Adsorption-Release Characteristics of Phosphorus and the Community of Phosphorus Accumulating Organisms of Sediments in a Shallow Lake
Abstract
1. Introduction
2. Materials and Methods
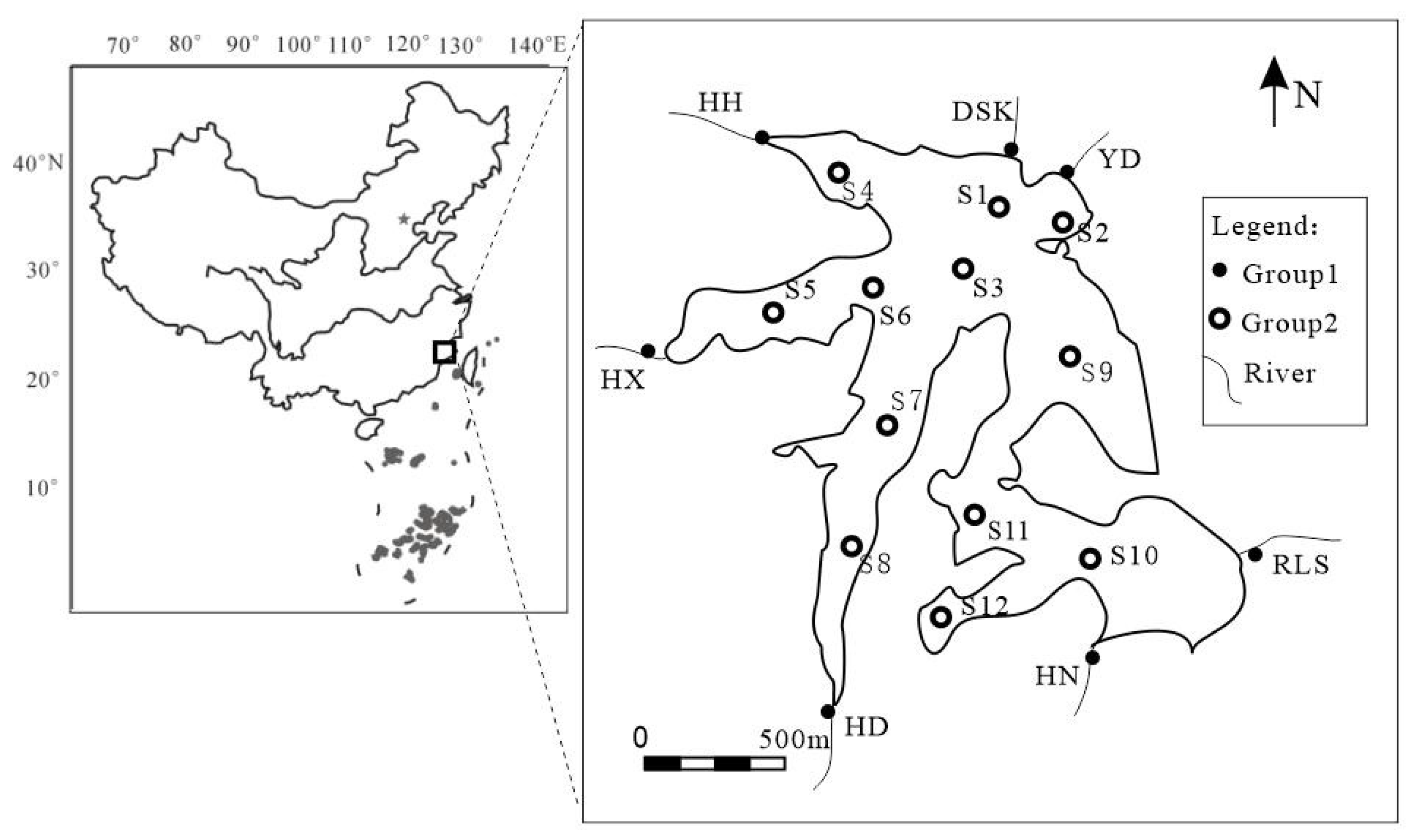
2.1. Study Site and Sampling
2.2. Simulation Experiment Design on Sorption Experiments
2.2.1. Kinetic Adsorption Experiments
2.2.2. Equilibrium Adsorption Experiments
2.2.3. Analyze Different Phosphorus Phases
2.3. Determination of Phosphorus Vertical Release Rate by Pore Water Diffusion Model
2.4. Phosphorus Accumulating Organisms (PAOs) in the Sediments
2.4.1. DNA Extraction and Purification of Sediment
2.4.2. Amplification, Recovery, and Enzymatic Digestion of PPK1 Gene in Phosphorus Accumulating Organisms
2.4.3. T-RFLP Detection and Pattern Analysis
2.4.4. Construction of Clone Libraries and Sequencing
2.4.5. Sequence Analysis of Functional Genes and Phylogenetic Tree Construction
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the P in Sediments
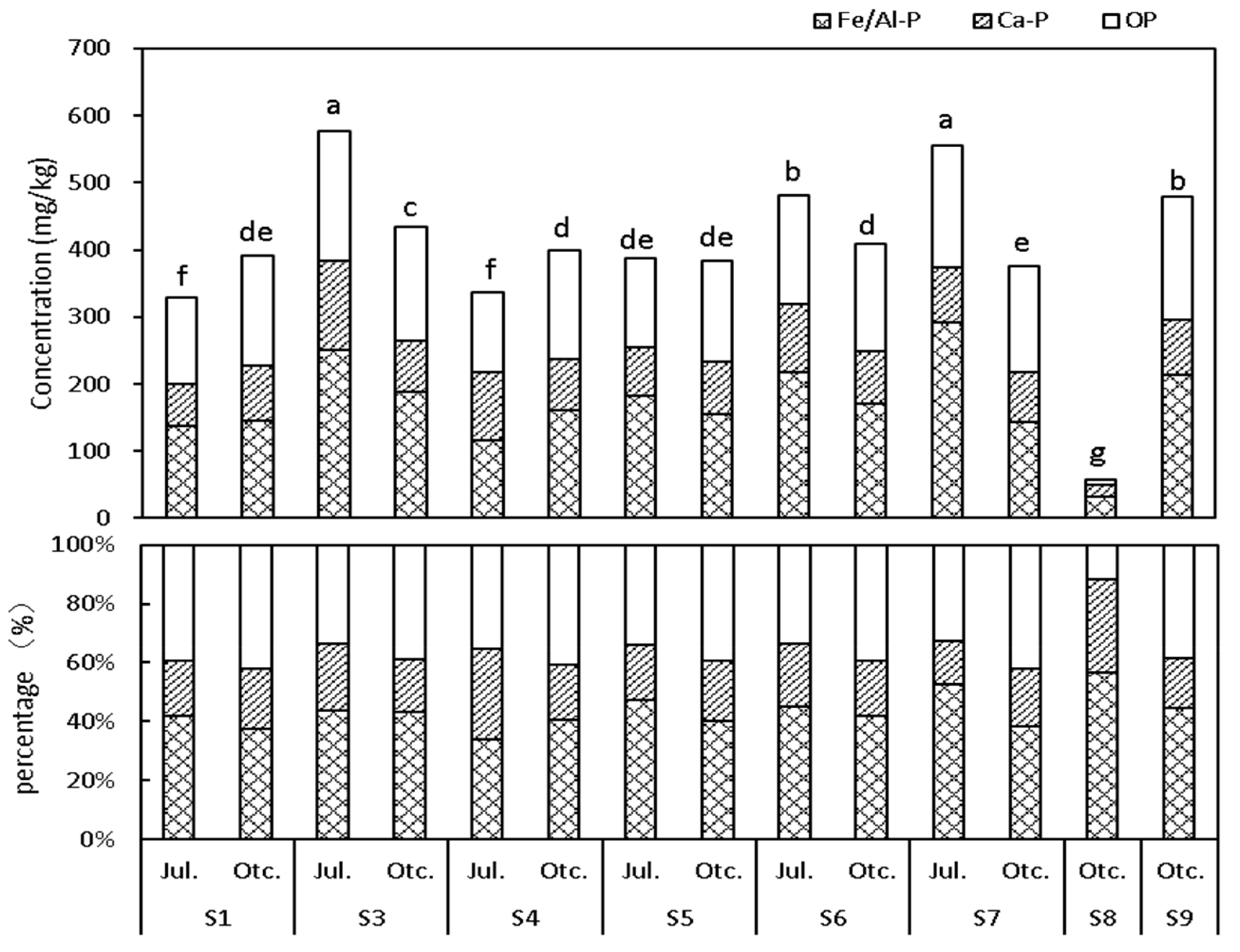
3.1.1. Spatial and Temporal Distribution of the P in the Surface Sediments
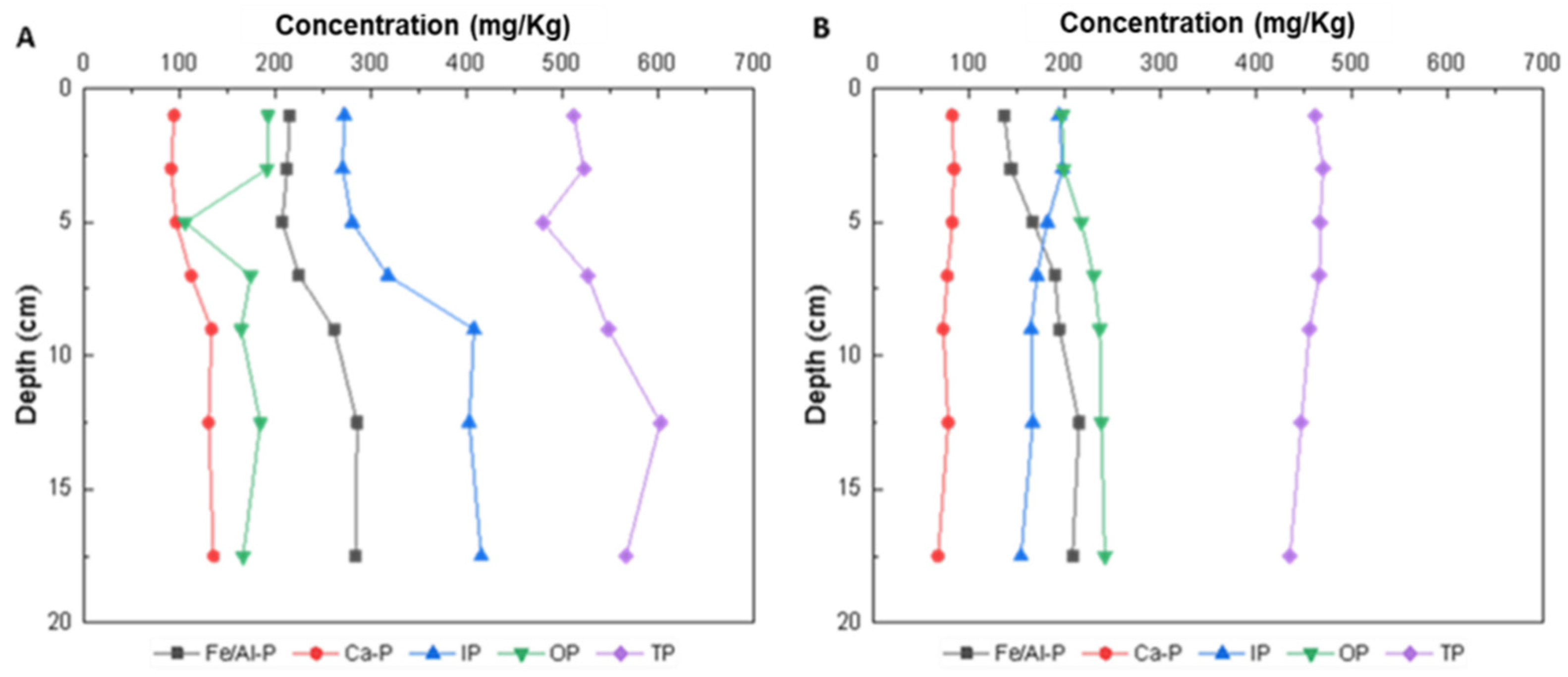
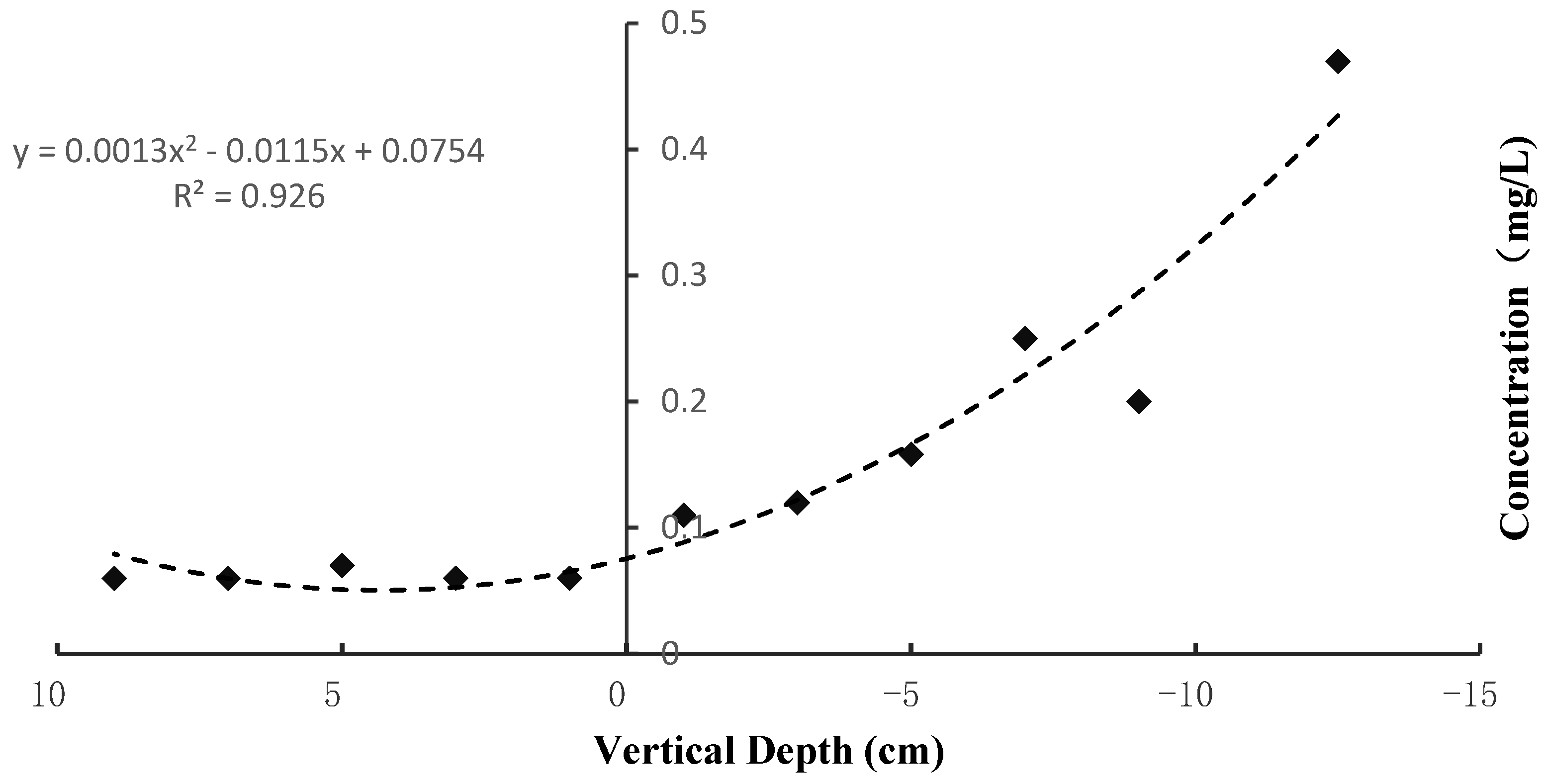
3.1.2. Distribution of the P Content in the Vertical Surface Sediments
3.1.3. Characteristics of P in the Surface Sediments
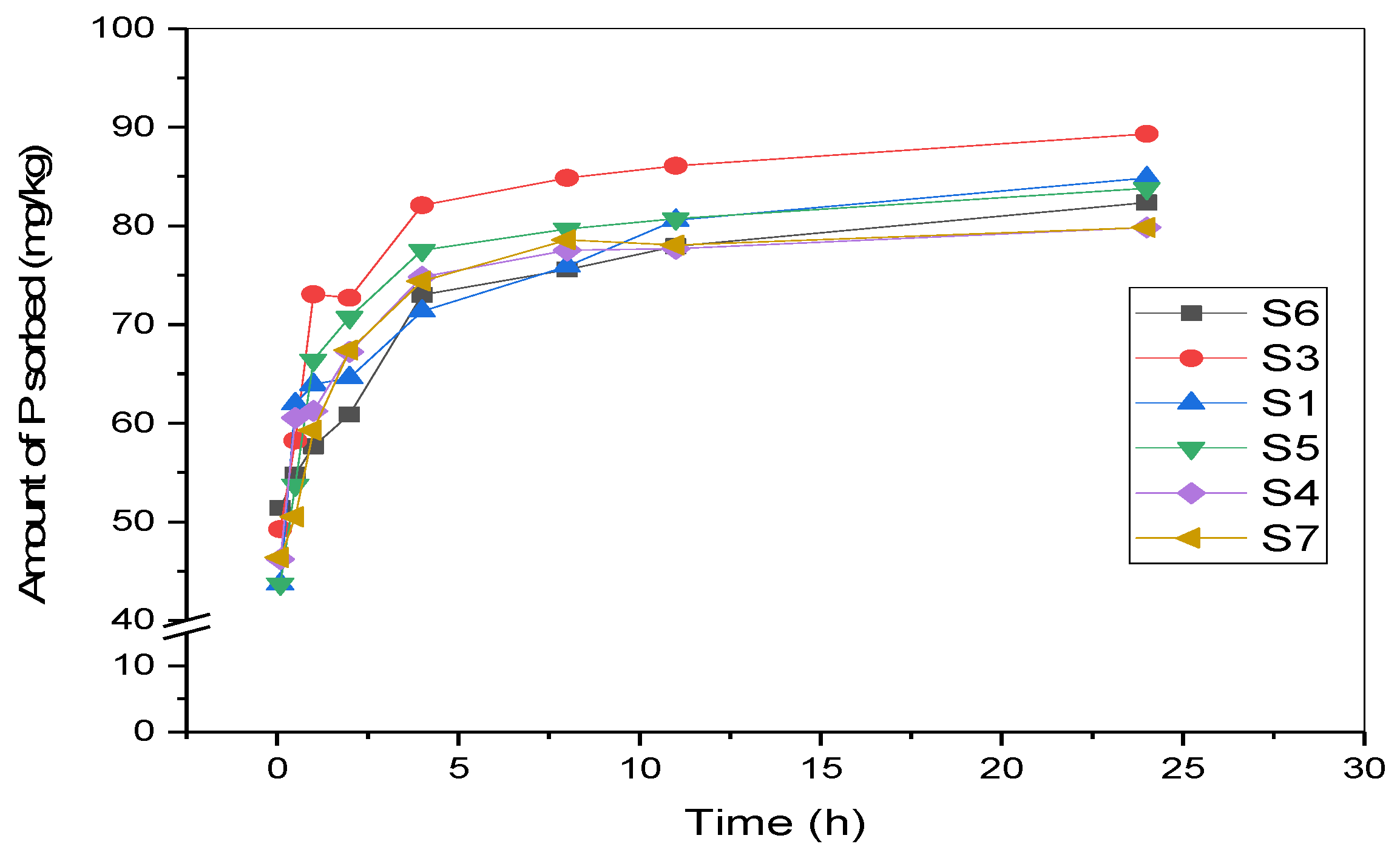
3.2. P Adsorption Kinetics of Surface Sediments
3.3. Isothermal Characteristics of Phosphorus Adsorption
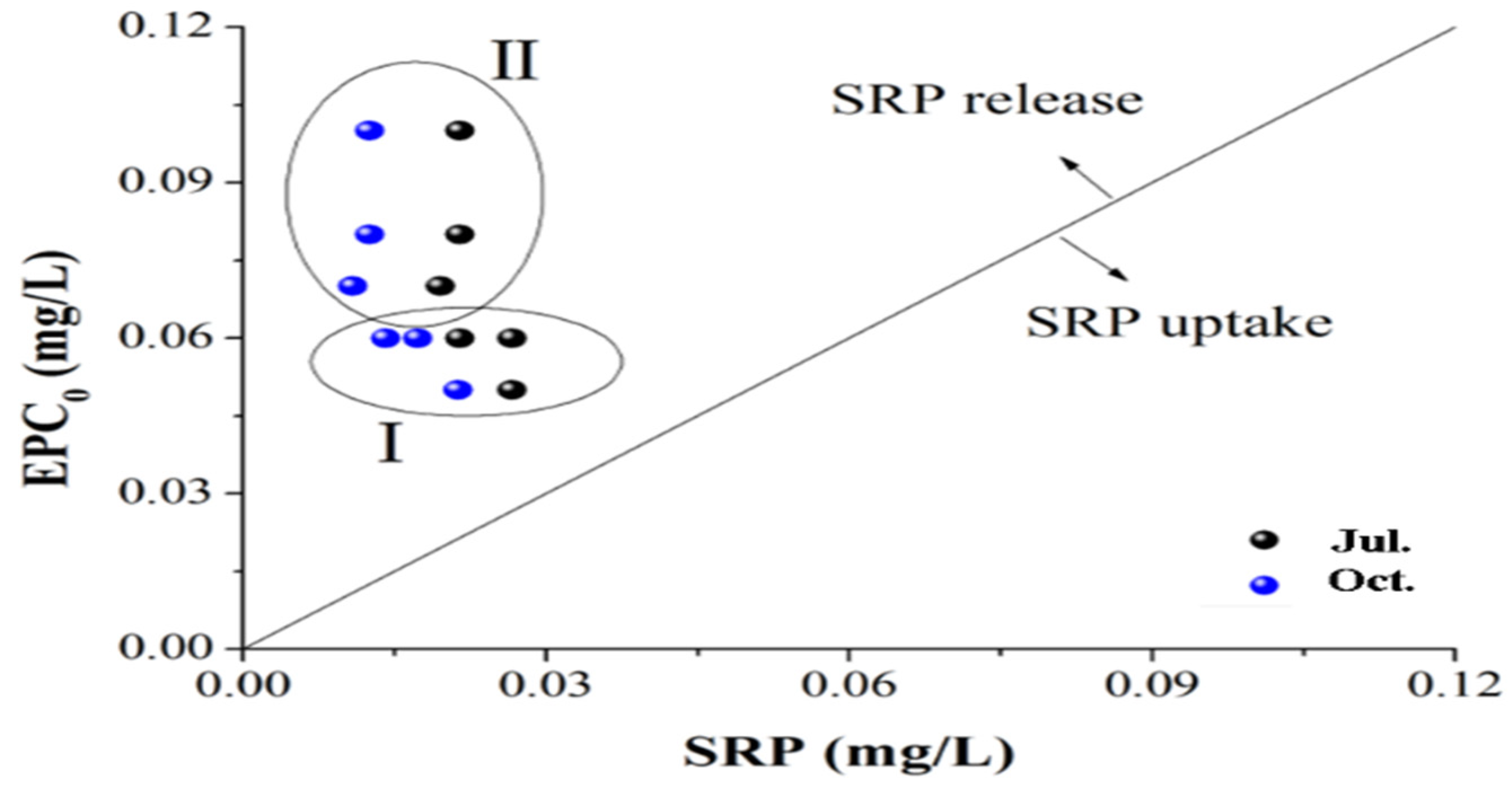
3.4. Risk of P Release from the Sediments of the Sanshiliujiao Lake
Risk of P Release from the Sediments of the Sanshiliujiao Lake
3.5. Diversity and Community Composition of Phosphorus Accumulating Organisms (PAOs) in the Sediments
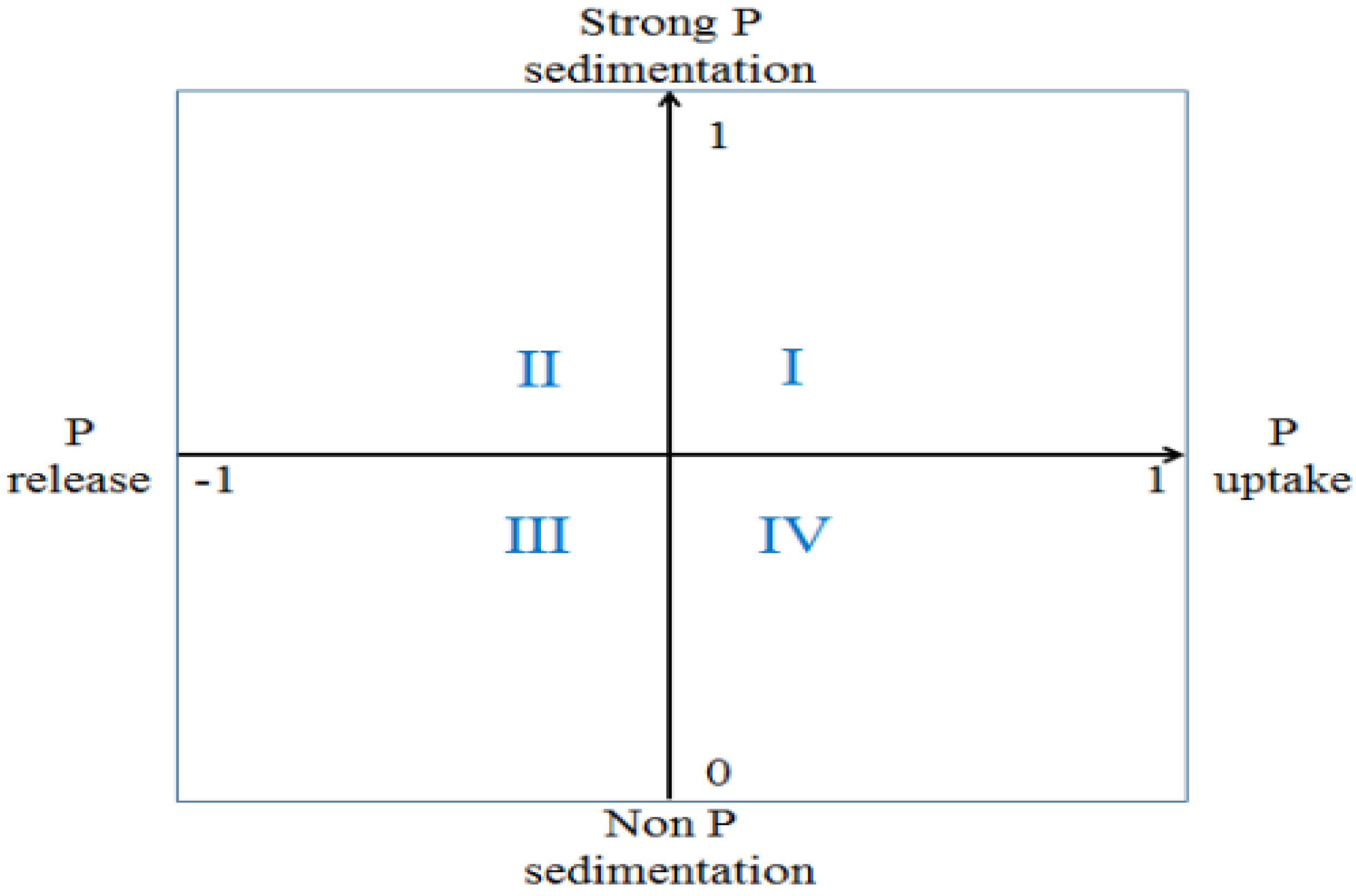
3.6. Migration and Deposition Mechanism of Phosphorus-Poor Sediments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Le Moal, M.; Gascuel-Odoux, C.; Menesguen, A.; Souchon, Y.; Etrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Xu, S.; Qin, G.; Sun, Y.; Wang, F. Spatial Distribution, Adsorption/Release Characteristics, and Environment Influence of Phosphorus on Sediment in Reservoir. Water 2017, 9, 724. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Tang, X.; Li, R.; Han, D.; Scholz, M. Response of Eutrophication Development to Variations in Nutrients and Hydrological Regime: A Case Study in the Changjiang River (Yangtze) Basin. Water 2020, 12, 1634. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.; Foy, B.; et al. Lake responses to reduced nutrient loading-an analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M. Phytoplankton blooms in Lake Erie impacted by both long-term and springtime phosphorus loading. J. Great Lakes Res. 2017, 43, 221–228. [Google Scholar] [CrossRef]
- Paytan, A.; Roberts, K.; Watson, S.; Peek, S.; Chuang, P.-C.; Defforey, D.; Kendall, C. Internal loading of phosphate in Lake erie central basin. Sci. Total Environ. 2017, 579, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Boström, B.; Andersen, J.M.; Fleischer, S.; Jansson, M.J.H. Exchange of phosphorus across the sediment water interface. Hydrobiologia 1998, 170, 229–244. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Hayashi, H.; Otsuki, A.; Aoyama, K.; Watanabe, Y.; Hanazato, T.; Iwakuma, T.; Yasuno, M. Role of Bottom Sediments in Sustaining Plankton Production in a Lake Ecosystem: Experimental Demonstration Using Enclosed Water Bodies in a Shallow Eutrophic Lake. Ecol. Res. 1989, 4, 1–16. [Google Scholar] [CrossRef]
- Kamiya, K.; Fukushima, T.; Ouchi, T.; Aizaki, M. Phosphorus budgetary analysis of sediment-water interface in a short-term anoxic condition in shallow Lake Kasumigaura, Japan. Limnology 2017, 18, 131–140. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Xiong, H.X.; Xiu, L.Z. Research progress of phosphorus adsorption and release from sediment. Chongqing Environ. Sci. 2003, 11, 147–149. [Google Scholar]
- Wang, L.; Liang, T. Distribution Characteristics of Phosphorus in the Sediments and Overlying Water of Poyang Lake. PLoS ONE 2015, 10, e0125859. [Google Scholar] [CrossRef]
- Pedro, T.; Kimberley, S.; Fernando, P. Dynamics of phosphorus in sediments of a naturally acidic lake. Int. J. Sediment Res. 2013, 28, 90–102. [Google Scholar] [CrossRef]
- An, W.; Li, X. Phosphate Adsorption Characteristics on Surface Sediments of Nansi Lake and Its Main Inflow Rivers. Environ. Sci. 2008, 291, 1295–1302. [Google Scholar]
- Huang, Q.H.; Wang, Z.J.; Wang, C.X.; Wang, S.R.; Jin, X.C. Phosphorus release in response to pH variation in the lake sediments with different ratios of iron-bound P to calcium-bound P. Chem. Spec. Bioavail. 2005, 17, 55–61. [Google Scholar] [CrossRef]
- Kisand, A.; Nõges, P. Sediment phosphorus release in phytoplankton dominated versus macrophyte dominated shallow lakes: Importance of oxygen conditions. Hydrobiologia 2003, 506–509, 129–133. [Google Scholar] [CrossRef]
- Gallego, I.; Venail, P.; Ibelings, B.W. Size differences predict niche and relative fitness differences between phytoplankton species but not their coexistence. ISME J. 2019, 13, 1133–1143. [Google Scholar] [CrossRef]
- Cao, X.; Xiaoyue, L.; Jiamei, Z.; Lisha, W.; Sumei, L.; Guipeng, Y. Characterization of phosphorus sorption on the sediments of Yangtze River Estuary and its adjacent areas. Mar. Pollut. Bull. 2017, 114, 277–284. [Google Scholar] [CrossRef]
- Li, M.; Hou, Y.L.; Zhu, B. Phosphorus sorption-desorption by purple soils of China in relation to their properties. Aust. J. Soil Res. 2007, 45, 182–189. [Google Scholar] [CrossRef]
- Chardon, W.J.; Blaauw, D. Kinetic Freundlich equation applied to soils with a high residual phosphorus content. Soil Sci. 1998, 163, 30–35. [Google Scholar] [CrossRef]
- Nürnberg, G.K.; Tarvainen, M.; Ventellä, A.-M.; Sarvala, J. Internal phosphorus load estimation during biomanipulation in a large polymictic and mesotrophic lake. Inland Waters 2012, 2, 147–162. [Google Scholar] [CrossRef]
- Bao, L.L.; Li, X.Y.; Su, J.J. Alteration in the potential of sediment phosphorus release along series of rubber dams in a typical urban landscape river. Sci. Rep. 2020, 10, 2714. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Chen, X.Y.; Song, C.L.; Zhou, Y.Y. Comparison of phosphorus sorption characteristics in the soils of riparian buffer strips with different land use patterns and distances from the shoreline around Lake Chaohu. J. Soils Sediments 2019, 19, 2322–2329. [Google Scholar] [CrossRef]
- Wen, C.-Y. Brief analysis on the environmental problems of 36 feet lake in pingtan county and the countermeasures of surrounding projects. Straits Sci. 2010, 3, 11–13. [Google Scholar]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 41–52. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; He, J.; Luo, X.; Zheng, Z. Phosphorus mobility among sediments, water and cyanobacteria enhanced by cyanobacteria blooms in eutrophic Lake Dianchi. Environ. Pollut. 2016, 219, 580–587. [Google Scholar] [CrossRef]
- Ruban, V.; López-Sánchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—A synthesis of recent works. Fresenius J. Anal. Chem. 2001, 370, 224–228. [Google Scholar] [CrossRef]
- Mittal, M.; Rockne, K.J. Diffusional losses of amended anaerobic electron acceptors in sediment field microcosms. Mar. Pollut. Bull. 2010, 60, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Ullman, W.J. Diffusion coefficients in near-shore marine sediments. Limnol. Oceanogr. 1982, 27, 552–556. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Islas-Espinoza, M.; Solís-Mejía, L.; Esteller, M.V. Phosphorus release kinetics in a soil amended with biosolids and vermicompost. Environ. Earth Sci. 2013, 71, 1441–1451. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, Y. Variation laws and release characteristics of phosphorus on surface sediment of Dongting Lake. Environ. Sci. Pollut. Res. 2018, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yao, Q.; Yu, Z. Particulate phosphorus speciation and phosphate adsorption characteristics associated with sediment grain size. Ecol. Eng. 2014, 70, 140–145. [Google Scholar] [CrossRef]
- Weng, Y.; Su, Y.; Zhang, Y.; Shao, K.; Zhan, X.; Liu, Y.; Jiang, B. Analysis of endogenous phosphorus load in surface sediments of Shanzi Reservoir in Fujian in different seasons. J. Lake Sci. 2014, 26, 871–878. [Google Scholar]
- Tammeorg, O.; Haldna, M.; Nõges, P.; Appleby, P.; Horppila, J. Factors behind the variability of phosphorus accumulation in Finnish lakes. J. Soils Sediments 2018, 18, 2117–2129. [Google Scholar] [CrossRef]
- Al-Enezi, E.; Bockelmann-Evans, B.; Falconer, R. Phosphorus adsorption/desorption processes of estuarine sediment: A case study-Loughor Estuary, UK. Arab. J. Geosci. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Tang, X.; Wu, M.; Dai, X.; Chaia, P. Phosphorus storage dynamics and adsorption characteristics for sediment from a drinking water source reservoir and its relation with sediment compositions. Ecol. Eng. 2014, 64, 276–284. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.; Deng, W.; He, J.; Wng, S.; Jiao, L.; Li, S.; Xu, D. Adsorption characteristics of phosphorus on the surface sediments of Dianchi Lake. Acta Sci. Circum. 2014, 34, 3065–3075. [Google Scholar]
- Sun, W.; Du, B.; Zhao, X.; He, B. Study on the Forms and Adsorption Characteristics of Phosphorus in the Sediment of Pengxi River in the Three Gorges Reservoir Area and the Soil Phosphorus in the Drawdown Zone. Environ. Sci. 2013, 34, 1107–1113. [Google Scholar]
- Garcia-Ruiz, R.; Parra, G.; Guerrero, F.; Lucena, J. Sedimentation of phosphorus fractions and temporal variation in the C:P ratio in La Concepcion reservoir, southern Spain. N. Z. J. Mar. Freshw. Res. 2001, 35, 711–723. [Google Scholar] [CrossRef][Green Version]
- Poister, D.; Armstrong, D.E. Seasonal sedimentation trends in a mesotrophic lake: Influence of diatoms and implications for phosphorus dynamics. Biogeochemistry 2003, 65, 1–13. [Google Scholar] [CrossRef]
- Brock, J.R.; Schulz-Vogt, H.N. Sulfide induces phosphate release from polyphosphate in cultures of a marine Beggiatoa strain. ISME J. 2010, 5, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hanke, A.; Tegetmeyer, H.E.; Kattelmann, I.; Sharma, R.; Hamann, E.; Hargesheimer, T.; Kraft, B.; Lenk, S.; Geelhoed, J.S.; et al. Impacts of chemical gradients on microbial community structure. ISME J. 2017, 11, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F.; Littmann, S.; Lavik, G.; Escrig, S.; Meibom, A.; Kuypers, M.M.M.; Ackermann, M. Phenotypic heterogeneity driven by nutrient limitation promotes growth in fluctuating environments. Nat. Microbiol. 2016, 1, 16055. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Deng, Y.; Ye, P.D. Semiconducting black phosphorus: Synthesis, transport properties and electronic applications. Chem. Soc. Rev. 2015, 44, 2732–2743. [Google Scholar] [CrossRef]
- Martinez, R.J.; Wu, C.H.; Beazley, M.J.; Andersen, G.L.; Conrad, M.E.; Hazen, T.C. Microbial Community Responses to Organophosphate Substrate Additions in Contaminated Subsurface Sediments. PLoS ONE 2014, 9, e100383. [Google Scholar] [CrossRef]


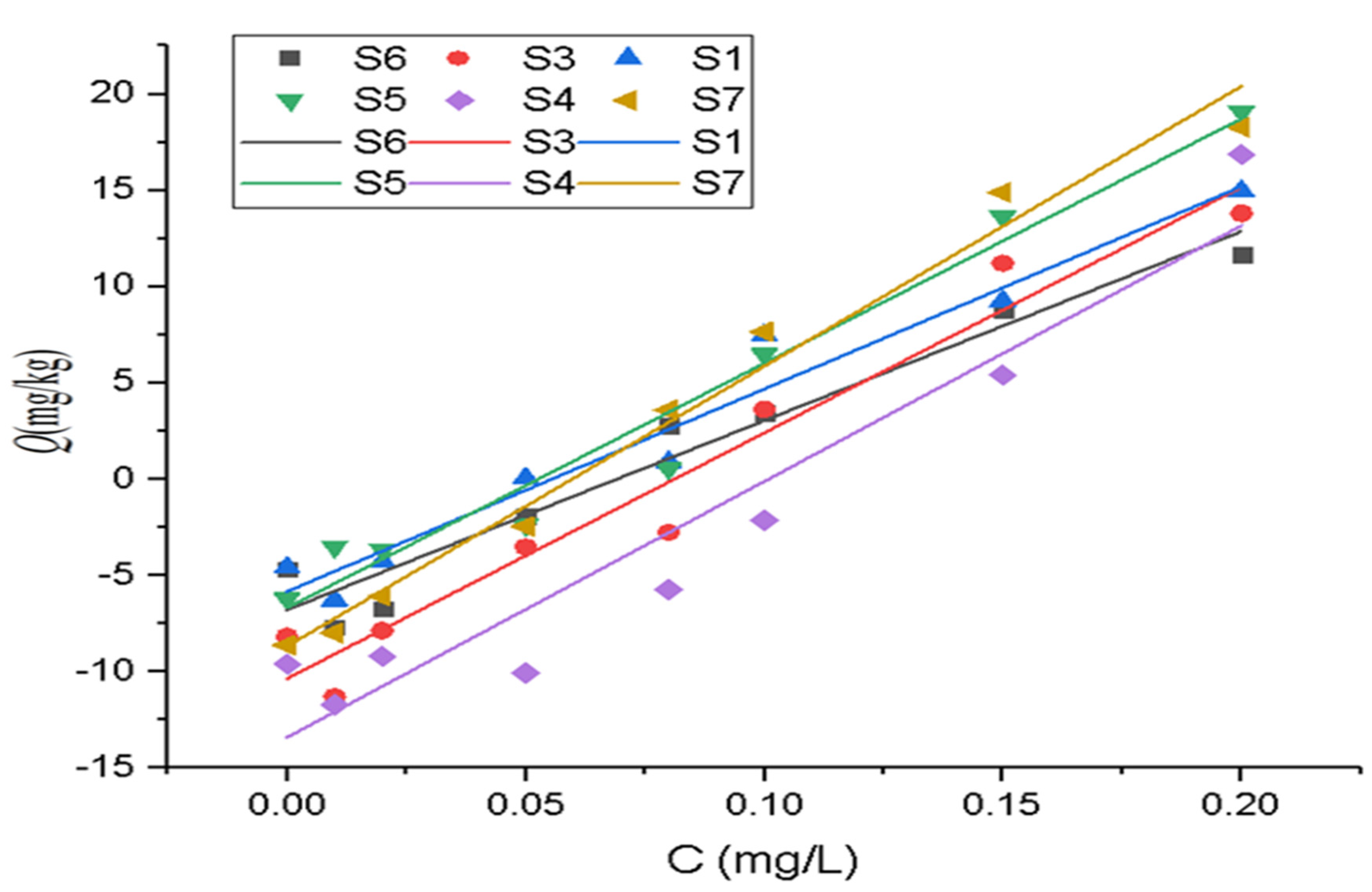

| Site | ln q = B + kt | q = a + kt1/2 | q = a + b ln t | ln q = a + b ln t | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | k | R2 | a | k | R2 | a | b | R2 | a | b | R2 | |
| S1 | 4.09 | 0.02 | 0.46 | 51.77 | 7.26 | 0.88 | 61.68 | 6.12 | 0.90 | 4.12 | 0.11 | 0.94 |
| S3 | 4.18 | 0.02 | 0.37 | 58.03 | 7.98 | 0.71 | 68.26 | 7.55 | 0.94 | 4.20 | 0.11 | 0.93 |
| S4 | 4.11 | 0.02 | 0.37 | 52.72 | 7.60 | 0.80 | 62.70 | 6.90 | 0.97 | 4.13 | 0.10 | 0.95 |
| S5 | 4.10 | 0.02 | 0.33 | 53.38 | 7.86 | 0.68 | 63.34 | 7.56 | 0.95 | 4.12 | 0.12 | 0.93 |
| S6 | 4.07 | 0.02 | 0.62 | 54.73 | 6.52 | 0.71 | 63.05 | 6.21 | 0.96 | 4.11 | 0.09 | 0.92 |
| S7 | 4.07 | 0.02 | 0.40 | 51.37 | 7.50 | 0.72 | 61.11 | 6.96 | 0.92 | 4.09 | 0.11 | 0.92 |
| Site | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | K (L/mg) | c | R2 | Kf | 1/n | R2 | |
| S1 | 1.60 | 0.05 | 0.22 | 0.999 | 0.08 | 0.7 | 0.993 |
| S3 | 1.00 | 0.09 | 0.01 | 0.997 | 0.08 | 0.77 | 0.984 |
| S4 | 3.30 | 0.02 | 0.28 | 0.996 | 0.07 | 0.73 | 0.987 |
| S5 | 3.36 | 0.02 | 0.29 | 0.994 | 0.07 | 0.71 | 0.990 |
| S6 | 1.18 | 0.08 | 0.07 | 0.999 | 0.08 | 0.77 | 0.986 |
| S7 | 5.00 | 0.02 | 0.26 | 0.996 | 0.08 | 0.76 | 0.988 |
| Point | a | B (L/g) | R2 | EPC0 (mg/L) | NAP (mg/kg) |
|---|---|---|---|---|---|
| S1 | −5.82 | 105.12 | 0.96 | 0.06 | 5.82 |
| S3 | −10.34 | 127.62 | 0.95 | 0.08 | 10.34 |
| S4 | −13.4 | 132.88 | 0.91 | 0.1 | 13.4 |
| S5 | −6.68 | 127.03 | 0.96 | 0.05 | 6.68 |
| S6 | −6.78 | 98.28 | 0.95 | 0.07 | 6.78 |
| S7 | −8.67 | 145.41 | 0.98 | 0.06 | 8.67 |
| Seasons and Sample | Length of T-RFs Fragments(bp) | |
|---|---|---|
| Summer | S3 | 106 107 109 110 172 225 229 236 240 242 244 248 249 253 255 259 260 282 286 481 |
| S1 | 43 48 62 63 65 92 102 107 110 112 126 132 135 140 181 224 228 230 232 236 238 243 245 246 247 251 252 253 262 264 294 319 321 322 474 476 562 | |
| Autumn | S3 | 35 39 40 48 55 58 177 179 180 181 182 184 |
| S1 | 38 46 54 57 176 178 179 180 181 183 | |
| Seasons and Sample | Shannon Index | Pielou Index | Dominance Index | |
|---|---|---|---|---|
| Summer | S3 | 2.73 | 0.916 | 0.790 |
| S1 | 2.87 | 0.834 | 0.765 | |
| Autumn | S3 | 2.19 | 0.876 | 0.691 |
| S1 | 2.07 | 0.890 | 0.674 | |
| Sample | Fe/Al-P (mg/kg) | Ca-P (mg/kg) | IP (mg/kg) | OP (mg/kg) | TP (mg/kg) | |
|---|---|---|---|---|---|---|
| Summer | S3 | 215.4 ± 6.9 | 94.5 ± 4.5 | 272.5 ± 2.5 | 192.6 ± 1.2 | 512.3 ± 7.2 |
| S1 | 129.9 ± 6.7 | 74.2 ± 2.9 | 199.1 ± 4.9 | 159.3 ± 1.2 | 362.1 ± 7.4 | |
| Autumn | S3 | 164.6 ± 6.5 | 88.7 ± 0.9 | 210.4 ± 2.4 | 218.4 ± 1.2 | 522.9 ± 7.0 |
| S1 | 152.6 ± 3.3 | 86.2 ± 1.4 | 222.0 ± 9.4 | 208.8 ± 3.5 | 503.7 ± 2.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaji Prasath, B.; Lin, Z.-R.; Su, Y.-P.; She, C.-X.; Lin, H.; Zhang, C.-W.; Yang, H. Adsorption-Release Characteristics of Phosphorus and the Community of Phosphorus Accumulating Organisms of Sediments in a Shallow Lake. Sustainability 2021, 13, 11501. https://doi.org/10.3390/su132011501
Balaji Prasath B, Lin Z-R, Su Y-P, She C-X, Lin H, Zhang C-W, Yang H. Adsorption-Release Characteristics of Phosphorus and the Community of Phosphorus Accumulating Organisms of Sediments in a Shallow Lake. Sustainability. 2021; 13(20):11501. https://doi.org/10.3390/su132011501
Chicago/Turabian StyleBalaji Prasath, Barathan, Zhi-Rong Lin, Yu-Ping Su, Chen-Xing She, Hong Lin, Chao-Wei Zhang, and Hong Yang. 2021. "Adsorption-Release Characteristics of Phosphorus and the Community of Phosphorus Accumulating Organisms of Sediments in a Shallow Lake" Sustainability 13, no. 20: 11501. https://doi.org/10.3390/su132011501
APA StyleBalaji Prasath, B., Lin, Z.-R., Su, Y.-P., She, C.-X., Lin, H., Zhang, C.-W., & Yang, H. (2021). Adsorption-Release Characteristics of Phosphorus and the Community of Phosphorus Accumulating Organisms of Sediments in a Shallow Lake. Sustainability, 13(20), 11501. https://doi.org/10.3390/su132011501








