An Experimental Study on the Melting Solidification of Municipal Solid Waste Incineration Fly Ash
Abstract
:1. Introduction
2. Method
2.1. Materials and Characterization
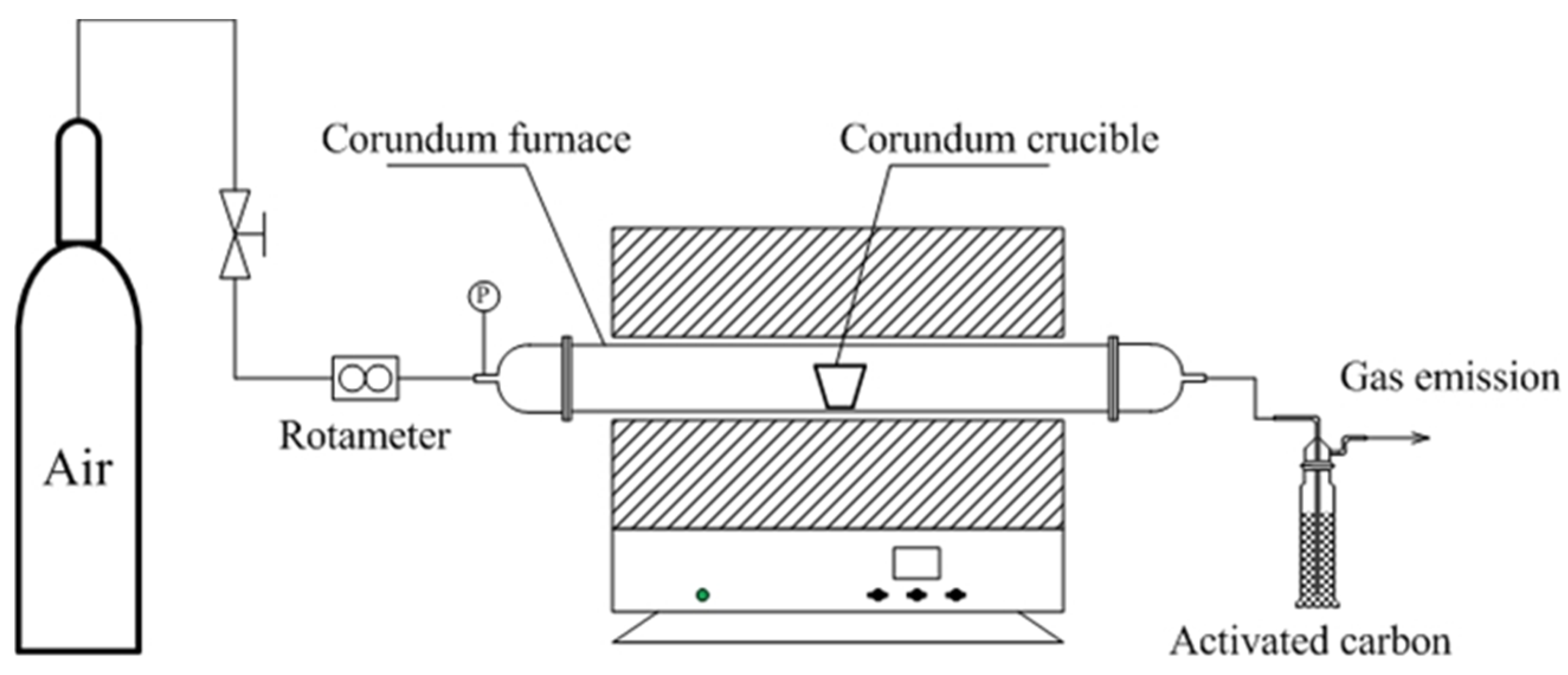
2.2. Experimental Devices and Methods
2.3. Heavy Metal Concentration
2.4. Leaching Tests
3. Results and Discussion
3.1. Characteristics of the MSWI Fly Ash
3.2. Mineral Transformation Behavior
3.3. Volatilization of Heavy Metals
3.4. Leaching Behavior of Heavy Metals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Ecology and Environment of the People’s Republic of China. Hazardous Waste List. 2016. Available online: http://www.mee.gov.cn/gkml/hbb/bl/201606/t20160621_354852.htm (accessed on 20 December 2020).
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of Incineration MSW Ash: A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Xiang, J.; Hu, S.; Yao, H. Mechanism on heavy metals vaporization from municipal solid waste fly ash by MgCl2⋅6H2O. Waste Manag. 2016, 49, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Li, R.D.; Nie, Y.F.; Wang, L.; Li, A.M.; Chi, Y.; Cen, K.F. Migration characteristics experiment of heavy metal in the vitrification course of fly ash from municipal solid waste incineration. China Environ. Sci. 2004, 24, 480–483. [Google Scholar]
- Katou, K.; Asou, T.; Kurauchi, Y.; Sameshima, R. Melting municipal solid waste incineration residue by plasma melting furnace with a graphite electrode. Thin Solid Film. 2001, 386, 183–188. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G.; Gagnepain, B.; Gauthier, D. Fate of heavy metals during municipal solid waste incineration. Waste Manag. Res. 2002, 20, 55–68. [Google Scholar] [CrossRef]
- Evans, J.; Williams, P.T. Heavy Metal Adsorption onto Fly ash in Waste Incineration Flue Gases. Process Saf. Environ. Prot. 2000, 78, 40–46. [Google Scholar] [CrossRef]
- Yu, J.; Qiao, Y.; Jin, L.; Ma, C.; Paterson, N.; Sun, L. Removal of toxic and alkali/alkaline earth metals during co-thermal treatment of two types of MSWI fly ashes in China. Waste Manag. 2015, 46, 287–297. [Google Scholar] [CrossRef]
- Tian, S.L. Thermal-Separation Process and Evaporation Mechanism of Heavy Metal from MSWI Fly Ash; Harbin Institute of Technology: Harbin, China, 2007. [Google Scholar]
- Ma, W.; Fang, Y.; Chen, D.; Chen, G.; Zhou, Z. Volatilization and leaching behavior of heavy metals in MSW incineration fly ash in a DC arc plasma furnace. Fuel 2017, 210, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Xu, Y.; Sun, J.; Cao, Z.; Zhou, J.; Pan, Y.; Qian, G. Inhibiting evaporation of heavy metal by controlling its chemical speciation in MSWI fly ash. Fuel 2015, 158, 764–769. [Google Scholar] [CrossRef]
- Kirk, D.W.; Chan, C.C.Y.; Marsh, H. Chromium behavior during thermal treatment of MSW fly ash. J. Hazard. Mater. 2002, 90, 39–49. [Google Scholar] [CrossRef]
- Chan, C.C.Y.; Kirk, D.W.; Marsh, H. The behaviour of Al in MSW incinerator fly ash during thermal treatment. J. Hazard. Mater. 2000, 76, 103–111. [Google Scholar] [CrossRef]
- Tian, S.; Li, J.; Liu, F.; Guan, J.; Dong, L.; Wang, Q. Behavior of Heavy Metals in the Vitrification of MSWI Fly Ash with a Pilot-scale Diesel Oil Furnace. Procedia Environ. Sci. 2012, 16, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Jakob, A.; Stucki, S.; Struis, R.P.W.J. Complete heavy metal removal from fly ash by heat treatment: Influences of chlorides on evaporation rates. Environ. Sci. Technol. 1996, 30, 3275–3283. [Google Scholar] [CrossRef]
- Nowak, B.; Rocha, S.F.; Aschenbrenner, P.; Rechberger, H.; Winter, F. Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: Influence of the chloride type. Chem. Eng. J. 2012, 179, 178–185. [Google Scholar] [CrossRef]
- Yen, C.-P.; Zhou, S.-Y.; Shen, Y.-H. The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash. Sustainability 2020, 12, 9086. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Shen, W.; Luo, G.; Li, A.; Lu, Z.; Yao, H. Comparison of CaO’s effect on the fate of heavy metals during thermal treatment of two typical types of MSWI fly ashes in China. Chemosphere 2013, 93, 590–596. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Determination of Fusibility of Coal Ash; Standards Press of China: Beijing, China, 2008. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. Soil Quality—Determination of Lead, Cadmium—Graphite Furnace Atomic Absorption Spectrophotometry; China Environmental Press: Beijing, China, 1997.
- Ministry of Ecology and Environment of the People’s Republic of China. Solid Waste-Extraction Procedure for Leaching Toxicity—Acetic Acid Buffer Solution Method; China Environmental Press: Beijing, China, 2007.
- Ministry of Ecology and Environment of the People’s Republic of China. Solid Waste—Determination of Lead, Cadmium—Graphite Furnace Atomic Absorption Spectrophotometry; China Environmental Science Press: Beijing, China, 2016.
- Li, H.; Wang, H.; Geng, H.Y.; Fang, J.H.; Bai, L.C. A study on physical and chemical characteristics of fly ash from municipal solid waste incinerator. Chin. J. Environ. Eng. 2007, 1, 137–140. [Google Scholar]
- Wang, K.-S.; Sun, C.-J.; Yeh, C.-C. The thermotreatment of MSW incinerator fly ash for use as an aggregate: A study of the characteristics of size-fractioning. Resour. Conserv. Recycl. 2002, 35, 177–190. [Google Scholar] [CrossRef]
- Guo, Y.W.; Wang, W.; Gao, X.B.; Qiao, W. Microstructure chatacteristic and X-ray energy dispersive microanalysis of MSW incineration fly ash. J. Fuel Chem. Technol. 2005, 33, 703–707. [Google Scholar]
- Zheng, B.; Luo, Y.; Liao, H.; Zhang, C. Investigation of the crystallinity of suspension plasma sprayed hydroxyapatite coatings. J. Eur. Ceram. Soc. 2017, 37, 5017–5021. [Google Scholar] [CrossRef]
- Xie, K.; Hu, H.; Xu, S.; Chen, T.; Huang, Y.; Yang, Y.; Yang, F.; Yao, H. Fate of heavy metals during molten salts thermal treatment of municipal solid waste incineration fly ashes. Waste Manag. 2020, 103, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; He, P.-J.; Xia, Y.; Lu, W.-T.; Shao, L.-M.; Zhang, H. Role of sodium chloride and mineral matrixes in the chlorination and volatilization of lead during waste thermal treatment. Fuel Process. Technol. 2016, 143, 130–139. [Google Scholar] [CrossRef]
- Wang, S.J.; He, P.J.; Lu, W.T.; Shao, L.M.; Zhang, H. Comparison of Pb, Cd, Zn, and Cu chlorination during pyrolysis and incineration. Fuel 2017, 194, 257–265. [Google Scholar] [CrossRef]
- Cao, L. Study on the Partitioning and Speciation of Elements from Municipal Solid Waste Incineration and Their Environmental Effects by Synchrotron Techniques; Chinese Academy of Sciences: Shanghai, China, 2015. [Google Scholar]
- Pinzani, M.C.C.; Somogyi, A.; Simionovici, A.S.; Ansell, S.; Steenari, B.; Lindqvist, O. Direct Determination of Cadmium Speciation in Municipal Solid Waste Fly Ashes by Synchrotron Radiation Induced μ-X-ray Fluorescence and μ-X-ray Absorption Spectroscopy. Environ. Sci. Technol. 2002, 36, 3165–3169. [Google Scholar] [PubMed]
- Pinzani, M.C.C.; Ansell, S.; Somogyi, A.; Steenari, B.M.; Lindqvist, O. Microextended X-ray Absorption Fine Structure Studies of Cadmium Speciation within Single Municipal Solid Waste Fly Ash Particles. Anal. Chem. 2004, 76, 1596–1602. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Kinetic vaporization of heavy metals during fluidized bed thermal treatment of municipal solid waste. Waste Manag. 2013, 33, 340–346. [Google Scholar] [CrossRef]
- Wang, X.T.; Jin, B.S.; Zhong, Z.P.; Dang, X.J. Influence of atmospheres on behavior of heavy metals during melting process of fly ashes from municipal solid waste incinerator. Proc. CSEE 2006, 26, 47–52. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China. Standard for Pollution Control on the Landfill Site of Municipal Solid Waste; China Environmental Science Press: Beijing, China, 2008.




| Chemical compositions | CaO | SiO2 | Al2O3 | Na2O | K2O | SO3 | Cl | Fe2O3 | MgO |
| Content (wt.%) | 30.09 | 17.91 | 11.9 | 1.26 | 2.35 | 5.45 | 5.23 | 4.4 | 1.97 |
| Heavy metal | Cd | Pb | Zn | Cu | |||||
| Content (mg/kg) | 60.9 | 677.4 | 1166.3 | 1070.2 | |||||
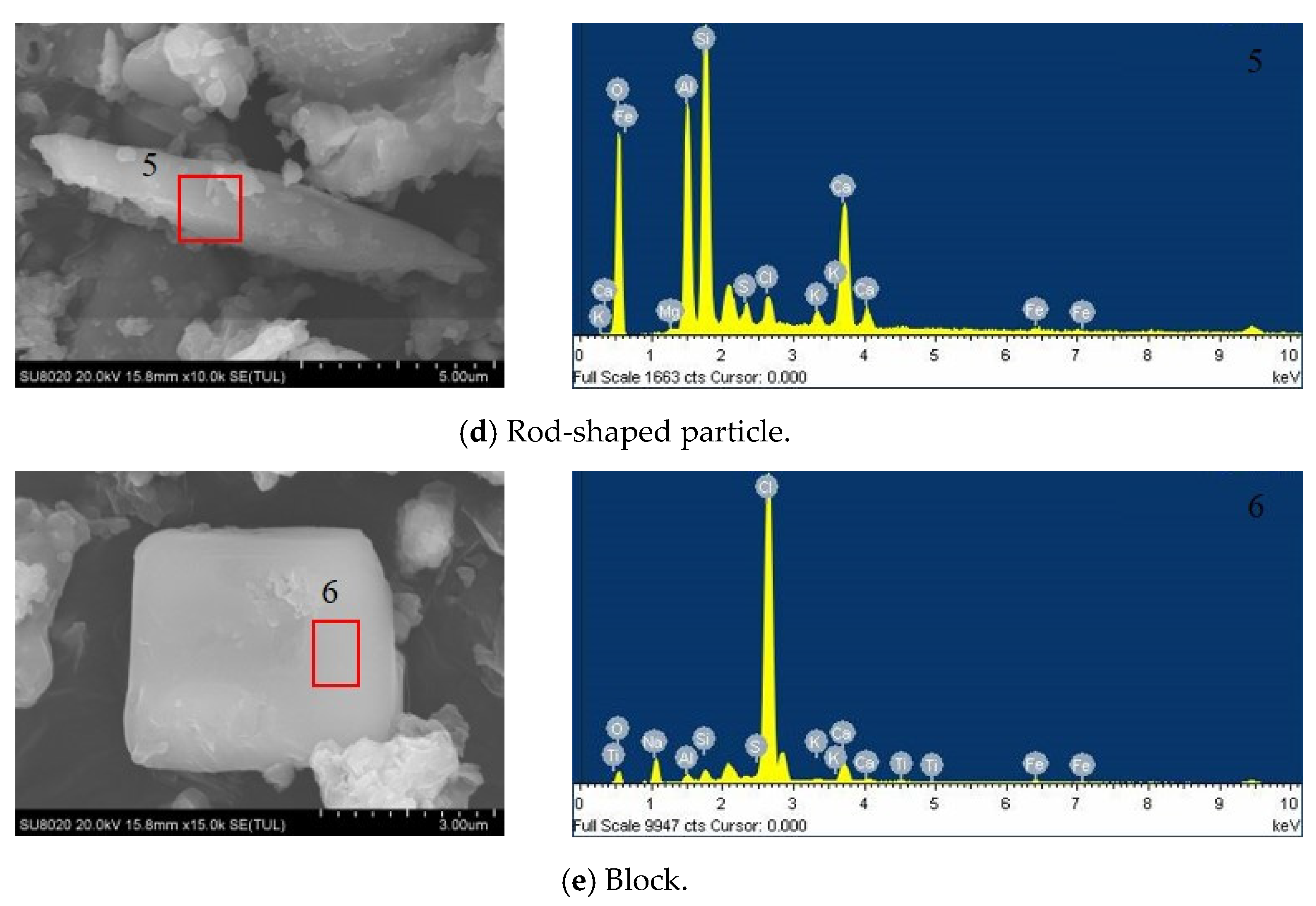
| Element | Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Zone 6 |
|---|---|---|---|---|---|---|
| Weight (%) | ||||||
| O | 41.75 | 38.67 | 42.41 | 37.12 | 53.33 | 20.17 |
| Na | 0.39 | 0.58 | 0 | 0 | 0 | 6.45 |
| Mg | 0.23 | 0.38 | 0.52 | 0 | 0.18 | 0 |
| Al | 8.13 | 8.79 | 1.53 | 2.25 | 11.42 | 1.17 |
| Si | 15.55 | 14.4 | 2.5 | 4.33 | 18.3 | 1.7 |
| S | 0.79 | 1.92 | 1.01 | 6.1 | 1.63 | 0.61 |
| Cl | 0.76 | 3.94 | 14.6 | 9.82 | 2.28 | 62.17 |
| K | 2.49 | 6.33 | 0.42 | 1.25 | 1.21 | 0.58 |
| Ca | 26.58 | 22.02 | 35.49 | 37.33 | 11.02 | 5.76 |
| Ti | 1.45 | 1.02 | 0.53 | 0.85 | 0 | 0.23 |
| Fe | 1.45 | 1.31 | 0.99 | 0.94 | 0.62 | 1.15 |
| Cu | 0.43 | 0.65 | 0 | 0 | 0 | 0 |
| AFTs (°C) | IDT | ST | HT | FT |
|---|---|---|---|---|
| FA | 1167 | 1180 | 1189 | 1211 |
| Chemical Compositions (wt.%) | CaO | SiO2 | Al2O3 | Na2O | K2O | SO3 | Cl | Fe2O3 | MgO | |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat Temperature | 950 °C | 28.29 | 27.8 | 19.53 | 2.63 | 1.99 | 1.73 | 5.75 | 4.13 | 3.56 |
| 1250 °C | 28.82 | 34.57 | 21.41 | 1.62 | 0.679 | 0.503 | 1.65 | 3.07 | 3.54 | |
| 1300 °C | 28.44 | 34.35 | 21.42 | 1.53 | 0.62 | 0.426 | 1.45 | 4.1 | 3.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wang, T.; Zhao, J.; Hu, X.; Dong, C. An Experimental Study on the Melting Solidification of Municipal Solid Waste Incineration Fly Ash. Sustainability 2021, 13, 535. https://doi.org/10.3390/su13020535
Gao J, Wang T, Zhao J, Hu X, Dong C. An Experimental Study on the Melting Solidification of Municipal Solid Waste Incineration Fly Ash. Sustainability. 2021; 13(2):535. https://doi.org/10.3390/su13020535
Chicago/Turabian StyleGao, Jing, Tao Wang, Jie Zhao, Xiaoying Hu, and Changqing Dong. 2021. "An Experimental Study on the Melting Solidification of Municipal Solid Waste Incineration Fly Ash" Sustainability 13, no. 2: 535. https://doi.org/10.3390/su13020535




