Potential of On-the-Go Gamma-Ray Spectrometry for Estimation and Management of Soil Potassium Site Specifically
Abstract
1. Introduction
2. Materials and Methods
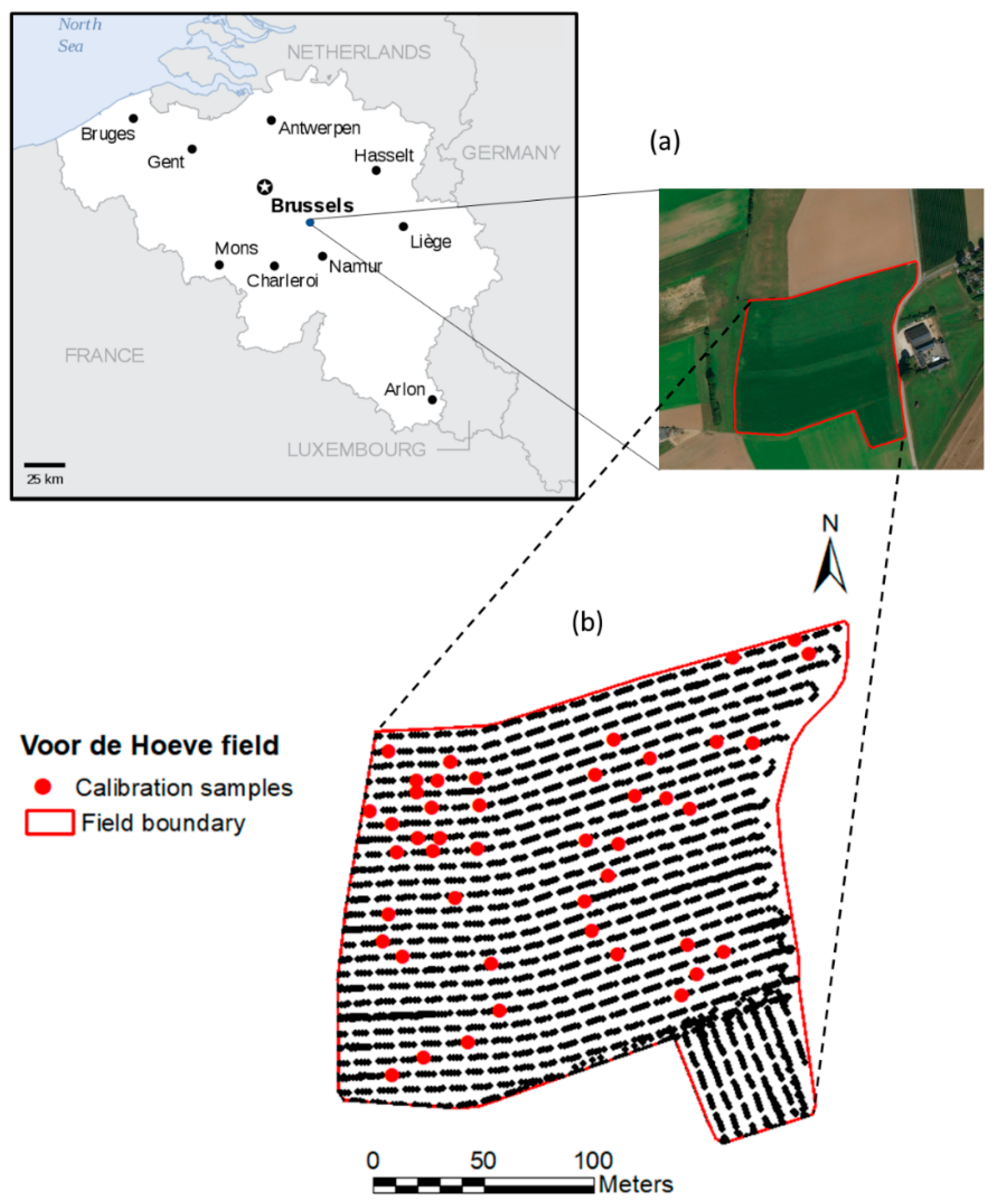
2.1. Study Area
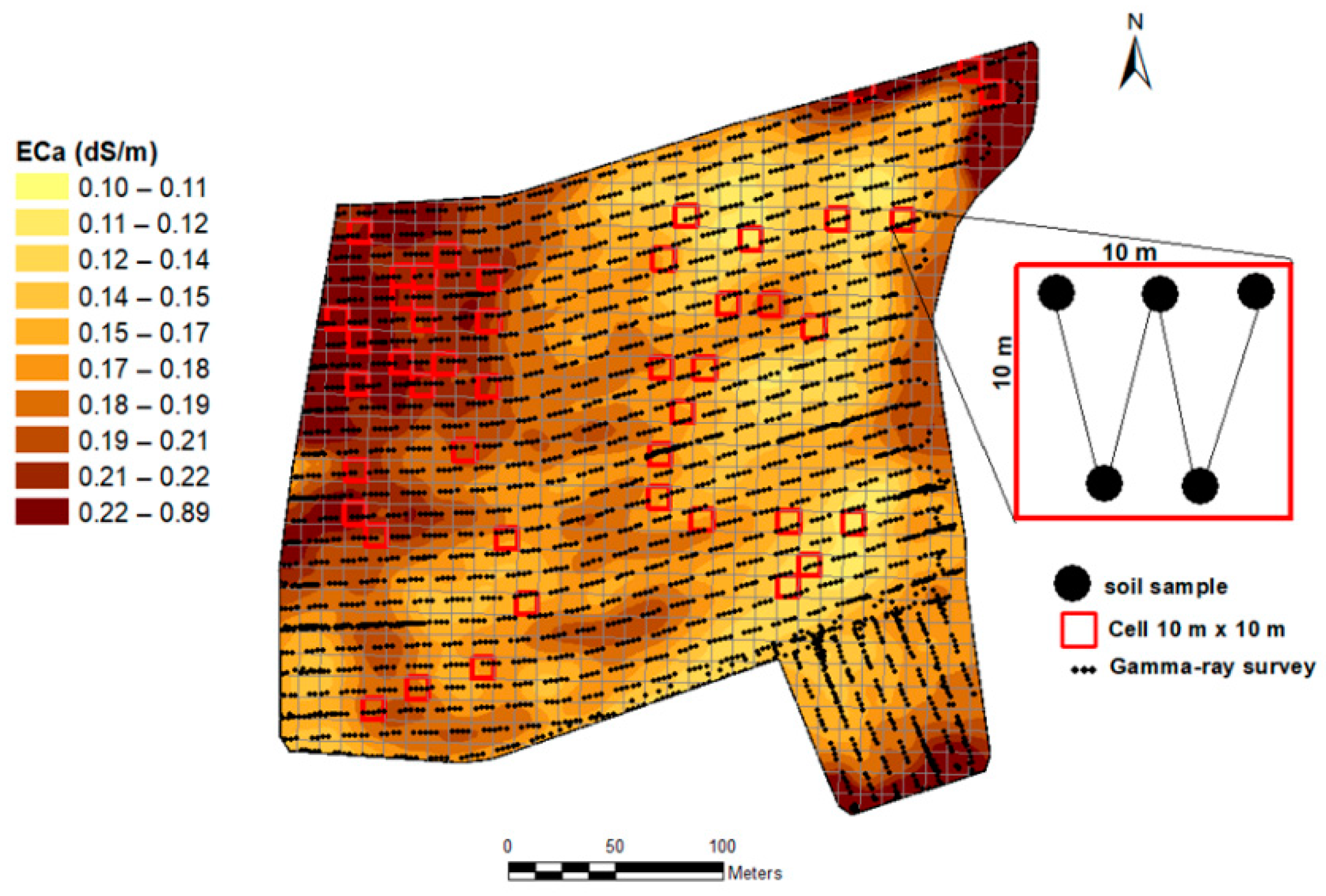
2.2. On-The-Go Gamma-Ray Spectrometer and Measurement
2.3. Soil Samples and Laboratory Analysis
2.4. Analyses
2.4.1. Univariate Analysis
2.4.2. Multivariate Analysis
- Dataset collected from the VH field only, using the W-shape sampling method in Figure 3. This dataset was designated as single (target) field (SF) (n = 45),
- Dataset combining samples from two fields, e.g., 45 samples from the VH field and 5 samples from the Bottelare field. This dataset was designated as single field-combined with Bottelare field (SF-CB) (n = 50 samples).
- Spiking of soil samples from the VH field (25 samples) into the soil spectral library, collected from Bottelare and other three fields. This dataset was designated as single field-spectral library (SF-SL) (n = 138 samples).
2.4.3. Evaluation of Model Accuracy
2.5. Development of Measured and On-The-Go Predicted Ka Map
2.6. Development of K2O Fertilizer Recommendation Maps
3. Results
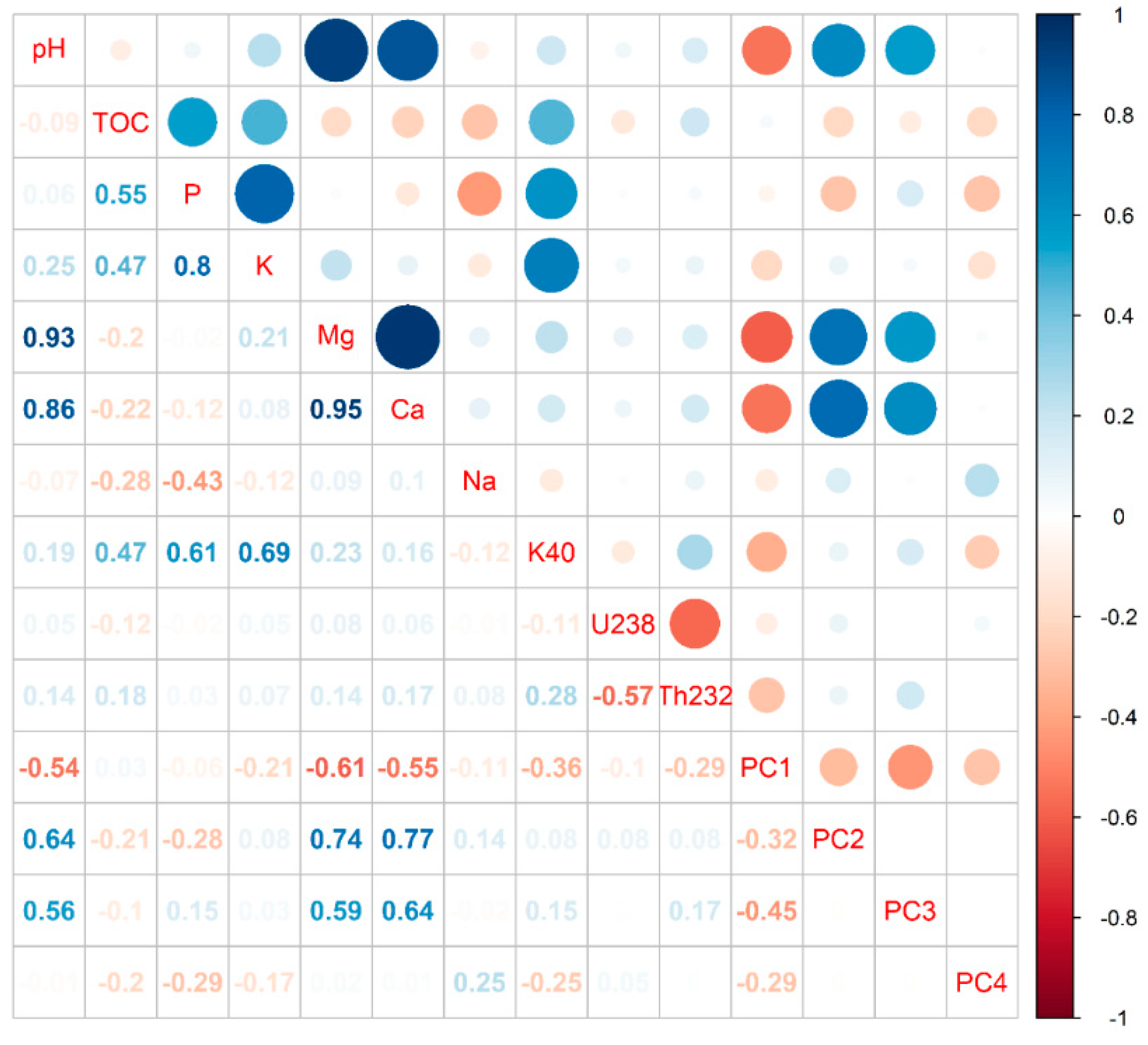
3.1. Soil and Spectral Analysis
3.2. Modeling Results with the Univariate and Multivariate Analyses
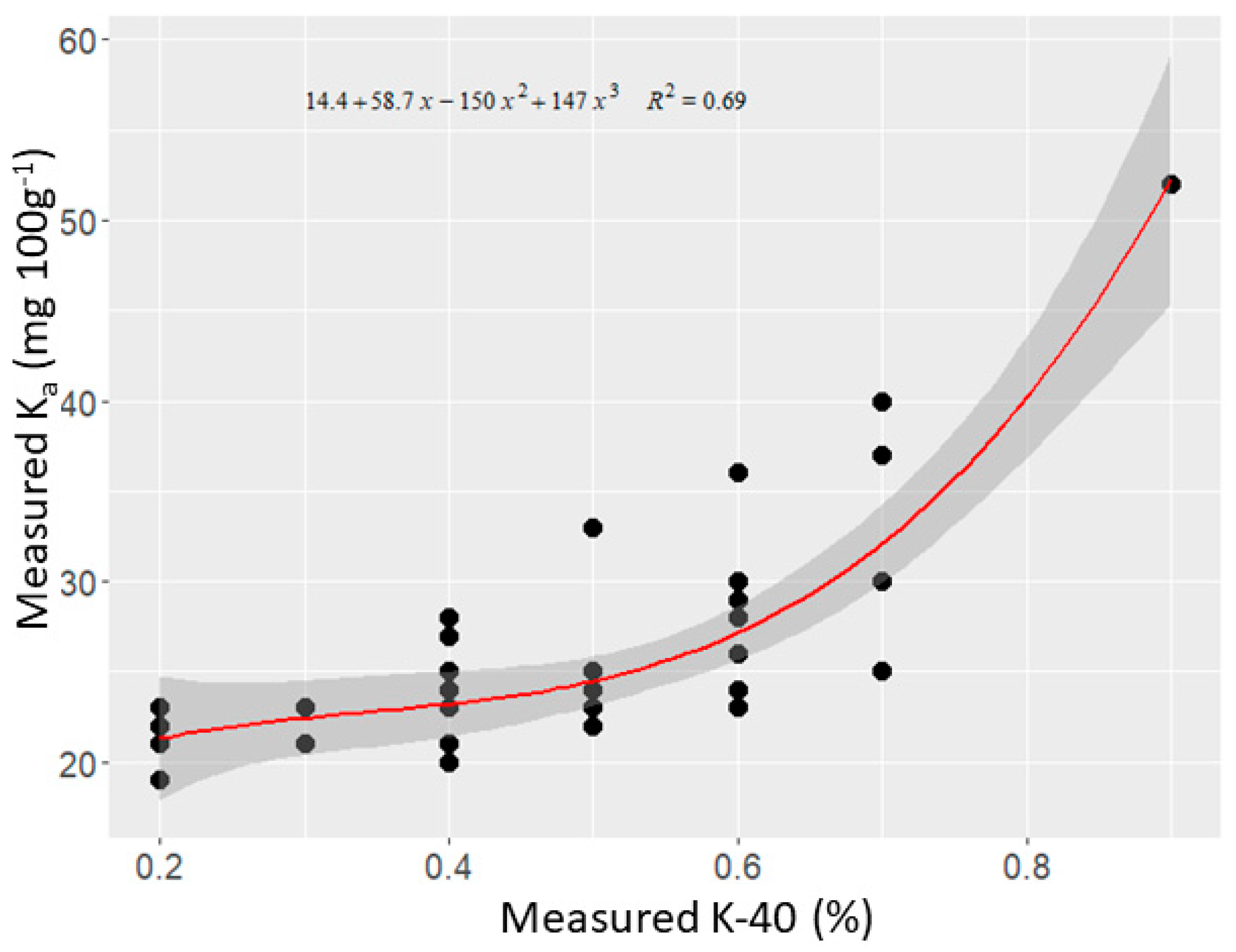
3.2.1. Univariate Regression Analysis
3.2.2. Multivariate Analysis
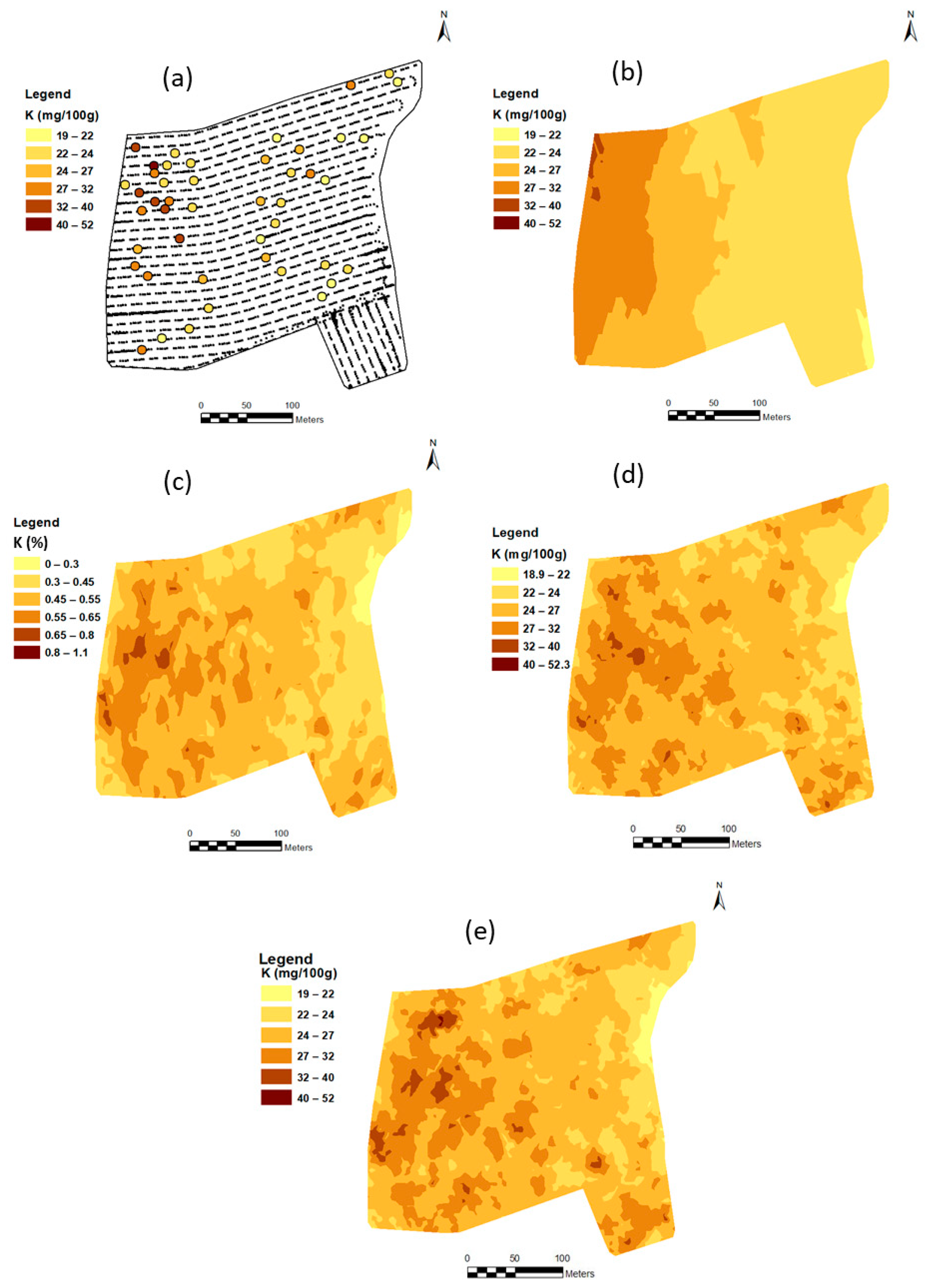
3.3. Soil Maps
3.3.1. Map of Measured Soil K-40 from Gamma-Ray Spectrometer Output
3.3.2. On-The-Go Predicted Maps of Ka
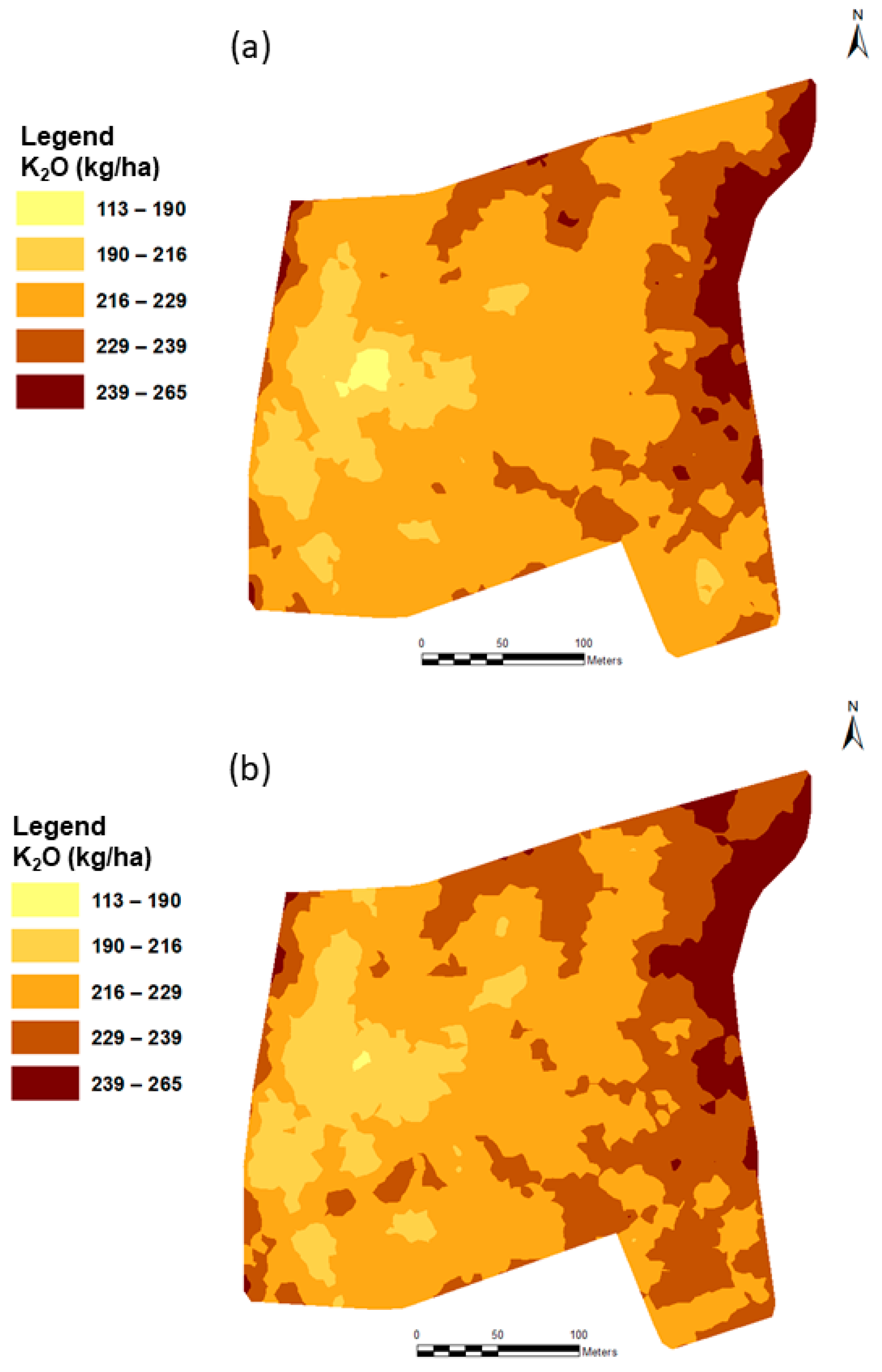
3.4. Fertilization Recommendation
4. Discussion
4.1. Univariate Analysis
4.2. Multivariate Analyses
4.3. Soil Maps
4.4. Fertilization Recommendation
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meena, V.S.; Bahadur, I.; Maurya, B.R.; Kumar, A.; Meena, R.K.; Meena, S.K.; Verma, J.P. Potassium-solubilizing microorganism in evergreen agriculture: An overview. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2006; pp. 1–20. [Google Scholar]
- Sparks, D.L. Potassium Dynamics in Soils. Adv. Soil Sci. 1987, 6, 1–63. [Google Scholar]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Kuang, B.; Mahmood, H.; Quraishi, Z.; Hoogmoed, W.; Mouazen, A.; Van Henten, E.J. Sensing Soil Properties in the Laboratory, In Situ, and On-Line. Adv. Agron. 2012, 14, 155–224. [Google Scholar]
- Farrell, R.E.; Scott, A.D. Ion-selective electrode determinations of exchangeable potassium in soils. Soil Sci. Soc. Am. J. 1987, 51, 594–598. [Google Scholar] [CrossRef]
- Brouder, S.M.; Thom, M.; Adamchuk, V.I.; Morgan, M.T. Potential uses of ion-selective K electrodes in soil fertility management. Commun. Soil Sci. Plant Anal. 2003, 34, 2699–2726. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Janik, L.J.; Viscarra Rossel, R.A.; Macdonald, L.M.; McLaughlin, M.J. The performance of visible, near-, and mid-infrared reflectance spectroscopy for prediction of soil physical, chemical, and biological properties. Appl. Spectrosc. Rev. 2014, 49, 139–186. [Google Scholar] [CrossRef]
- Adamchuk, V.I.; Lund, E.; Sethuramasamyraja, B.; Dobermann, A.; Marx, D.B. Direct measurement of soil chemical properties on-the-go using ion selective electrodes. Comput. Electron. Agric. 2005, 48, 272–294. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Taylor, H.J.; McBratney, A.B. Multivariate calibration of hyperspectral γ-ray energy spectra for proximal soil sensing. Eur. J. Soil Sci. 2007, 58, 343–353. [Google Scholar] [CrossRef]
- Priori, S.; Bianconi, N.; Costantini, E.A.C. Can γ-radiometrics predict soil textural data and stoniness in different parent materials? A comparison of two machine-learning methods. Geoderma 2014, 226, 354–364. [Google Scholar] [CrossRef]
- Heggemann, T.; Welp, G.; Amelung, W.; Angst, G.; Franz, S.; Koszinski, S.; Schmidt, K.; Pätzold, S. Proximal gamma-ray spectrometry for site-independent in situ prediction of soil texture on ten heterogeneous fields in Germany using support vector machines. Soil Tillage Res. 2017, 168, 99–109. [Google Scholar] [CrossRef]
- Van Egmond, F.M.; Loonstra, E.H.; Limburg, J. Gamma-ray Sensor for Topsoil Mapping: The Mole. In Proximal Soil Sensing, 1st ed.; Rossel, R.A.V., McBratney, A.B., Minasny, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 323–332. [Google Scholar]
- Wong, M.T.F.; Harper, R.J. Use of on-ground gamma-ray spectrometry to measure plant-available potassium and other topsoil attributes. Aust. J. Soil Res. 1999, 37, 267–277. [Google Scholar] [CrossRef]
- Pracilio, G.; Adams, M.L.; Smettem, K.R.J.; Harper, R.J. Determination of spatial distribution patterns of clay and plant available potassium contents in surface soils at the farm scale using high resolution gamma ray spectrometry. Plant Soil. 2006, 282, 67–82. [Google Scholar] [CrossRef]
- Taylor, M.J.; Smettem, K.; Pracilio, G.; Verboom, W. Relationships between soil properties and high-resolution radiometrics, central eastern Wheatbelt, western Australia. Explor. Geophys. 2002, 33, 95–102. [Google Scholar] [CrossRef]
- Mahmood, H.S.; Hoogmoed, W.B.; van Henten, E.J. Proximal gamma-ray spectroscopy to predict soil propertiesusing windows and full-spectrum analysis methods. Sensors 2013, 13, 16263–16280. [Google Scholar] [CrossRef] [PubMed]
- Dierke, C.; Werben, U. Relationships between gamma-ray data and soil properties at an agricultural test site. Geoderma 2013, 199, 90–98. [Google Scholar] [CrossRef]
- Castrignano, A.; Wong, M.T.F.; Stelluti, M.; De Benedetto, D.; Sollitto, D. Use of EMI, gamma-ray emission and GPS height as multi-sensor data for soil characterisation. Geoderma 2012, 175, 78–89. [Google Scholar] [CrossRef]
- Royal Meteorological Institute of Belgium (RMI). Klimaatatlas, May 2015. Available online: www.meteo.be/klimaatlas (accessed on 20 August 2019).
- Baeyens, L.; Dudal, R. Verklarende Tekst en Het Kaartblad Duisburg 103W Comité Voor Het Opnemen Van de Bodemkaart Van Belgë, Ghent; Ghent University: Gent, Belgium, 1959. [Google Scholar]
- Dondeyne, S.; Vanierscot, L.; Langohr, R.; Van Ranst, E.; Deckers, J. The Soil Map of the Flemish Region Converted to the 3rd Edition of the World Reference Base for Soil Resources; Ghent University: Gent, Belgium, 2014. [Google Scholar]
- Duivenstijn, A.J.; Venverloo, L. Practical gamma spectrometry. Charles Thomas Springf. 1963, III, 156. [Google Scholar]
- Sudduth, K.A.; Kitchen, N.R.; Drummond, S.T. Soil conductivity sensing on claypan soils: Comparison of electromagnetic in duction and direct methods. In Proceedings of the 4th International Conference on Precision Agriculture, St. Paul, MN, USA, 19–22 July 1999; Robert, P.C., Ed.; ASA, CSSA, and SSSA: Madison, WI, USA, 1999; pp. 979–990. [Google Scholar]
- Franzen, D.W.; Kitchen, N.R. Developing management zones to target nitrogen applications. In Site-Specific Management Guidelines.SSMG-5; Potash and Phosphate Inst.: Norcross, GA, USA, 1999. [Google Scholar]
- Heiniger, R.; Mcbride, R.; Clay, D. Using Soil Electrical Conductivity to Improve Nutrient Management. Agron. J. 2003, 95, 508–519. [Google Scholar] [CrossRef]
- Jaynes, D.B.; Colvin, T.S.; Ambuel, J. Yield mapping by electromagnetic induction. In Proceedings of the International Conference Site-Specific Management for Agricultural Systems, Minneapolis, MN, USA, 27–30 March 1995; Robert, P.C., Ed.; ASA, CSSA, and SSSA: Madison, WI, USA, 1995; pp. 383–394. [Google Scholar]
- Sudduth, K.A.; Kitchen, N.R.; Hughes, D.F.; Drummond, S.T. Electromagnetic induction sensing as an indicator of productivity on claypan soils. In Proceedings of the 3rd International Conference on Precision Agriculture, Minneapolis, MN, USA, 23–26 June 1996; Robert, P.C., Ed.; ASA, CSSA, and SSSA: Madison, WI, USA, 1996; pp. 671–681. [Google Scholar]
- Kitchen, N.R.; Sudduth, K.A.; Drummond, S.T. Soil electrical conductivity as a crop productivity measure for claypan soils. J. Prod. Agric. 1999, 12, 607–617. [Google Scholar] [CrossRef]
- Martens, H.; Næs, T. Multivariate Calibration; John Wiley & Sons: Hoboken, NJ, USA, 1989. [Google Scholar]
- Stevens, A.; Ramirez-Lopez, L.; Vignette, R. An Introduction to the Prospectr Package. 2013. Available online: https://github.com/antoinestevens/prospectr (accessed on 21 July 2019).
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; McGlynn, R.N.; McBratney, A.B. Determining the composition of mineral-organic mixes using UV–vis–NIR diffuse reflectance spectroscopy. Geoderma 2006, 137, 70–82. [Google Scholar] [CrossRef]
- Chiozzi, P.; Pasquale, V.; Verdoya, M. Naturally occurring radioactivity at the Alps-Apennines transition. Radiat. Meas. 2002, 35, 147–154. [Google Scholar] [CrossRef]
- Baeza, A.; Del-Rio, M.; Miro, C. Natural radioactivity in soils of the province of Caceres (Spain). Radiat. Prot. Dosim. 1992, 45, 261–263. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and effects of ionizing radiation. In Report to General Assembly, with Scientific Annexes; United Nations: New York, NY, USA, 2000. [Google Scholar]
- Grasty, R.L. Applications of gamma radiation in remote sensing. In Remote Sensing for Environmental Science; Schanda, E., Ed.; Springer: New York, NY, USA, 1997; pp. 257–276. [Google Scholar]
- Cook, S.E.; Corner, R.J.; Groves, P.R.; Grealish, G.J. Use of airborne gamma radiometric data for soil mapping. Aust. J. Soil Res. 1996, 34, 183–194. [Google Scholar] [CrossRef]
- de Groot, A.V.; van der Graaf, E.R.; de Meijer, R.J.; Maučec, M. Sensitivity of in-situ γ-ray spectra to soil density and water content. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 600, 519–523. [Google Scholar] [CrossRef]
- Manning, D.A.C. Mineral sources of potassium for plant nutrition. A review. Agron. Sustain. Dev. 2010, 30, 281–294. [Google Scholar] [CrossRef]
- Ciceri, D.; Allanore, A. Microfluidic leaching of soil minerals: Release of K+ from K feldspar. PLoS ONE 2015, 10, e0139979. [Google Scholar] [CrossRef]
- Megumi, K.; Mamuro, T. Concentrations of uranium series nuclides in soil particles in relation to their size. J. Geophys. Res. 1977, 82, 353–356. [Google Scholar] [CrossRef]


| Dataset | No | Min | Median | Mean | Max | 1stQ | 3rdQ | SD | |
|---|---|---|---|---|---|---|---|---|---|
| (a) SF | K-40 (%) | 45 | 0.10 | 0.50 | 0.47 | 1.10 | 0.40 | 0.60 | 0.16 |
| Ka (mg 100 g−1) | 45 | 19.0 | 24.0 | 26.2 | 52.0 | 23.0 | 29.0 | 6.1 | |
| pH | 45 | 6.0 | 6.4 | 6.6 | 7.7 | 6.2 | 7.1 | 0.5 | |
| TOC (%) | 45 | 0.8 | 1.0 | 1.1 | 2.0 | 1.0 | 1.1 | 0.2 | |
| P (mg 100 g−1) | 45 | 9.0 | 15.0 | 16.0 | 29.0 | 14.0 | 18.0 | 4.2 | |
| Mg (mg 100 g−1) | 45 | 10.0 | 14.0 | 18.7 | 44.0 | 12.0 | 26.0 | 9.4 | |
| Ca (mg 100 g−1) | 45 | 144.0 | 194.0 | 285.8 | 962.0 | 174.0 | 338.0 | 184.2 | |
| Na (mg 100 g−1) | 45 | 1.3 | 2.3 | 2.3 | 3.2 | 1.9 | 2.6 | 0.5 | |
| (b) SF-CB | Ka (mg 100 g−1) | 50 | 19.0 | 24.0 | 26.4 | 52.0 | 23.0 | 28.7 | 6.2 |
| (c) SF-SL | Ka (mg 100 g−1) | 138 | 10.0 | 24.1 | 25.4 | 54.0 | 22.0 | 29.0 | 7.1 |
| Data Set | RMSECV (mg 100 g−1) | R2 | RPD | RPIQ |
|---|---|---|---|---|
| (a) SF | 2.29 | 0.85 | 2.67 | 2.61 |
| (b) SF-CB | 3.40 | 0.70 | 1.82 | 1.69 |
| (c) SF-SL | 6.07 | 0.27 | 1.18 | 1.27 |
| Recommendation Method | Application Method | Applied Amount (kg/ha) | Applied Amount (kg/Field) |
|---|---|---|---|
| Traditional soil analysis | Homogeneous rate | 217.2 | 1824.5 |
| Third order polynomial function (3DPF) | Variable rate | 216.0 | 1814.4 |
| =Partial least squares regression (PLSR) model | Variable | 218.7 | 1837.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassim, A.M.; Nawar, S.; Mouazen, A.M. Potential of On-the-Go Gamma-Ray Spectrometry for Estimation and Management of Soil Potassium Site Specifically. Sustainability 2021, 13, 661. https://doi.org/10.3390/su13020661
Kassim AM, Nawar S, Mouazen AM. Potential of On-the-Go Gamma-Ray Spectrometry for Estimation and Management of Soil Potassium Site Specifically. Sustainability. 2021; 13(2):661. https://doi.org/10.3390/su13020661
Chicago/Turabian StyleKassim, Anuar Mohamed, Said Nawar, and Abdul M. Mouazen. 2021. "Potential of On-the-Go Gamma-Ray Spectrometry for Estimation and Management of Soil Potassium Site Specifically" Sustainability 13, no. 2: 661. https://doi.org/10.3390/su13020661
APA StyleKassim, A. M., Nawar, S., & Mouazen, A. M. (2021). Potential of On-the-Go Gamma-Ray Spectrometry for Estimation and Management of Soil Potassium Site Specifically. Sustainability, 13(2), 661. https://doi.org/10.3390/su13020661







