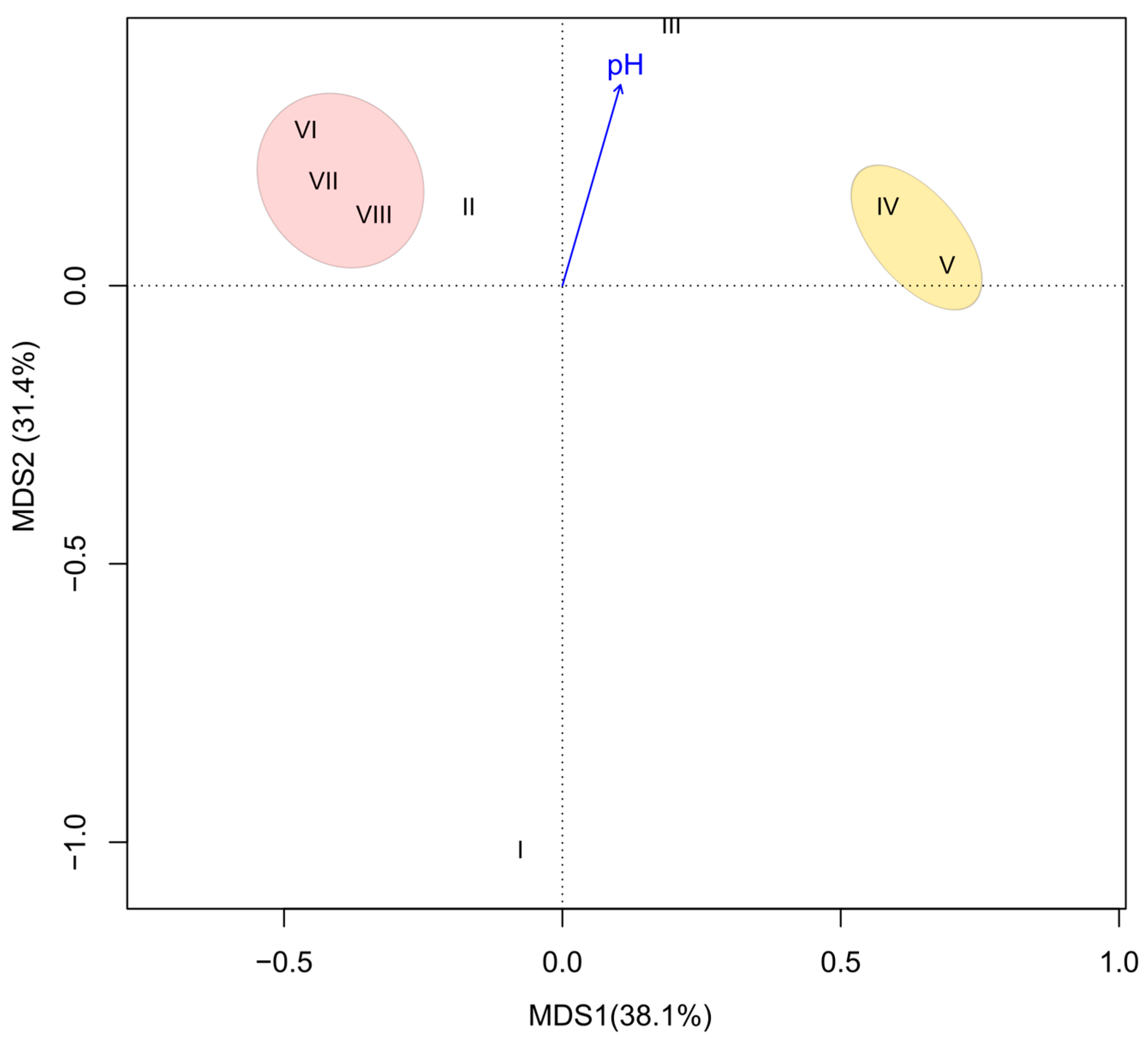
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.; Chen, W.; Sun, J.; Liu, L.; Huang, X. Spatial Variation in Bacterioplankton Communities in the Pearl River, South China: Impacts of Land Use and Physicochemical Factors. Microorganisms 2020, 8, 814. [Google Scholar] [CrossRef]
- Gotkowska-Płachta, A.; Gołaś, I.; Korzeniewska, E.; Koc, J.; Rochwerger, A.; Solarski, K. Evaluation of the distribution of fecal indicator bacteria in a river system depending on different types of land use in the southern watershed of the Baltic Sea. Environ. Sci. Pollut. Res. 2015, 23, 4073–4085. [Google Scholar] [CrossRef]
- Tissera, S.; Lee, S.M. Isolation of Extended Spectrum beta-lactamase (ESBL) producing bacteria from urban surface waters in Malaysia. Malays. J. Med. Sci. 2013, 20, 14–22. [Google Scholar]
- Jeffries, T.C.; Schmitz Fontes, M.L.; Harrison, D.P.; Van-Dongen-Vogels, V.; Eyre, B.D.; Ralph, P.J.; Seymour, J.R. Bacterioplankton Dynamics within a Large Anthropogenically Impacted Urban Estuary. Front. Microbiol. 2016, 6, 1438. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A Guide to the Natural History of Freshwater Lake Bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef] [Green Version]
- Beck, H.J.; Birch, G.F. Metals, nutrients and total suspended solids discharged during different flow conditions in highly urbanised catchments. Environ. Monit. Assess. 2012, 184, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Gregoracci, G.B.; Nascimento, J.R.; Cabral, A.S.; Paranhos, R.; Valentin, J.L.; Thompson, C.C.; Thompson, F.L. Structuring of bacterioplankton diversity in a large tropical bay. PLoS ONE 2012, 7, e31408. [Google Scholar] [CrossRef] [Green Version]
- McCready, S.; Birch, G.F.; Long, E.R. Metallic and organic contaminants in sediments of Sydney Harbour, Australia and vicinity—A chemical dataset for evaluating sediment quality guidelines. Environ. Int. 2006, 32, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Senjarini, K.; Karsten, U.; Schumann, R. Application of Fluorescence Markers for the Diagnosis of Bacterial Abundance and Viability in Aquatic Ecosystem. J. Microbiol. Res. 2013, 3, 143–147. [Google Scholar]
- Cunha, M.A.; Almeida, M.A.; Alcântara, F. Short-term responses of the natural planktonic bacterial community to the changing water properties in an estuarine environment: Ectoenzymatic activity, glucose incorporation, and biomass production. Microb. Ecol. 2001, 42, 69–79. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Chen, H.; Yang, J. Spatiotemporal dynamics and determinants of planktonic bacterial and microeukaryotic communities in a Chinese subtropical river. Appl. Microbiol. Biotechnol. 2015, 99, 9255–9266. [Google Scholar] [CrossRef]
- Du, Y.; Yang, E.; Ding, X.; Zhang, J.; Zheng, Z.; Zhu, J. High heterogeneity of bacterioplankton community shaped by spatially structured environmental factors in West Lake, a typical urban lake in eastern China. Environ. Sci. Pollut. Res. 2020, 27, 42283–42293. [Google Scholar] [CrossRef] [PubMed]
- Meiling Zhang, M.; Yu, N.; Chen, L.; Jiang, C.; Tao, Y.; Zhang, T.; Chen, J.; Xue, D. Structure and seasonal dynamics of bacterial communities in three urban rivers in China. Aquat. Sci. 2012, 74, 113–120. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, Y.; Wu, Z.; Feng, Q.; Xie, S.; Liu, Y. Spatiotemporal variation of planktonic and sediment bacterial assemblages in two plateau freshwater lakes at different trophic status. Appl. Microbiol. Biotechnol. 2016, 100, 4161–4175. [Google Scholar] [CrossRef] [PubMed]
- Krevš, A.; Kučinskienė, A. Microbial decomposition of sedimentary organic matter in small temperate lakes. Fundam. Appl. Limnol. 2018, 191, 239–251. [Google Scholar] [CrossRef]
- Mansour, I.; Heppell, C.M.; Ryo, M.; Rillig, M.C. Application of the microbial community coalescence concept to riverine networks. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1832–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiersztyn, B.; Chróst, R.; Kaliński, T.; Siuda, W.; Bukowska, A.; Kowalczyk, G.; Grabowska, K. Structural and functional microbial diversity along a eutrophication gradient of interconnected lakes undergoing anthropopressure. Sci. Rep. 2019, 9, 11144. [Google Scholar] [CrossRef]
- Zhang, H.; Sekar, R.; Visser, P.M. Editorial: Microbial Ecology in Reservoirs and Lakes. Front. Microbiol. 2020, 11, 1348. [Google Scholar] [CrossRef]
- Lu, Q.; Song, Y.; Mao, G.; Lin, B.; Wang, Y.; Gao, G. Spatial variation in bacterial biomass, community composition and driving factors across a eutrophic river. Ecotoxicol. Environ. Saf. 2020, 205, 111113. [Google Scholar] [CrossRef]
- Kubera, Ł. Spread Patterns of Antibiotic Resistance in Faecal Indicator Bacteria Contaminating an Urbanized Section of the Brda River. Microb. Ecol. 2021, 81, 592–600. [Google Scholar] [CrossRef]
- Hoppe, H.G. Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 423–432. [Google Scholar]
- Yamada, N.; Fukuda, H.; Ogawa, H.; Saito, H.; Suzumura, M. Heterotrophic bacteria production and extracellular enzymatic activity in sinking particulate matter in the western North Pacific Ocean. Front. Microbiol. 2012, 3, 379. [Google Scholar] [CrossRef] [Green Version]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends. Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Reynoso, G.; Smith, M.R.; Holmes, C.P.; Keelan, C.R.; McGrath, S.E.; Alvarez, G.H.; Coceano, M.A.; Eldridge, K.A.; Fried, H.I.; Gilbert, N.E.; et al. Bacterial community structure and response to nitrogen amendments in Lake Shenandoah (VA, USA). Water Sci. Technol. 2019, 80, 675–684. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tang, X.; Shao, K.; Hu, Y.; Gao, G. Dynamics of bacterial abundance and the related environmental factors in large shallow eutrophic Lake Taihu. J. Freshw. Ecol. 2017, 32, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tu, T.; Gao, G.; Bartlam, M.; Wang, Y. Biogeography and Diversity of Freshwater Bacteria on a River Catchment Scale. Microb. Ecol. 2019, 78, 324–335. [Google Scholar] [CrossRef]
- Xie, G.; Tang, X.; Shao, K.; Hu, Y.; Martin, R.M.; Gao, G. Spatiotemporal patterns and environmental drivers of total and active bacterial abundances in Lake Taihu, China. Ecol. Indicat. 2020, 114, 106335. [Google Scholar] [CrossRef]
- Kopylov, A.I.; Ievleva, T.V.; Romanenko, A.V.; Zabotkina, E.A. Structural and functional characteristics of the bacterioplankton of rivers flowing through a large city (Cherepovets, Upper Volga Region). Biol. Bull. Russ. Acad. Sci. 2016, 43, 1350–1356. [Google Scholar] [CrossRef]
- Hu, W.; Murata, K.; Zhang, D. Applicability of LIVE/DEAD BacLight stain with glutaraldehyde fixation for the measurement of bacterial abundance and viability in rainwater. J. Environ. Sci. 2017, 51, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, T.; Govender, A.; Dobretsov, S.; Abed, R.M.M. Evaluating the Reliability of Counting Bacteria Using Epifluorescence Microscopy. J. Mar. Sci. Eng. 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Wen, B.; Zhang, X.; Yang, Z.; Xiong, H.; Qiu, Y. Influence of tourist disturbance on soil properties, plant communities, and surface water quality in the Tianchi scenic area of Xinjiang, China. J. Arid. Land. 2016, 8, 304–313. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Wang, C.; Miao, L.; Hou, J.; Yuan, Q. Shift in bacterioplankton diversity and structure: Influence of anthropogenic disturbances along the Yarlung Tsangpo River on the Tibetan Plateau, China. Sci. Rep. 2017, 7, 12529. [Google Scholar] [CrossRef]
- Lin, W.; Wang, Y.; Pan, Y. Short-term effects of temperature on the abundance and diversity of magnetotactic cocci. MicrobiologyOpen 2012, 1, 53–63. [Google Scholar] [CrossRef]
- Kubera, Ł.; Donderski, W. Distribution and activity of benthic bacteria in four lakes in the Bory Tucholskie National Park (Poland). Aquat. Microb. Ecol. 2017, 79, 127–135. [Google Scholar] [CrossRef]
- Luo, X.; Xiang, X.; Huang, G.; Song, X.; Wang, P.; Fu, K. Bacterial Abundance and Physicochemical Characteristics of Water and Sediment Associated with Hydroelectric Dam on the Lancang River China. Int. J. Environ. Res. Public Health 2019, 16, 2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew, S.; Glińska-Lewczuk, K.; Lew, M. The effects of environmental parameters on the microbial activity in peat-bog lakes. PLoS ONE 2019, 14, e0224441. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Chao, Y.; Farrara, J.D.; Hatosy, S.; Martiny, A.C. Fine-scale temporal variation in marine extracellular enzymes of coastal southern California. Front. Microbiol. 2012, 3, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, G. Microbial parameters as a practical tool for the functional characterization and ecological status assessment of transitional areas. J. Ecosyst. Ecograph. 2015, 5, e124. [Google Scholar] [CrossRef] [Green Version]
- Arnosti, C. Speed bumps and barricades in the carbon cycle: Substrate structural effects on carbon cycling. Mar. Chem. 2004, 92, 263–273. [Google Scholar] [CrossRef]
- Perliński, P.; Mudryk, Z.J. Enzymatic biodegradation of high molecular weight polymers in the sediment–water interface in the coastal river estuary. River Res. Applic. 2018, 34, 745–754. [Google Scholar] [CrossRef]
- Mudryk, Z.J.; Skórczewski, P. Extracellular enzyme activity at the air-water interface of an estuarine lake. Estuar. Coast. Shelf Sci. 2004, 59, 59–67. [Google Scholar] [CrossRef]
- Kalwasińska, A.; Swiontek Brzezinska, M. Extracellular enzymatic activities in subsurface water of eutrophic Lake Chełmżyńskie, Poland. J. Freshw. Ecol. 2013, 28, 517–527. [Google Scholar] [CrossRef]
- Kubera, Ł.; Donderski, W. Distribution and physiological activity of heterotrophic benthic bacteria in lakes with different trophic conditions located in the Bory Tucholskie National Park (Poland). Pol. J. Natur. Sci. 2017, 32, 549–559. [Google Scholar]
- Perliński, P.; Mudryk, Z.J.; Antonowicz, J. Enzymatic activity in the surface microlayer and subsurface water in the harbour channel. Estuar. Coast. Shelf Sci. 2017, 196, 150–158. [Google Scholar] [CrossRef]
- Caruso, G. Leucine Aminopeptidase, beta-Glucosidase and Alkaline Phosphatase activity rates and their significance in nutrient cycles in some coastal Mediterranean sites. Mar. Drugs 2010, 8, 916–940. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, A.M.; Goulder, R. Downstream change in leucine aminopeptidase activity and leucine assimilation by epilithic microbiota along the River Swale, northern England. Sci. Total. Environ. 2000, 251–252, 191–204. [Google Scholar] [CrossRef]
- Jaramillo-Salazar, M.T.; Aguirre-Ramírez, N.J.; Galvis-García, J.H. Using extracellular enzyme activity as a pollutant indicator: A field study in Chinchiná River, Caldas–Colombia. Int. J. Environ. Prot. 2016, 6, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Zaccone, R.; Caruso, G. Microbial enzymes in the Mediterranean Sea: Relationship with climate changes. AIMS Microbiol. 2019, 5, 251–271. [Google Scholar] [CrossRef]
- Cunha, A.; Almeida, A.; Coelho, F.J.R.C.; Gomes, N.C.M.; Oliveira, V.; Santos, A.L. Bacterial extracellular enzymatic activity in globally changing aquatic ecosystems. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; pp. 124–135. [Google Scholar]
- Caruso, G.; Azzaro, F.; Azzaro, M.; Decembrini, F.; La Ferla, R.; Maimone, G.; De Pasquale, F.; Monticelli, L.S.; Zaccone, R.; Zappalà, G.; et al. Environmental variability in a transitional Mediterranean system (Oliveri-Tindari, Italy): Focusing on the response of microbial activities and prokaryotic abundance. Estuar. Coast. Shelf Sci. 2013, 135, 158–170. [Google Scholar] [CrossRef]
- Zaccone, R.; Azzaro, M.; Azzaro, F.; Bergamasco, A.; Caruso, G.; Leonardi, M.; La Ferla, R.; Maimone, G.; Mancuso, M.; Monticelli, L.S.; et al. Seasonal Dynamics of Prokaryotic Abundance and Activities in Relation to Environmental Parameters in a Transitional Aquatic Ecosystem (Cape Peloro, Italy). Microb. Ecol. 2014, 67, 45–56. [Google Scholar] [CrossRef]
- Hassell, N.; Tinker, K.A.; Moore, T.; Ottesen, E.A. Temporal and spatial dynamics in microbial community composition within a temperate stream network. Environ. Microbiol. 2018, 20, 3560–3572. [Google Scholar] [CrossRef]
- Jordaan, K.; Bezuidenhout, C.C. Bacterial community composition of an urban river in the North West Province, South Africa, in relation to physico-chemical water quality. Environ. Sci. Pollut. Res. 2016, 23, 5868–5880. [Google Scholar] [CrossRef]
- Hanashiro, F.T.T.; Mukherjee, S.; Souffreau, C.; Engelen, J.; Brans, K.I.; Busschaert, P.; De Meester, L. Freshwater Bacterioplankton Metacommunity Structure Along Urbanization Gradients in Belgium. Front. Microbiol. 2019, 10, 743. [Google Scholar] [CrossRef] [Green Version]
- Drury, B.; Rosi-Marshall, E.; Kelly, J.J. Wastewater Treatment Effluent Reduces the Abundance and Diversity of Benthic Bacterial Communities in Urban and Suburban Rivers. Appl. Environ. Microbiol. 2013, 79, 1897–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, L.L.; Sun, Y.Y.; Cha, Q.Q.; Li, C.Y.; Zhao, D.L.; Song, X.Y.; Wang, M.; McMinn, A.; Chen, X.L.; et al. Extracellular Enzyme Activity and Its Implications for Organic Matter Cycling in Northern Chinese Marginal Seas. Front. Microbiol. 2019, 10, 2137. [Google Scholar] [CrossRef] [Green Version]
- Nupur Bhumika, V.; Srinivas, T.N.R.; Kumar, P.A. Flavobacterium nitratireducens sp. nov., an amylolytic bacterium of the family Flavobacteriaceae isolated from coastal surface seawater. Int. J. Syst. Evol. Microbiol. 2013, 63, 2490–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.M.; Xie, Y.R.; Kwon, S.W.; Sheu, S.Y. Flavobacterium sufflavum sp. nov., isolated from a freshwater lake. Int. J. Syst. Evol. Microbiol. 2019, 69, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, L.; Guo, J.; Yang, J.; Zhang, J.; He, B.; Xu, L. Responses of spatial-temporal dynamics of bacterioplankton community to large-scale reservoir operation: A case study in the Three Gorges Reservoir, China. Sci. Rep. 2017, 7, 42469. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, P.; Dorador, C.; Vila, I.; Sommaruga, R. Bacterial Communities Associated with Spherical Nostoc Macrocolonies. Front. Microbiol. 2019, 10, 483. [Google Scholar] [CrossRef]
- Ghai, R.; Mizuno, C.M.; Picazo, A.; Camacho, A.; Rodriguez-Valera, F. Key roles for freshwater Actinobacteria revealed by deep metagenomic sequencing. Mol. Ecol. 2014, 23, 6073–6090. [Google Scholar] [CrossRef]
- Karayanni, H.; Macingo, S.C.; Tolis, V.; Alivertis, D. Diversity of Bacteria in Lakes with Different Chlorophyll Content and Investigation of Their Respiratory Activity through a Long-Term Microcosm Experiment. Water 2019, 11, 467. [Google Scholar] [CrossRef] [Green Version]

| Enzyme | Parameter | p-Value | Used Statistical Test |
|---|---|---|---|
| Lipase | season | 0.0008 | Kruskal–Wallis |
| sites | 0.8891 | ||
| α-glucosidase | season | 0.0399 | Main Effect AVOVA |
| sites | 0.8112 | ||
| β-glucosidase | season | 0.0272 | Kruskal–Wallis |
| sites | 0.9851 | ||
| Aminopeptidase | season | 0.2027 | Main Effect ANOVA |
| sites | 0.0010 |
| Sampling Site | OTUs | 97% Similarity | |||
|---|---|---|---|---|---|
| Shannon–Wiener | Simpson | Chao1 | ACE | ||
| I | 728 | 7.592323 | 0.986510 | 729.885 | 731.492 |
| II | 541 | 7.538064 | 0.989000 | 543.769 | 543.032 |
| III | 424 | 7.233476 | 0.986403 | 424.750 | 424.860 |
| IV | 719 | 7.493382 | 0.987583 | 723.550 | 722.216 |
| V | 774 | 7.774601 | 0.989369 | 787.80 | 780.825 |
| VI | 457 | 7.308804 | 0.987690 | 457.111 | 457.380 |
| VII | 476 | 7.182502 | 0.986323 | 484.076 | 481.115 |
| VIII | 518 | 7.325249 | 0.987742 | 525.50 | 522.690 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małecka-Adamowicz, M.; Kubera, Ł. Patterns of Structural and Functional Bacterioplankton Metacommunity along a River under Anthropogenic Pressure. Sustainability 2021, 13, 11518. https://doi.org/10.3390/su132011518
Małecka-Adamowicz M, Kubera Ł. Patterns of Structural and Functional Bacterioplankton Metacommunity along a River under Anthropogenic Pressure. Sustainability. 2021; 13(20):11518. https://doi.org/10.3390/su132011518
Chicago/Turabian StyleMałecka-Adamowicz, Marta, and Łukasz Kubera. 2021. "Patterns of Structural and Functional Bacterioplankton Metacommunity along a River under Anthropogenic Pressure" Sustainability 13, no. 20: 11518. https://doi.org/10.3390/su132011518
APA StyleMałecka-Adamowicz, M., & Kubera, Ł. (2021). Patterns of Structural and Functional Bacterioplankton Metacommunity along a River under Anthropogenic Pressure. Sustainability, 13(20), 11518. https://doi.org/10.3390/su132011518







