Assuaging Microalgal Harvesting Woes via Attached Growth: A Critical Review to Produce Sustainable Microalgal Feedstock
Abstract
:1. Introduction
2. Suspended Microalgal Cultivation
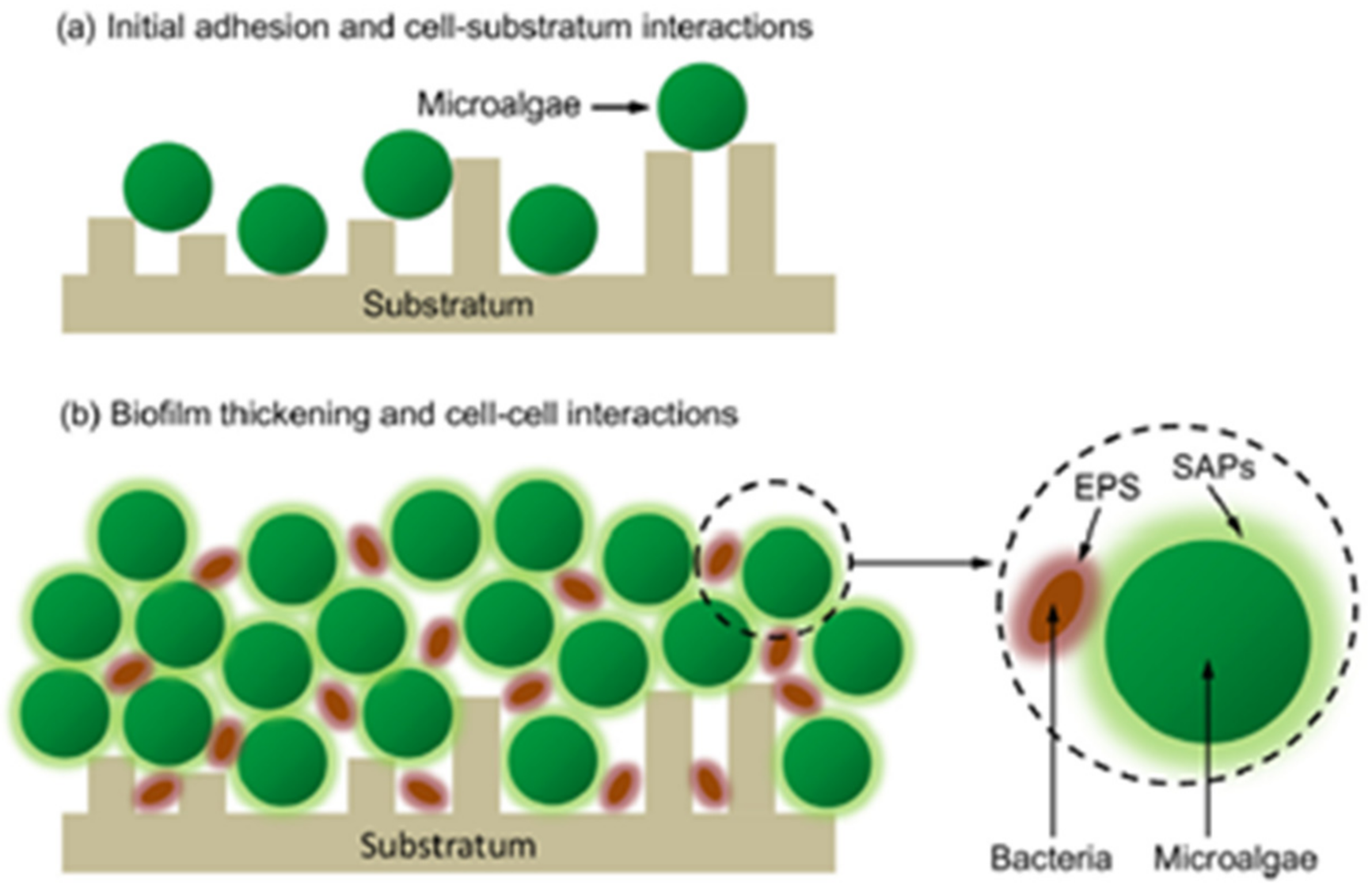
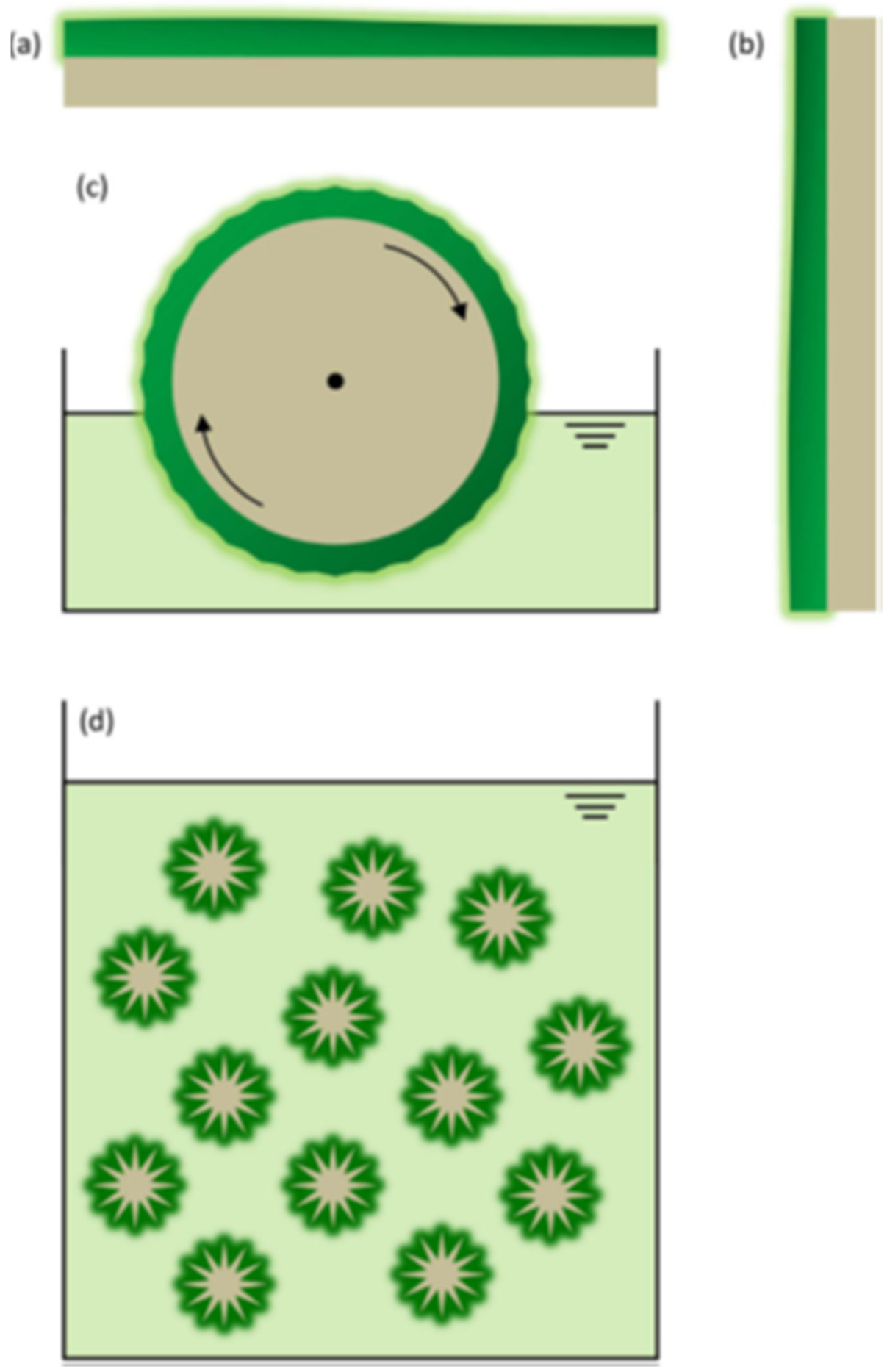
3. Attached Microalgal Cultivation
| Algal Species | Attachment Material | Biomass (gm−2) | Productivity (gm−2 day−1) | Reference |
|---|---|---|---|---|
| Botryococcus braunii | Concrete | 25 | 0.71 | [75] |
| Chlorella sp. | Polystyrene | 25.65 | 2.59 | [79] |
| Scenedesmus obliquus | Glass | 29.4 | 2.10 | [98] |
| Nitzschia palea | Glass | 39.2 | 2.80 | [98] |
| Scenedesmus obliquus, Chlorella vulgaris, Coccomyxa sp., Nannochloris sp., Nitschia palea, Oocystis sp., Oocystis polymorpha | Polycarbonate | 1.58 | 1.25 | [99] |
| Isochrysis sp. | Printing paper | 10 | 0.6 | [100] |
| Tetraselmi ssuecica | Printing paper | 15 | 1.5 | [100] |
| Phaeodactylum tricornutum | Printing paper | 12.4 | 1.8 | [100] |
| Chlorella vulgaris | Cotton duct | 25 | 3.51 | [101] |
| Scenedesmus obliquus | Filter paper | 10.6–83.7 | 1.33–10.46 | [102] |
| Botryococcus braunii | Cellulose acetate | 10–51 | 1–6.45 | [103] |
4. Effect of pH on Attached Microalgal Growth
5. Effect of Hydrophobicity and Hydrophilicity on Attached Microalgae Growth
6. Effect of Substratum Surface Properties on Attached Microalgal Growth
7. Effect of Photoperiod and Light Intensity on Attached Microalgal Growth
8. Ways Forward for Sustainable Cultivation of Attached Microalgae
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dragone, G.; Fernandes, B.; Vicente, A.; Teixeira, J. Third generation biofuels from microalgae. Microb. Biotechnol. 2010, 2, 1355–1366. [Google Scholar]
- Alam, F.; Mobin, S.; Chowdhury, H. Third Generation Biofuel from Algae. Procedia Eng. 2015, 105, 763–768. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Lee, R.; Lavoie, J.-M. From First- to Third-Generation Biofuels: Challenges of Producing a Commodity from a Biomass of Increasing Complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for emergy evaluation of first, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms. Water 2012, 4, 460–473. [Google Scholar] [CrossRef]
- Guzzon, A.; Di Pippo, F.; Congestri, R. Wastewater Biofilm Photosynthesis in Photobioreactors. Microorganisms 2019, 7, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeselers, G.; Loosdrecht, M.C.; Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 2008, 20, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, S.S.; Bong, K.X.; Mubashir, M.; Cheng, C.K.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Munawaroh, H.S.H.; Show, P.L. Microalgae Cultivation in Palm Oil Mill Effluent (POME) Treatment and Biofuel Production. Sustainability 2021, 13, 3247. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.-L.; Lau, B.F.; Chang, J.-S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A realistic scenario on microalgae based biodiesel production: Third generation biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Molina-Miras, A.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. A new approach to finding optimal centrifugation conditions for shear-sensitive microalgae. Algal Res. 2019, 44, 101677. [Google Scholar] [CrossRef]
- Rossi, S.; Visigalli, S.; Castillo Cascino, F.; Mantovani, M.; Mezzanotte, V.; Parati, K.; Canziani, R.; Turolla, A.; Ficara, E. Metal-based flocculation to harvest microalgae: A look beyond separation efficiency. Sci. Total Environ. 2021, 799, 149395. [Google Scholar] [CrossRef]
- Foteinis, S.; Chatzisymeon, E.; Litinas, A.; Tsoutsos, T. Used-cooking-oil biodiesel: Life cycle assessment and comparison with first- and third-generation biofuel. Renew. Energy 2020, 153, 588–600. [Google Scholar] [CrossRef]
- Sydney, E.B.; Sturm, W.; de Carvalho, J.C.; Thomaz-Soccol, V.; Larroche, C.; Pandey, A.; Soccol, C.R. Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [Google Scholar] [CrossRef]
- Kebelmann, K.; Hornung, A.; Karsten, U.; Griffiths, G. Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 2013, 49, 38–48. [Google Scholar] [CrossRef]
- Han, F.; Huang, J.; Li, Y.; Wang, W.; Wang, J.; Fan, J.; Shen, G. Enhancement of microalgal biomass and lipid productivities by a model of photoautotrophic culture with heterotrophic cells as seed. Bioresour. Technol. 2012, 118, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Brilman, D.W.F.; Withag, J.A.M.; Brem, G.; Kersten, S. Assessment of a dry and a wet route for the production of biofuels from microalgae: Energy balance analysis. Bioresour. Technol. 2011, 102, 5113–5122. [Google Scholar] [CrossRef] [PubMed]
- Biapa, P.; Moukette, B. Chemical Composition of Spirulina Platensis of Nomayos-Yaounde (Cameroon). Annals. Food Sci. Technol. 2016, 17, 524–528. [Google Scholar]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef]
- Yadavalli, R.; Rao, C.S.; Rao, R.S.; Potumarthi, R. Dairy effluent treatment and lipids production by Chlorella pyrenoidosa and Euglena gracilis: Study on open and closed systems. Asia-Pac. J. Chem. Eng. 2014, 9, 368–373. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, W.; Qiu, Y.A.; Wang, S.; Peng, Y.; Zhu, Y.; Zhuang, S. Lipids monitoring in Scenedesmus obliquus based on terahertz technology. Biotechnol. Biofuels 2020, 13, 161. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El Baroty, G.S.; El Baz, F.K.; Abd El Baky, H.H.; Murad, S.A. Scenedesmus obliquus: Antioxidant and antiviral activity of proteins hydrolyzed by three enzymes. J. Genet. Eng. Biotechnol. 2018, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-K.; Yun, H.-S.; Hwang, J.-H.; Salama, E.-S.; Jeon, B.-H.; Choi, J. Effect of flue gas CO2 on the growth, carbohydrate and fatty acid composition of a green microalga Scenedesmus obliquus for biofuel production. Environ. Technol. 2016, 38, 1–8. [Google Scholar] [CrossRef]
- Andreotti, V.; Solimeno, A.; Rossi, S.; Ficara, E.; Marazzi, F.; Mezzanotte, V.; García, J. Bioremediation of aquaculture wastewater with the microalgae Tetraselmis suecica: Semi-continuous experiments, simulation and photo-respirometric tests. Sci. Total Environ. 2020, 738, 139859. [Google Scholar] [CrossRef] [PubMed]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; García-Camacho, F.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass nutrient profiles of the microalga Nannochloropsis. J. Agric. Food Chem. 2001, 49, 2966–2972. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Chua, E.T.; Schenk, P.M. A biorefinery for Nannochloropsis: Induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernaerts, T.M.M.; Verstreken, H.; Dejonghe, C.; Gheysen, L.; Foubert, I.; Grauwet, T.; Van Loey, A.M. Cell disruption of Nannochloropsis sp. improves in vitro bioaccessibility of carotenoids and ω3-LC-PUFA. J. Funct. Foods 2020, 65, 103770. [Google Scholar] [CrossRef]
- Ashour, M.; Kamel, A. Enhance Growth and Biochemical Composition of Nannochloropsis oceanica, Cultured under Nutrient Limitation, Using Commercial Agricultural Fertilizers. J. Mar. Sci. Res. Dev. 2017, 7, 233. [Google Scholar]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Bjornsson, W.J.; McGinn, P.J. Biochemical composition and amino acid profiles of Nannochloropsis granulata algal biomass before and after supercritical fluid CO2 extraction at two processing temperatures. Anim. Feed Sci. Technol. 2015, 204, 62–71. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Borowitzka, L.J. Micro-Algal Biotechnology; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Safafar, H.; Hass, M.Z.; Møller, P.; Holdt, S.L.; Jacobsen, C. High-EPA biomass from Nannochloropsis salina cultivated in a flat-panel photo-bioreactor on a process water-enriched growth medium. Mar. Drugs 2016, 14, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molino, A.; Martino, M.; Larocca, V.; Di Sanzo, G.; Spagnoletta, A.; Marino, T.; Karatza, D.; Iovine, A.; Mehariya, S.; Musmarra, D. Eicosapentaenoic acid extraction from Nannochloropsis gaditana using carbon dioxide at supercritical conditions. Mar. Drugs 2019, 17, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, M.; Patidar, S.K.; George, B.; Shah, F.; Mishra, S. A euryhaline Nannochloropsis gaditana with potential for nutraceutical (EPA) and biodiesel production. Algal Res. 2015, 8, 161–167. [Google Scholar] [CrossRef]
- Katarzyna, L.; Sai, G.; Singh, O.A. Non-enclosure methods for non-suspended microalgae cultivation: Literature review and research needs. Renew. Sustain. Energy Rev. 2015, 42, 1418–1427. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.-L.; Wang, J.-H.; Hu, H.-Y. Differences between attached and suspended microalgal cells in ssPBR from the perspective of physiological properties. J. Photochem. Photobiol. B Biol. 2018, 181, 164–169. [Google Scholar]
- Gross, M.; Jarboe, D.; Wen, Z. Biofilm-based algal cultivation systems. Appl. Microbiol. Biotechnol. 2015, 99, 5781–5789. [Google Scholar] [CrossRef]
- Gross, M.A. Development and Optimization of Algal Cultivation Systems; Iowa State University: Ames, IW, USA, 2013. [Google Scholar]
- Pal, P.; Chew, K.W.; Yen, H.-W.; Lim, J.W.; Lam, M.K.; Show, P.L. Cultivation of Oily Microalgae for the Production of Third-Generation Biofuels. Sustainability 2019, 11, 5424. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Tao, W.; Chen, F. High-value biomass from microalgae production platforms: Strategies and progress based on carbon metabolism and energy conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef] [Green Version]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Sforza, E.; Pastore, M.; Bellucci, M.; Casagli, F.; Marazzi, F.; Ficara, E. Photo-respirometry to shed light on microalgae-bacteria consortia—A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 43–72. [Google Scholar] [CrossRef]
- Leong, W.H.; Zaine, S.N.A.; Ho, Y.C.; Uemura, Y.; Lam, M.K.; Khoo, K.S.; Kiatkittipong, W.; Cheng, C.K.; Show, P.L.; Lim, J.W. Impact of various microalgal-bacterial populations on municipal wastewater bioremediation and its energy feasibility for lipid-based biofuel production. J. Environ. Manag. 2019, 249, 109384. [Google Scholar] [CrossRef]
- Pate, R.; Klise, G.; Wu, B. Resource demand implications for US algae biofuels production scale-up. Appl. Energy 2011, 88, 3377–3388. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Wang, J.-H.; Zhuang, L.-L.; Xu, X.-Q.; Deantes-Espinosa, V.M.; Wang, X.-X.; Hu, H.-Y. Microalgal attachment and attached systems for biomass production and wastewater treatment. Renew. Sustain. Energy Rev. 2018, 92, 331–342. [Google Scholar] [CrossRef]
- Lau, K.Y.; Pleissner, D.; Lin, C.S.K. Recycling of food waste as nutrients in Chlorella vulgaris cultivation. Bioresour. Technol. 2014, 170, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Mirzaie, M.A.; Kalbasi, M.; Mousavi, S.M.; Ghobadian, B. Investigation of mixotrophic, heterotrophic, and autotrophic growth of Chlorella vulgaris under agricultural waste medium. Prep. Biochem. Biotechnol. 2016, 46, 150–156. [Google Scholar] [CrossRef]
- Manzoor, M.; Jabeen, F.; Ahmad, Q.-U.-A.; Younis, T.; Eltanahy, E.; Schenk, P.M. Sugarcane Bagasse Hydrolysate as Organic Carbon Substrate for Mixotrophic Cultivation of Nannochloropsis sp. BR2. Waste Biomass Valorization 2021, 12, 2321–2331. [Google Scholar] [CrossRef]
- Ran, W.; Wang, H.; Liu, Y.; Qi, M.; Xiang, Q.; Yao, C.; Zhang, Y.; Lan, X. Storage of starch and lipids in microalgae: Biosynthesis and manipulation by nutrients. Bioresour. Technol. 2019, 291, 121894. [Google Scholar] [CrossRef]
- Leong, W.-H.; Lim, J.-W.; Lam, M.-K.; Uemura, Y.; Ho, Y.-C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Gross, M.; Wen, Z. Yearlong evaluation of performance and durability of a pilot-scale Revolving Algal Biofilm (RAB) cultivation system. Bioresour. Technol. 2014, 171, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Balancing the organic load and light supply in symbiotic microalgal–bacterial biofilm reactors treating synthetic municipal wastewater. Ecol. Eng. 2014, 64, 213–221. [Google Scholar] [CrossRef]
- Gross, M.; Zhao, X.; Mascarenhas, V.; Wen, Z. Effects of the surface physico-chemical properties and the surface textures on the initial colonization and the attached growth in algal biofilm. Biotechnol. Biofuels 2016, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rinanti, A.; Purwadi, R. Harvesting of freshwater microalgae biomass by Scenedesmus sp. as bioflocculant. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 27–28 September 2018; p. 012087. [Google Scholar]
- Molina-Miras, A.; López-Rosales, L.; Sánchez-Mirón, A.; Cerón-García, M.C.; Seoane-Parra, S.; García-Camacho, F.; Molina-Grima, E. Long-term culture of the marine dinoflagellate microalga Amphidinium carterae in an indoor LED-lighted raceway photobioreactor: Production of carotenoids and fatty acids. Bioresour. Technol. 2018, 265, 257–267. [Google Scholar] [CrossRef]
- Xu, Y.; Milledge, J.J.; Abubakar, A.; Swamy, R.A.R.; Bailey, D.; Harvey, P.J. Effects of centrifugal stress on cell disruption and glycerol leakage from Dunaliella salina. Microalgae Biotechnol. 2015, 1, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Maybury, J.P.; Hoare, M.; Dunnill, P. The use of laboratory centrifugation studies to predict performance of industrial machines: Studies of shear-insensitive and shear-sensitive materials. Biotechnol. Bioeng. 2000, 67, 265–273. [Google Scholar] [CrossRef]
- Molina-Miras, A.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. CFD-aided optimization of a laboratory-scale centrifugation for a shear-sensitive insect cell line. Food Bioprod. Process. 2018, 107, 113–120. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visigalli, S.; Barberis, M.G.; Turolla, A.; Canziani, R.; Berden Zrimec, M.; Reinhardt, R.; Ficara, E. Electrocoagulation–flotation (ECF) for microalgae harvesting—A review. Sep. Purif. Technol. 2021, 271, 118684. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Ferrer, I.; González-Molina, A.; Salvadó, H.; García, J.; Uggetti, E. Microalgae recycling improves biomass recovery from wastewater treatment high rate algal ponds. Water Res. 2016, 106, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Gerde, J.A.; Yao, L.; Lio, J.; Wen, Z.; Wang, T. Microalgae flocculation: Impact of flocculant type, algae species and cell concentration. Algal Res. 2014, 3, 30–35. [Google Scholar] [CrossRef]
- Sanyano, N.; Chetpattananondh, P.; Chongkhong, S. Coagulation–flocculation of marine Chlorella sp. for biodiesel production. Bioresour. Technol. 2013, 147, 471–476. [Google Scholar] [CrossRef]
- Surendhiran, D.; Vijay, M. Study on flocculation efficiency for harvesting Nannochloropsis oculata for biodiesel production. Int. J. Chem. Technol. Res. 2013, 5, 1761–1769. [Google Scholar]
- Leong, H.Y.; Chang, C.-K.; Lim, J.W.; Show, P.L.; Lin, D.-Q.; Chang, J.-S. Liquid Biphasic Systems for Oil-Rich Algae Bioproducts Processing. Sustainability 2019, 11, 4682. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Khoiroh, I.; Ling, T.C.; Show, P.L. Aqueous Two-Phase Flotation for the Recovery of Biomolecules. Sep. Purif. Rev. 2016, 45, 81–92. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.-L.; Yu, D.; Zhang, J.; Liu, F.-F.; Wu, Y.-H.; Zhang, T.-Y.; Dao, G.-H.; Hu, H.-Y. The characteristics and influencing factors of the attached microalgae cultivation: A review. Renew. Sustain. Energy Rev. 2018, 94, 1110–1119. [Google Scholar] [CrossRef]
- Ozkan, A.; Kinney, K.; Katz, L.; Berberoglu, H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012, 114, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Wen, Z. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010, 85, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Cerón-García, M.C.; Camacho, F.; Mirón, A.; Fernandez-Sevilla, J.M.; Chisti, Y.; Molina-Grima, E. Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. J. Microbiol. Biotechnol. 2006, 16, 689. [Google Scholar]
- Abiusi, F.; Wijffels, R.H.; Janssen, M. Doubling of Microalgae Productivity by Oxygen Balanced Mixotrophy. ACS Sustain. Chem. Eng. 2020, 8, 6065–6074. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.T.; Bangert, K.; Wilkinson, S.J.; Gilmour, D.J. Synergistic carbon metabolism in a fast growing mixotrophic freshwater microalgal species Micractinium inermum. Biomass Bioenergy 2015, 82, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Grama, B.S.; Agathos, S.N.; Jeffryes, C.S. Balancing Photosynthesis and Respiration Increases Microalgal Biomass Productivity during Photoheterotrophy on Glycerol. ACS Sustain. Chem. Eng. 2016, 4, 1611–1618. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.P. Growth of Chlorella sorokiniana on a mixture of volatile fatty acids: The effects of light and temperature. Bioresour. Technol. 2015, 198, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Marquez, F.J.; Nishio, N.; Nagai, S.; Sasaki, K. Enhancement of biomass and pigment production during growth of Spirulina platensis in mixotrophic culture. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1995, 62, 159–164. [Google Scholar]
- Ratnapuram, H.P.; Vutukuru, S.S.; Yadavalli, R. Mixotrophic transition induced lipid productivity in Chlorella pyrenoidosa under stress conditions for biodiesel production. Heliyon 2018, 4, e00496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajadian, S.F.; Morowvat, M.H.; Ghasemi, Y. Investigation of autotrophic, heterotrophic, and mixotrophic modes of cultivation on lipid and biomass production in Chlorella vulgaris. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 594–599. [Google Scholar] [CrossRef]
- Alonso, D.L.; Belarbi, E.-H.; Fernández-Sevilla, J.M.; Rodríguez-Ruiz, J.; Grima, E.M. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 2000, 54, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Ning, K.; Zeng, X.; Luo, Y.; Wang, D.; Hu, J.; Li, J.; Xu, H.; Huang, J.; Wan, M. Genomic foundation of starch-to-lipid switch in oleaginous Chlorella spp. Plant Physiol. 2015, 169, 2444–2461. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, G.; Sekoulov, I. Polishing of secondary effluent by an algal biofilm process. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2002, 46, 83–90. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol. Bioeng. 2012, 109, 1674–1684. [Google Scholar] [CrossRef]
- Ozkan, A.; Berberoglu, H. Cell to substratum and cell to cell interactions of microalgae. Colloids Surf. B Biointerfaces 2013, 112, 302–309. [Google Scholar] [CrossRef]
- Bernard, O.; Rémond, B. Validation of a simple model accounting for light and temperature effect on microalgal growth. Bioresour. Technol. 2012, 123, 520–527. [Google Scholar] [CrossRef]
- Rossi, S.; Casagli, F.; Mantovani, M.; Mezzanotte, V.; Ficara, E. Selection of photosynthesis and respiration models to assess the effect of environmental conditions on mixed microalgae consortia grown on wastewater. Bioresour. Technol. 2020, 305, 122995. [Google Scholar] [CrossRef] [PubMed]
- Ippoliti, D.; Gómez, C.; del Mar Morales-Amaral, M.; Pistocchi, R.; Fernández-Sevilla, J.M.; Acién, F.G. Modeling of photosynthesis and respiration rate for Isochrysis galbana (T-Iso) and its influence on the production of this strain. Bioresour. Technol. 2016, 203, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rosli, S.-S.; Lim, J.-W.; Uemura, Y.; Lam, M.-K.; Isa, M.H.; Oh, W.-D.; Sakidin, H. pH optimization to promote attached growth of microalgae biomass onto polyurethane foam material. AIP Conf. Proc. 2018, 2016, 020123. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R. Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Rep. 2019, 5, 327–336. [Google Scholar] [CrossRef]
- Schnurr, P.J.; Espie, G.S.; Allen, D.G. Algae biofilm growth and the potential to stimulate lipid accumulation through nutrient starvation. Bioresour. Technol. 2013, 136, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Genin, S.N.; Stewart Aitchison, J.; Grant Allen, D. Design of algal film photobioreactors: Material surface energy effects on algal film productivity, colonization and lipid content. Bioresour. Technol. 2014, 155, 136–143. [Google Scholar] [CrossRef]
- Naumann, T.; Çebi, Z.; Podola, B.; Melkonian, M. Growing microalgae as aquaculture feeds on twin-layers: A novel solid-state photobioreactor. J. Appl. Phycol. 2013, 25, 1413–1420. [Google Scholar] [CrossRef]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Hu, Q.; Cheng, P.; Ji, B.; Liu, J.; Chen, Y.; Zhang, W.; Chen, X.; Chen, L. Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour. Technol. 2013, 127, 216–222. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Liu, T. Effects of nitrogen source and nitrogen supply model on the growth and hydrocarbon accumulation of immobilized biofilm cultivation of B. braunii. Bioresour. Technol. 2014, 166, 527–533. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Hu, H.-Y.; Wu, Y.-H.; Wang, T.; Zhang, T.-Y. A novel suspended-solid phase photobioreactor to improve biomass production and separation of microalgae. Bioresour. Technol. 2014, 153, 399–402. [Google Scholar] [CrossRef]
- Economou, C.N.; Marinakis, N.; Moustaka-Gouni, M.; Kehayias, G.; Aggelis, G.; Vayenas, D.V. Lipid production by the filamentous cyanobacterium Limnothrix sp. growing in synthetic wastewater in suspended- and attached-growth photobioreactor systems. Ann. Microbiol. 2015, 65, 1941–1948. [Google Scholar] [CrossRef]
- Lam, M.K.; Yusoff, M.I.; Uemura, Y.; Lim, J.W.; Khoo, C.G.; Lee, K.T.; Ong, H.C. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renew. Energy 2017, 103, 197–207. [Google Scholar] [CrossRef]
- Gonçalves, A.; Ferreira, C.; Loureiro, J.; Pires, J.; Simões, M. Surface physicochemical properties of selected single and mixed cultures of microalgae and cyanobacteria and their relationship with sedimentation kinetics. Bioresour. Bioprocess. 2015, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sekar, R.; Venugopalan, V.P.; Satpathy, K.K.; Nair, K.V.K.; Rao, V.N.R. Laboratory studies on adhesion of microalgae to hard substrates. Hydrobiologia 2004, 512, 109–116. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, X.; Xu, Y.; Ding, Y.; Geng, Y.; Li, Y. Maximizing CO2 biofixation and lipid productivity of oleaginous microalga Graesiella sp. WBG 1 via CO2-regulated pH in indoor and outdoor open reactors. Sci. Total Environ. 2018, 619–620, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Irving, T.E.; Allen, D.G. Species and material considerations in the formation and development of microalgal biofilms. Appl. Microbiol. Biotechnol. 2011, 92, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yuan, W.; Pei, Z.J.; Davis, T.; Cui, Y.; Beltran, M. A Preliminary Study of the Effect of Surface Texture on Algae Cell Attachment for a Mechanical-Biological Energy Manufacturing System. J. Manuf. Sci. Eng. Trans. ASME 2009, 131, 064505. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Romero-Ogawa, M.A.; Vannela, R.; Lai, Y.S.; Rittmann, B.E.; Parra-Saldivar, R. Effects of light intensity and carbon dioxide on lipids and fatty acids produced by Synechocystis sp. PCC6803 during continuous flow. Algal Res. 2015, 12, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Xu, K.; Chang, W.; Qu, Y.; Li, Y. A novel microalgal biofilm reactor using walnut shell as substratum for microalgae biofilm cultivation and lipid accumulation. Renew. Energy 2021, 175, 676–685. [Google Scholar] [CrossRef]
- Yoshimura, T.; Okada, S.; Honda, M. Culture of the hydrocarbon producing microalga Botryococcus braunii strain Showa: Optimal CO2, salinity, temperature, and irradiance conditions. Bioresour. Technol. 2013, 133, 232–239. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, S.; Ho, S.-H.; Xie, Y.; Chen, J. Enhancing lipid production in attached culture of a thermotolerant microalga Desmodesmus sp. F51 using light-related strategies. Biochem. Eng. J. 2018, 129, 119–128. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Liu, T. The difference in effective light penetration may explain the superiority in photosynthetic efficiency of attached cultivation over the conventional open pond for microalgae John Sheehan. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Wang, Y.; Osei-Wusu, D.; Liu, T.; Liu, D. Effects of seed age, inoculum density, and culture conditions on growth and hydrocarbon accumulation of Botryococcus braunii SAG807-1 with attached culture. Bioresour. Bioprocess. 2018, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 282, 245–253. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Guan, L.; Wang, X.; Wang, Y.; Jiang, Z.; Cao, L.; Zhang, X. Influence of Photoperiods on Microalgae Biofilm: Photosynthetic Performance, Biomass Yield, and Cellular Composition. Energies 2019, 12, 3724. [Google Scholar] [CrossRef] [Green Version]
- Martín-Girela, I.; Curt, M.D.; Fernández, J. Flashing light effects on CO2 absorption by microalgae grown on a biofilm photobioreactor. Algal Res. 2017, 25, 421–430. [Google Scholar] [CrossRef]
- Toninelli, E.; Wang, J.; Liu, M.; Wu, H.; Liu, T. Scenedesmus dimorphus biofilm: Photoefficiency and biomass production under intermittent lighting. Sci. Rep. 2016, 6, 32305. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Ho, S.-H.; Chen, C.-N.N.; Chen, C.-Y.; Ng, I.S.; Jing, K.-J.; Chang, J.-S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.; Kim, J.-D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Murphy, T.; Fleming, E.; Berberoglu, H. Vascular Structure Design of an Artificial Tree for Microbial Cell Cultivation and Biofuel Production. Transp. Porous Media 2014, 104, 25–41. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Cui, J.; Yao, X.; Sun, Z.; Feng, Y.; Cui, Q. Simultaneous accumulation of neutral lipids and biomass in Nannochloropsis oceanica IMET1 under high light intensity and nitrogen replete conditions. Algal Res. 2015, 11, 55–62. [Google Scholar] [CrossRef]
- Cheng, P.; Ji, B.; Gao, L.; Zhang, W.; Wang, J.; Liu, T. The growth, lipid and hydrocarbon production of Botryococcus braunii with attached cultivation. Bioresour. Technol. 2013, 138C, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Hou, D.; Li, Y.; Fan, J.; Huang, J.; Liang, S.; Wang, W.; Pan, R.; Wang, J.; Li, S. The effective photoinduction of Haematococcus pluvialis for accumulating astaxanthin with attached cultivation. Bioresour. Technol. 2014, 163, 26–32. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Wang, J.; Chen, Y.; Gao, X.; Zhang, Z.; Liu, T. Attached cultivation for improving the biomass productivity of Spirulina platensis. Bioresour. Technol. 2015, 181, 136–142. [Google Scholar] [CrossRef]
- Ji, C.; Wang, J.; Zhang, W.; Liu, J.; Wang, H.; Gao, L.; Liu, T. An applicable nitrogen supply strategy for attached cultivation of Aucutodesmus obliquus. J. Appl. Phycol. 2014, 26, 173–180. [Google Scholar] [CrossRef]
- Kumar, M.S.; Hwang, J.-H.; Abou-Shanab, R.A.; Kabra, A.N.; Ji, M.-K.; Jeon, B.-H. Influence of CO2 and light spectra on the enhancement of microalgal growth and lipid content. J. Renew. Sustain. Energy 2014, 6, 063107. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Lai, Z.; Zhang, X.; Jiang, Z.; Zhang, X. Analyzing microalgal biofilm structures formed under different light conditions by evaluating cell–cell interactions. J. Colloid Interface Sci. 2021, 583, 563–570. [Google Scholar] [CrossRef]
- Das, P.; Lei, W.; Aziz, S.S.; Obbard, J.P. Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour. Technol. 2011, 102, 3883–3887. [Google Scholar] [CrossRef]
- Baba, M.; Kikuta, F.; Suzuki, I.; Watanabe, M.M.; Shiraiwa, Y. Wavelength specificity of growth, photosynthesis, and hydrocarbon production in the oil-producing green alga Botryococcus braunii. Bioresour. Technol. 2012, 109, 266–270. [Google Scholar] [CrossRef] [Green Version]
- de Mooij, T.; de Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of light color on photobioreactor productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Jafari, N.; Shafiee Alavijeh, R.; Abdolahnejad, A.; Farrokhzadeh, H.; Amin, M.M.; Ebrahimi, A. An innovative approach to attached cultivation of Chlorella vulgaris using different materials. Environ. Sci. Pollut. Res. Int. 2018, 25, 20097–20105. [Google Scholar] [CrossRef]
- Rosli, S.S.; Amalina Kadir, W.N.; Wong, C.Y.; Han, F.Y.; Lim, J.W.; Lam, M.K.; Yusup, S.; Kiatkittipong, W.; Kiatkittipong, K.; Usman, A. Insight review of attached microalgae growth focusing on support material packed in photobioreactor for sustainable biodiesel production and wastewater bioremediation. Renew. Sustain. Energy Rev. 2020, 134, 110306. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, L.; Sun, D.Z. Functions and behaviors of activated sludge extracellular polymeric substances (EPS): A promising environmental interest. J. Environ. Sci. 2006, 18, 420–427. [Google Scholar]
- Shen, Y.; Zhu, W.; Chen, C.; Nie, Y.; Lin, X. Biofilm formation in attached microalgal reactors. Bioprocess Biosyst. Eng. 2016, 39, 1281–1288. [Google Scholar] [CrossRef]
- Chuah, S.Y.; Dasan, Y.K.; Cheng, Y.W.; Lim, J.W.; Ho, Y.C.; Tan, I.S.; Yew Foo, H.C.; Kiew, P.L.; Leong, S.S.; Lam, M.K. The potential of attached growth of microalgae on solid surface for biomass and lipid production. IOP Conf. Ser. Mater. Sci. Eng. 2020, 965, 012001. [Google Scholar] [CrossRef]
- Leong, W.H.; Kiatkittipong, K.; Kiatkittipong, W.; Cheng, Y.W.; Lam, M.K.; Shamsuddin, R.; Mohamad, M.; Lim, J.W. Comparative performances of microalgal-bacterial co-cultivation to bioremediate synthetic and municipal wastewaters whilst producing biodiesel sustainably. Processes 2020, 8, 1427. [Google Scholar] [CrossRef]
- Muys, M.; Coppens, J.; Boon, N.; Vlaeminck, S.E. Photosynthetic oxygenation for urine nitrification. Water Sci. Technol. 2018, 78, 183–194. [Google Scholar] [CrossRef]
- Choudhary, P.; Prajapati, S.K.; Kumar, P.; Malik, A.; Pant, K.K. Development and performance evaluation of an algal biofilm reactor for treatment of multiple wastewaters and characterization of biomass for diverse applications. Bioresour. Technol. 2017, 224, 276–284. [Google Scholar] [CrossRef]
- Liu, J.; Danneels, B.; Vanormelingen, P.; Vyverman, W. Nutrient removal from horticultural wastewater by benthic filamentous algae Klebsormidium sp., Stigeoclonium spp. and their communities: From laboratory flask to outdoor Algal Turf Scrubber (ATS). Water Res. 2016, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Berner, F.; Heimann, K.; Sheehan, M. Microalgal biofilms for biomass production. J. Appl. Phycol. 2014, 27, 1–12. [Google Scholar] [CrossRef]
- Schnurr, P.J.; Allen, D.G. Factors affecting algae biofilm growth and lipid production: A review. Renew. Sustain. Energy Rev. 2015, 52, 418–429. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, J.; Garbayo, I.; Cuaresma, M.; Montero Lobato, Z.; González-delValle, M.A.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [Green Version]
- Croft, M.; Lawrence, A.; Deery, E.; Warren, M.; Smith, A. Algae acquire Vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Teplitski, M.; Rajamani, S. Signal and Nutrient Exchange in the Interactions between Soil Algae and Bacteria. In Biocommunication in Soil Microorganisms; Springer: Berlin/Heidelberg, Germany, 2011; Volume 23, pp. 413–426. [Google Scholar]
- Kim, B.-H.; Ramanan, R.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Bolch, C.J.; Subramanian, T.A.; Green, D.H. The Toxic Dinoflagellate Gymnodinium Catenatum (Dinophyceae) Requires Marine Bacteria For Growth. J. Phycol. 2011, 47, 1009–1022. [Google Scholar] [CrossRef]
- Kazamia, E.; Czesnick, H.; Nguyen, T.T.; Croft, M.T.; Sherwood, E.; Sasso, S.; Hodson, S.J.; Warren, M.J.; Smith, A.G. Mutualistic interactions between vitamin B12 -dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 2012, 14, 1466–1476. [Google Scholar] [CrossRef]
- Czaczyk, K.; Myszka, K. Biosynthesis of Extracellular Polymeric Substances (EPS) and Its Role in Microbial Biofilm Formation. Pol. J. Environ. Stud. 2007, 16, 799–806. [Google Scholar]
- Milferstedt, K.; Kuo-Dahab, W.C.; Butler, C.S.; Hamelin, J.; Abouhend, A.S.; Stauch-White, K.; McNair, A.; Watt, C.; Carbajal-González, B.I.; Dolan, S. The importance of filamentous cyanobacteria in the development of oxygenic photogranules. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Abouhend, A.S.; McNair, A.; Kuo-Dahab, W.C.; Watt, C.; Butler, C.S.; Milferstedt, K.; Hamelin, J.; Seo, J.; Gikonyo, G.J.; El-Moselhy, K.M.; et al. The Oxygenic Photogranule Process for Aeration-Free Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 3503–3511. [Google Scholar] [CrossRef]
- Kumar, R.; Venugopalan, V.P. Development of self-sustaining phototrophic granular biomass for bioremediation applications. Curr. Sci. 2015, 108, 1653–1661. [Google Scholar]
- Tiron, O.; Bumbac, C.; Manea, E.; Stefanescu, M.; Lazar, M.N. Overcoming microalgae harvesting barrier by activated algae granules. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Brockmann, D.; Gérand, Y.; Park, C.; Milferstedt, K.; Hélias, A.; Hamelin, J. Wastewater treatment using oxygenic photogranule-based process has lower environmental impact than conventional activated sludge process. Bioresour. Technol. 2021, 319, 124204. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y. Mass Flow and Energy Efficiency of Municipal Wastewater Treatment Plants; IWA Publishing: London, UK, 2011. [Google Scholar]
- Eom, H.; Brennan, A.; Watt, C.; Chon, D.-H.; Park, C. Performance of a Pilot-Scale High-Rate Anaerobic Side-stream Reactor (ASSR) Process: Minimized Sludge Production and Generation of Biogas. Proc. Water Environ. Fed. 2013, 2013, 2669–2686. [Google Scholar] [CrossRef]

| Microalgae | Lipid (%) | Protein (%) | Carbohydrate (%) | Reference |
|---|---|---|---|---|
| Botryococcus braunii | 33–86 | 4–40 | 20 | [15] |
| Chlamydomonas reinhardtii | 18–22 | 46–48 | 17 | [16] |
| Chlorella ellipsoidea | 10–30 | 34–35 | 24–51 | [17] |
| Chlorella pyrenoidosa | 8–35 | 31–47 | 20–57 | [17] |
| Chlorella vulgaris | 10–50 | 29–58 | 12–17 | [18] |
| Spirulina platensis | 30 | 38 | 24 | [19] |
| Dunaliella tertiolecta | 3–13 | 26–61 | 22 | [20] |
| Euglena gracilis | 11 | 29 | 32 | [21] |
| Scenedesmus obliquus | 18–52 | 34–41 | 22–24 | [22,23,24] |
| Tetraselmis suecica | 5–17 | 37–92 | 5–24 | [25] |
| Nannochloropsis sp. | 15–30 | 27–43 | 10–36 | [26,27,28,29,30] |
| Nannochloropsis oceanica | 31 | 15 | 8 | [31,32] |
| Nannochloropsis granulata | 29 | 46 | 15 | [33] |
| Haematococcus pluvialis | 20–25 | 21–45 | 15–74 | [34,35] |
| Nannochloropsis salina | 6–26 | 18–36 | 18–36 | [36] |
| Nannochloropsis gaditana | 17 | 47 | 22 | [37,38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosmahadi, N.A.; Leong, W.-H.; Rawindran, H.; Ho, Y.-C.; Mohamad, M.; Ghani, N.A.; Bashir, M.J.K.; Usman, A.; Lam, M.-K.; Lim, J.-W. Assuaging Microalgal Harvesting Woes via Attached Growth: A Critical Review to Produce Sustainable Microalgal Feedstock. Sustainability 2021, 13, 11159. https://doi.org/10.3390/su132011159
Rosmahadi NA, Leong W-H, Rawindran H, Ho Y-C, Mohamad M, Ghani NA, Bashir MJK, Usman A, Lam M-K, Lim J-W. Assuaging Microalgal Harvesting Woes via Attached Growth: A Critical Review to Produce Sustainable Microalgal Feedstock. Sustainability. 2021; 13(20):11159. https://doi.org/10.3390/su132011159
Chicago/Turabian StyleRosmahadi, Nurulfarah Adilah, Wai-Hong Leong, Hemamalini Rawindran, Yeek-Chia Ho, Mardawani Mohamad, Noraini A. Ghani, Mohammed J. K. Bashir, Anwar Usman, Man-Kee Lam, and Jun-Wei Lim. 2021. "Assuaging Microalgal Harvesting Woes via Attached Growth: A Critical Review to Produce Sustainable Microalgal Feedstock" Sustainability 13, no. 20: 11159. https://doi.org/10.3390/su132011159
APA StyleRosmahadi, N. A., Leong, W.-H., Rawindran, H., Ho, Y.-C., Mohamad, M., Ghani, N. A., Bashir, M. J. K., Usman, A., Lam, M.-K., & Lim, J.-W. (2021). Assuaging Microalgal Harvesting Woes via Attached Growth: A Critical Review to Produce Sustainable Microalgal Feedstock. Sustainability, 13(20), 11159. https://doi.org/10.3390/su132011159









