How Organic Substances Promote the Chemical Oxidative Degradation of Pollutants: A Mini Review
Abstract
:1. Introduction
2. Reaction Acceleration by Organic Small Molecular Substances
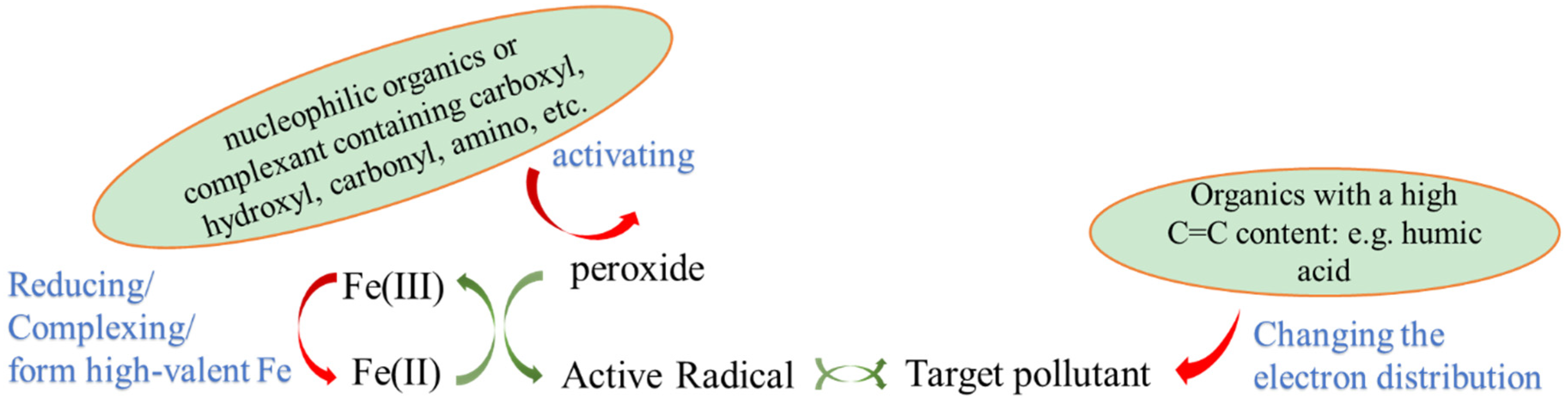
2.1. Accelerated the Metal Redox Cycle
2.2. Activated H2O2 or PDS to Construct Advanced Oxidation System
2.2.1. Organic Matters Activate H2O2
2.2.2. Organic Matter Activates PDS
2.3. Other Effects
3. The Promotion of Organic Chelating Agents
3.1. Humic Substances
3.2. Carboxylic Acid
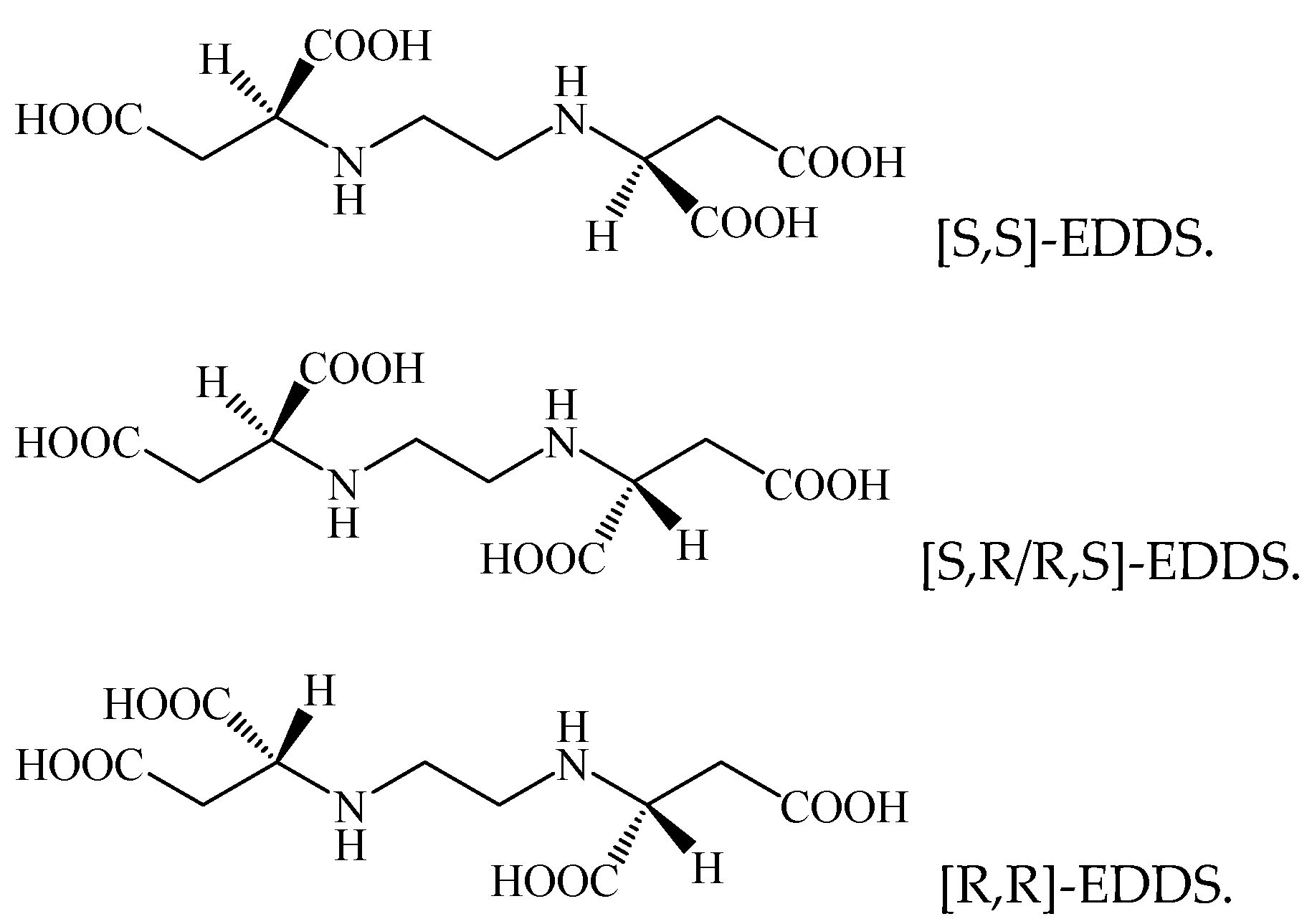
3.3. Amino Carboxylic Acid
4. Metal-Organic Framework Materials
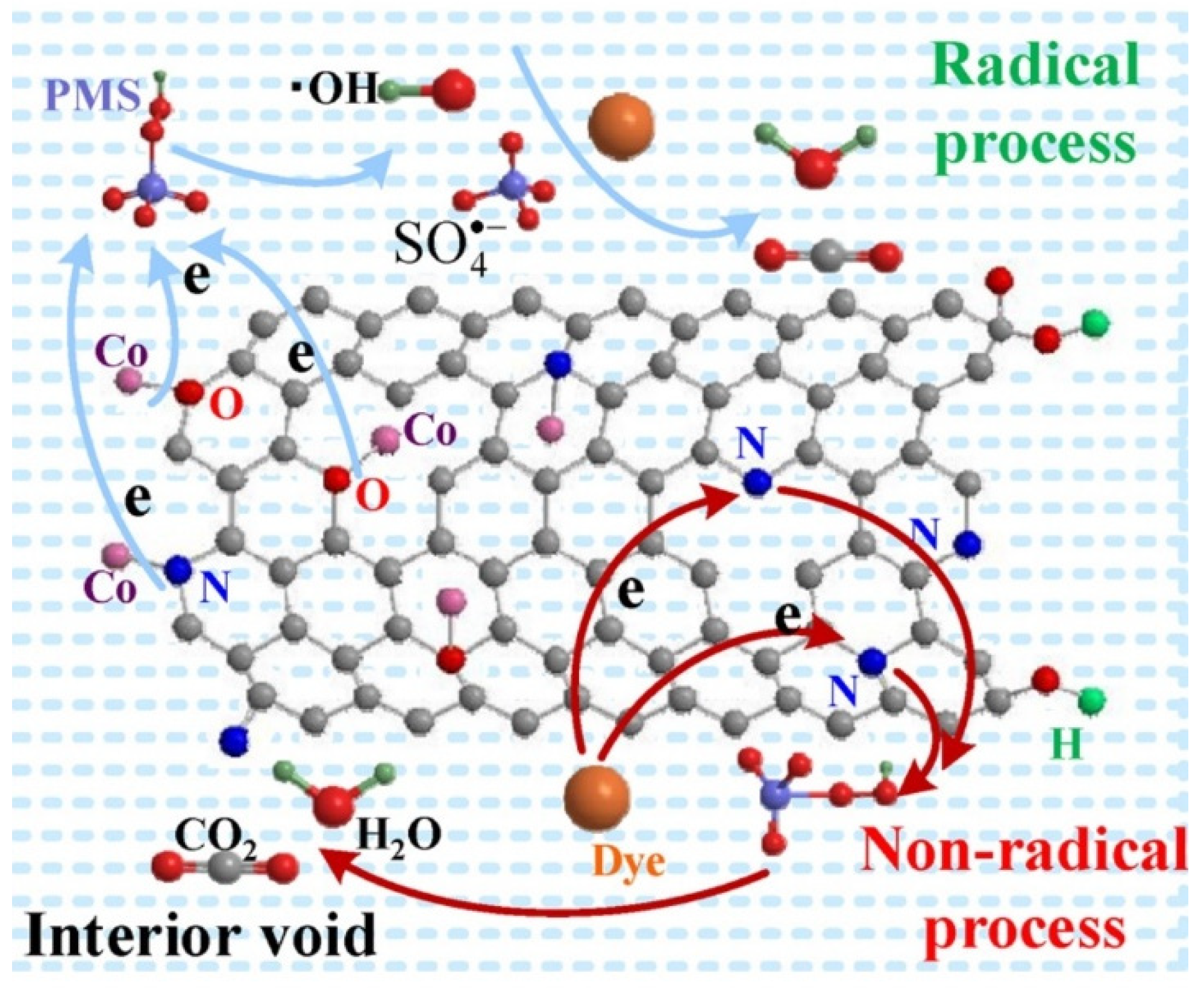
4.1. Fe-Based MOFs Catalytic Materials
4.2. Copper- and Cobalt-Based MOFs Catalytic Materials
4.3. Multi-Core MOFs Catalytic Materials
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matzek, L.W.; Carter, K.E. Activated Persulfate for Organic Chemical Degradation: A Review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.E. Decolourisation, mineralisation and detoxification of mixture of azo dyes using Fenton and Fenton-type advanced oxidation processes. Chemical. Pap. 2020, 74, 3145–3159. [Google Scholar] [CrossRef]
- Chávez, A.M. Simulated solar photocatalytic ozonation of contaminants of emerging concern and effluent organic matter in secondary effluents by a reusable magnetic catalyst. Chem. Eng. J. 2020, 398, 125–642. [Google Scholar] [CrossRef]
- Martins, A.F.; Vasconcelos, G.; Wilde, M.L. Influence of variables of the combined coagulation–Fenton-sedimentation process in the treatment of trifluraline effluent. J. Hazard. Mater. 2005, 127, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Baldrian, P.; Gabriel, J.; Trnka, T.; Nerud, F. Copper–ligand complex for the decolorization of synthetic dyes. Chemosphere 2004, 57, 1207–1211. [Google Scholar] [CrossRef]
- Costa, R.; Moura, F.; Ardisson, J.D.; Fabris, J.D.; Lago, R.M. Highly active heterogeneous Fenton-like systems based on FeO/Fe3O4 composites prepared by controlled reduction of iron oxides. Appl. Catal. B Environ. 2008, 83, 131–139. [Google Scholar] [CrossRef]
- Liu, J.; Du, Y.; Ye, T.; Cao, J.; Peng, C. Removal of orange II using an adsorbent-supported zero-valent iron as a heterogeneous Fenton-like catalyst. Desalination Water Treat. 2020, 175, 273–281. [Google Scholar] [CrossRef]
- Chen, R.; Pignatello, J.J. Role of Quinone Intermediates as Electron Shuttles in Fenton and Photoassisted Fenton Oxidations of Aromatic Compounds. Environ. Sci. Technol. 1997, 31, 2399–2406. [Google Scholar] [CrossRef]
- Jiang, C.; Pang, S.; Ouyang, F. A new insight into Fenton and Fenton-like processes for water treatment. J. Hazard. Mater. 2010, 174, 813–817. [Google Scholar] [CrossRef]
- Huang, W.Y.; Brigante, M.; Wu, F. Assessment of the Fe(III)-EDDS Complex in Fenton-like Processes: From the Radical Formation to the Degradation of Bisphenol, A. Environ. Sci. Technol. 2013, 47, 1952–1959. [Google Scholar] [CrossRef]
- Lien, H.L. Bimetallic Fe/Al system: An all-in-one solid-phase Fenton reagent for generation of hydroxyl radicals under oxic conditions. Sci. Total. Environ. 2019, 673, 480–488. [Google Scholar] [CrossRef]
- Shen, J.; Hou, L.; Sun, H. Effect of an electro-Fenton process on the biodegradation of lignin by Trametes versicolor. Bioresources 2020, 15, 8039–8050. [Google Scholar] [CrossRef]
- Subramanian, G.; Madras, G. Remarkable Enhancement of Fenton Degradation at a Wide pH Range Promoted by Thioglycolic Acid. Chem. Commun. 2017, 53, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, J.; Li, X. Strong Enhancement on Fenton Oxidation by Addition of Hydroxylamine to Accelerate the Ferric and Ferrous Iron Cycles. Environ. Sci. Technol. 2011, 45, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, F.T. Optimizing Operating Parameters of A Honeycomb Zeolite rotor Concentrator for Processing TFT-LCD Volatile Organic Compounds with Competitive Adsorption Characteristics. J. Hazard. Mater. 2009, 164, 517–526. [Google Scholar] [CrossRef]
- Teel, A.L.; Cutler, L.M.; Watts, R.J. Effect of Sorption on Contaminant Oxidation in Activated Persulfate Systems. J. Environ. Sci. Health Part A 2009, 44, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hui, Z.; Wang, J.; Jia, A. Rapid and Continuous Oxidation of Organic Contaminants with Ascorbic Acid and A Modified Ferric/Persulfate System. Chem. Eng. J. 2015, 270, 73–79. [Google Scholar]
- Sang, W.; Li, Z.; Huang, M.; Wu, X.; Cui, J. Enhanced transition metal oxide based peroxymonosulfate activation by hydroxylamine for the degradation of sulfamethoxazole. Chem. Eng. J. 2020, 383, 123057. [Google Scholar] [CrossRef]
- Minisci, F.; Citterio, A.; Giordano, C. Electron-transfer processes: Peroxydisulfate, a useful and versatile reagent in organic chemistry. Cheminform 1983, 16, 27–32. [Google Scholar] [CrossRef]
- Liang, C.; Liang, C.P.; Chen, C.C. pH Dependence of Persulfate Activation by EDTA/Fe(III) for Degradation of Trichloroethylene. J. Contam. Hydrol. 2009, 106, 173–182. [Google Scholar] [CrossRef]
- Di, H.; Xiaoiaohong, G.; Jun, M. Influence of different nominal molecular weight fractions of humic acids on phenol oxidation by permanganate. Environ. Sci. Technol. 2009, 43, 8332–8337. [Google Scholar]
- Xiao, J.; Wang, C.; Liu, H. Fenton-like degradation of dimethyl phthalate enhanced by quinone species. J. Hazard. Mater. 2019, 382, 121007. [Google Scholar] [CrossRef]
- Jiang, C.C.; Gao, Z.; Qu, H.L. A New Insight into Fenton and Fenton-Like Processes for Water Treatment: Part II. Influence of Organic Compounds on Fe(III)/Fe(II) Interconversion and the Course of Reactions. J. Hazard. Mater. 2013, 250–251, 76–81. [Google Scholar] [CrossRef]
- Sun, H.; Xie, G.; He, D. Ascorbic Acid Promoted Magnetite Fenton Degradation of Alachlor: Mechanistic Insights and Kinetic Modeling. Appl. Catal. B-Environ. 2020, 267, 118–383. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Cheng, M.; Lai, C.; Long, M. Improving the Fenton-like catalytic performance of MnOx-Fe3O4/biochar using reducing agents: A comparative study. J. Hazard. Mater. 2020, 406, 124333. [Google Scholar] [CrossRef]
- Nanny, M.A.; Maza, J.P. Noncovalent interactions between monoaromatic compounds and dissolved humic acids: A deuterium NMR T1 relaxation study. Environ. Sci. Technol. 2001, 35, 379–384. [Google Scholar] [CrossRef]
- Chen, L.W.; Li, X.C.; Zhang, J. Production of hydroxyl radical via the activation of hydrogen peroxide by hydroxylamine. Environ. Sci. Technol. 2015, 49, 10373–10379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Z.; Kalyanaraman, B.; Jiang, G.B. Molecular Mechanism for Metal independent Production of Hydroxyl Radicals by Hydrogen Peroxide and Halogenated Quinones. Proc. Natl. Acad. Sci. USA 2007, 104, 17575–17578. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.Z.; Zhao, H.T.; Kalyanaraman, B. Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: An ESR spin trapping study. Free. Radic. Biol. Med. 2002, 32, 465–473. [Google Scholar] [CrossRef]
- Fang, G.D.; Gao, J.; Dionysiou, D.D. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, Y.; Yang, F. FeOOH-catalyzed heterogeneous electro-Fenton system upon anthraquinone@graphene nanohybrid cathode in a divided electrolytic cell: Catholyte-regulated catalytic oxidation performance and mechanism. J. Electrochem. Soc. 2015, 162, H357–H365. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, W. Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle. Environ. Sci. Technol. 2009, 43, 878–883. [Google Scholar] [CrossRef]
- Leng, Y.; Guo, W.; Shi, X. Polyhydroquinone-coated Fe3O4 nanocatalyst for degradation of rhodamine B based on sulfate radicals. Ind. Eng. Chem. Res. 2013, 52, 13607–13612. [Google Scholar] [CrossRef]
- Hou, X.; Zhan, G.; Huang, X. Persulfate activation induced by ascorbic acid for efficient organic pollutants oxidation. Chem. Eng. J. 2020, 382, 122355. [Google Scholar] [CrossRef]
- Cao, M.; Hou, Y.; Zhang, E. Ascorbic acid induced activation of persulfate for pentachlorophenol degradation. Chemosphere 2019, 229, 200–205. [Google Scholar] [CrossRef]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Mechanism of persulfate activation by phenols. Environ. Sci. Technol. 2013, 47, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chiu, Y.T.; Ciou, C. Natural organic activator quercetin for persulfate oxidative degradation of halogenated hydrocarbons. Environ. Sci. Water Res. Technol. 2019, 5, 1064–1071. [Google Scholar] [CrossRef]
- Elloy, F.C.; Teel, A.L.; Watts, R.J. Activation of persulfate by surfactants under acidic and basic conditions. Groundw. Monit. Remediat. 2014, 34, 51–59. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.; Ma, J.; Liu, Z.-Q. Simultaneous oxidation of phenol and bisphenol a by permanganate: Synergetic or competitive effect. Sep. Purif. Technol. 2013, 116, 271–276. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Dong, W.; Ma, J.; Cao, J.; Li, T. Study on enhanced degradation of atrazine by ozonation in the presence of hydroxylamine. J. Hazard. Mater. 2016, 316, 110–121. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, X.; Sun, L.; Qiang, Z.; Xia, D. Insights into the activation of ozonation by hydroxylamine: Influential factors, degradation mechanism and reaction kinetics. J. Hazard. Mater. 2019, 373, 600–607. [Google Scholar] [CrossRef]
- Georgi, A.; Schierz, A.; Trommler, U.; Horwitz, C.P.; Collins, T.J.; Kopinke, F.D. Humic Acid Modified Fenton Reagent for Enhancement of the Working pH Range. Appl. Catal. B Environ. 2007, 72, 26–36. [Google Scholar] [CrossRef]
- Ying, Z.; Zhou, M.A. Critical Review of the Application of Chelating Agents to Enable Fenton and Fenton-like Reactions at High pH Values. J. Hazard. Mater. 2018, 362, 436–450. [Google Scholar]
- Davydova, N.D.; Znamenskaya, T.I. Barrier Functions of Soils of Natural and Technogenic Steppe Landscapes. Geogr. Nat. Resour. 2019, 40, 384–393. [Google Scholar] [CrossRef]
- Davies, G.; Fataftah, A.; Cherkasskiy, A.; Ghabbour, E.A.; Radwan, A.; Jansen, S.A. Tight metal binding by humic acids and its role in biomineralization. J. Chem. Soc. Dalton Trans. 1997, 21, 4047–4060. [Google Scholar] [CrossRef]
- Li, Z.M.; Shea, P.J.; Comfort, S.D. Fenton Oxidation of 2,4,6-trinitrotoluene in Contaminated Soil Slurries. Environ. Eng. Sci. 1997, 14, 55–66. [Google Scholar] [CrossRef]
- Yu, H.; Liu, G.; Jin, R.; Zhou, J. Goethite-humic acid coprecipitate mediated Fenton-like degradation of sulfanilamide: The role of coprecipitated humic acid in accelerating Fe(III)/Fe(II) cycle and degradation efficiency. J. Hazard. Mater. 2021, 403, 124026. [Google Scholar] [CrossRef]
- Lipczynsk, A.; Kochany, E.; Kochany, J. Effect of humic substances on the Fenton treatment of wastewater at acidic and neutral pH. Chemosphere 2008, 73, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Voelker, B.M.; Sulzberger, B. Effects of fulvic acid on Fe(II) oxidation by hydrogen peroxide. Environ. Sci. Technol. 1996, 30, 1106–1114. [Google Scholar] [CrossRef]
- Lindesy, M.E.; Tarr, M.A. Inhibition of Hydroxyl Radical Reaction with Aromatics by Dissolved Natural Organic Matter. Environ. Sci. Technol. 2000, 34, 444–449. [Google Scholar] [CrossRef]
- Lindsey, M.E.; Tarr, M.A. Inhibited hydroxyl radical degradation of aromatic hydrocarbons in the presence of dissolved fulvic acid. Water Res. 2000, 34, 2385–2389. [Google Scholar] [CrossRef]
- He, D.; Guan, X.; Ma, J. Influence of humic acids of different origins on oxidation of phenol and chlorophenols by permanganate. J. Hazard. 2010, 182, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.E.; Schwartz, F.W. Oxidative degradation and kinetics of chlorinated ethylenes by potassium permanganate. J. Contam. Hydrol. 1999, 37, 343–365. [Google Scholar] [CrossRef]
- Smejkalova, D.; Spaccini, R.; Fontaine, B. Binding of Phenol and Differently Halogenated Phenols to Dissolved Humic Matter as Measured by NMR Spectroscopy. Environ. Sci. Technol. 2009, 43, 5377–5382. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Graham, N.J.D. Degradation of atrazine by manganese-catalysed ozonation: Influence of humic substances. Water Res. 1999, 33, 785–793. [Google Scholar] [CrossRef]
- Warner, R.C.; Weber, I. The cupric and ferric citrate complexes. J. Am. Chem. Soc. 1953, 75, 5086–5094. [Google Scholar] [CrossRef]
- Field, T.B.; Mccourt, J.L.; Mcbryde, W.A.E. Composition and Stability of Iron and Copper Citrate Complexes in Aqueous Solution. Can. J. Chem. 1974, 52, 3119–3124. [Google Scholar] [CrossRef]
- Timberlake, C.F. Iron-Malate and Iron-Citrate Complexes. J. Chem. Soc. 1964, 8, 5078–5085. [Google Scholar] [CrossRef]
- Lewis, S.; Lynch, A.; Bachas, L. Chelate-modified Fenton Reaction for the Degradation of Trichloroethylene in Aqueous and Two-phase Systems. Environ. Eng. Sci. 2009, 26, 849–859. [Google Scholar] [CrossRef]
- Li, Y.; Bachas, L.G.; Bhattacharyya, D. Kinetics Studies of Trichlorophenol Destruction by Chelate-based Fenton Reaction. Environ. Eng. Sci. 2005, 22, 756–771. [Google Scholar] [CrossRef]
- Trovo, A.G.; Nogueira, R.F.P. Diclofenac Abatement Using Modified Solar Photo-Fenton Process with Ammonium Iron(III) Citrate. J. Braz. Chem. Soc. 2011, 22, 1033–1039. [Google Scholar] [CrossRef]
- Qin, Y.; Song, F.; Ai, Z. Protocatechuic Acid Promoted Alachlor Degradation in Fe(III)/H2O2 Fenton System. Environ. Sci. Technol. 2015, 49, 7948–7956. [Google Scholar] [CrossRef]
- Ren, H.; Jin, X.; Li, C.G. Rosmarinic Acid Enhanced Fe(III)-mediated Fenton Oxidation Removal of Organic Pollutants at Near Neutral pH. Sci. Total. Environ. 2020, 736, 139528. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.L.; He, J.Y. EDTA-Fe(III) Fenton-like Oxidation for the Degradation of Malachite Green. J. Environ. Manag. 2018, 226, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Seibig, S.; Eldik, R.V. Kinetics of [FeII(EDTA)] Oxidation by Molecular Oxygen Revisited. New Evid. A Multistep Mechanism. Inorg. Chem. 1997, 36, 4115. [Google Scholar]
- Brian, K.; Gullett, L.; Oudejans, D. Near-real-time combustion monitoring for PCDD/PCDF indicators by GC-REMPI-TOFMS. Environ. Sci. Technol. 2011, 46, 923–928. [Google Scholar]
- Tweedy, S.E.; Benitez, A.R.; Narayan, A.; Zimmerman, P.M.; Wymore, T. The hydroxyl radical coupled electron transfer mechanism of flavin-dependent hydroxylases. J. Phys. Chem. B 2019, 123, 8065–8073. [Google Scholar] [CrossRef]
- Banavath, R.; Srivastava, R.; Bhargava, P. Improved non-enzymatic H2O2 sensors using highly electroactive cobalt hexacyanoferrate nanostructures prepared through EDTA chelation route. Mater. Chem. Phys. 2021, 267, 124593. [Google Scholar] [CrossRef]
- Feng, D.; Hong, C.; Sida, Z. Effect of chelating agents on reactive Green 19 decolorization through Fe0-activated persulfate oxidation process. J. Environ. Manag. 2017, 200, 325–334. [Google Scholar]
- Pan, Y.; Bu, Z.; Sang, C. EDTA enhanced pre-magnetized Fe0/H2O2 process for removing sulfamethazine at neutral pH. Sep. Purif. Technol. 2020, 250, 117281. [Google Scholar] [CrossRef]
- Englehardt, J.D.; Meeroff, D.E.; Echegoye, N.L. Oxidation of aqueous EDTA and associated organics and coprecipitation of inorganics by ambient iron-mediated aeration. Environ. Sci. Technol. 2007, 41, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kari, F.G.; Giger, W. Speciation and Fate of Ethylenediaminetetraacetate (EDTA) in Municipal Wastewater Treatment. Water Res. 1996, 30, 122–134. [Google Scholar] [CrossRef]
- Sun, S.P.; Zeng, X.; Li, C. Enhanced heterogeneous and homogeneous Fenton-like degradation of carbamazepine by nano-Fe3O4/H2O2 with nitrilotriacetic acid. Chem. Eng. J. 2014, 244, 44–49. [Google Scholar] [CrossRef]
- Ye, Z.; Brillas, E.; Centellas, F. Expanding the Application of Photoelectro-Fenton Treatment to Urban Wastewater Using the Fe(III)-EDDS Complex. Water Res. 2020, 169, 115219. [Google Scholar] [CrossRef] [PubMed]
- Miralles-cuevas, S.; Oller, I.; Ruiz-delgado, A. EDDS as complexing agent for enhancing solar advanced oxidation processes in natural water: Effect of iron species and different oxidants. J. Hazard. Mater. 2019, 372, 129–136. [Google Scholar] [CrossRef]
- Schowanek, D.; Feijtel, T.C.J.; Perkins, C.M. Biodegradation of [s,s], [r,r] and Mixed Stereoisomers of Ethylene Diamine Disuccinic Acid (EDDS), A Transition Metal Chelator. Chemosphere 1997, 34, 2375–2391. [Google Scholar] [CrossRef]
- Orama, M.; Saarinen, H.; Aksela, R. Complexation of [s,s] and Mixed Stereoisomers of N,N’-Ethylenediaminedisuccinic Acid (EDDS) with Fe(III), Cu(II), Zn(II) and Mn(II) Ions in Aqueous Solution. J. Chem. Soc. Dalton Trans. 2002, 24, 4644–4648. [Google Scholar] [CrossRef]
- Sun, Y.; Pignatello, J.J. Chemical Treatment of Pesticide Wastes. Evaluation of Fe(III) Chelates for Catalytic Hydrogen Peroxide Oxidation of 2,4-D at Circumneutral pH. J. Agric. Food Chem. 1992, 40, 74–81. [Google Scholar] [CrossRef]
- Bates, G.W.; Billups, C.; Saltman, P. The Kinetics and Mechanism of Iron(III) Exchange Between Chelates and Transferrin, I. The Complexes of Citrate and Nitrilotriacetic Acid. J. Biol. Chem. 1971, 246, 2810. [Google Scholar] [CrossRef]
- White, V.E.; Knowles, C.J. Degradation of copper-NTA by Mesorhizobium sp. NCIMB 13524. Int. Biodeterior. Biodegrad. 2003, 52, 143–150. [Google Scholar] [CrossRef]
- Canals, M.; Gonzalez-olmos, R.; Costas, M. Robust Iron Coordination Complexes with N-Based Neutral Ligands as Efficient Fenton-Like Catalysts at Neutral pH. Environ. Sci. Technol. 2013, 47, 9918–9927. [Google Scholar] [CrossRef]
- Ghiselli, G.; Jardim, W.F.; Litter, M.I.; Mansilla, H.D. Destruction of EDTA using Fenton and photo-Fenton-like reactions under UV-A irradiation. J. Photochem. Photobiol. A Chem. 2004, 167, 59–67. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, D.H.; Xu, J. Flexible Metal–Organic Frameworks: Recent Advances and Potential Applications. Adv. Mater. 2015, 27, 5432–5441. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Yang, D.H.; Xu, J.; FDecoste, J.B.; Peterson, G.W. Metal–Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114, 5695. [Google Scholar]
- Zhu, L.; Meng, L.; Shi, J. Metal-organic frameworks/carbon-based materials for environmental remediation: A state-of-the-art mini-review. J. Environ. Manag. 2019, 232, 964–977. [Google Scholar] [CrossRef]
- Cheng, M.; Hu, L.; Huan, G.D. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Toyao, T.; Saito, M.; Dohshi, S. Development of a Ru Complex-Incorporated MOF Photocatalyst for Hydrogen Production under Visible-light Irradiation. Chem. Commun. 2014, 50, 6779–6781. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y. Mesoporous Metal–organic Framework Materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, Z.; Yu, H.; Hou, X. Synergistic effect of a magnetic field and carbon nanotubes on the adsorption performance of metal ions and phenols. Desalination Water Treat. 2019, 151, 295–306. [Google Scholar] [CrossRef]
- Yao, Y.; Hao, C.; Chao, L.; Wei, F.; Wang, S. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wu, M. Tuning lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Li, L. Iron Terephthalate Metal–organic Framework: Revealing the Effective Activation of Hydrogen Peroxide for the Degradation of Organic Dye under Visible Light Irradiation. Appl. Catal. B Environ. 2014, 148–149, 191–200. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X. Enhanced Fenton-like Catalysis by Iron-based Metal Organic Frameworks for Degradation of Organic Pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Cao, T. Efficient Degradation of High Concentration Azo-dye Wastewater by Heterogeneous Fenton Process with Iron-based Metal-organic Framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Li, W.J. A Nanoscale Fe(II) Metal-organic Framework with a Bipyridinedicarboxylate Ligand as a High Performance heterogeneous Fenton catalyst. Rsc. Adv. 2016, 6, 6756–6760. [Google Scholar] [CrossRef]
- Choi, K.; Lee, W. Enhanced Degradation of Trichloroethylene In Nano-scale zero-Valent Iron Fenton System with Cu(II). J. Hazard. Mater. 2012, 211–212, 146–153. [Google Scholar] [CrossRef]
- Guimaraes, I.R.; Giroto, A.; Oliveira, L.C.; Guerreiro, M.C.; Lima, D.Q.; Fabris, J.D. Synthesis and Thermal Treatment of Cu-doped Goethite: Oxidation of Quinoline through Heterogeneous Fenton Process. Appl. Catal. B Environ. 2009, 91, 581–586. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.F.; Iacob, M. Siloxane-based Metal–Organic Frameworks with Remarkable Catalytic Activity in Mild Environmental Photodegradation of Azo Dyes. Appl. Catal. B: Environ. 2017, 205, 78–92. [Google Scholar] [CrossRef]
- Pariyar, A.; Yaghoobnejad, A.H.; Choundhury, A. Tetragonal Versus Hexagonal: Structure-dependent Catalytic Activity of Co/Zn Bimetallic Metal-Organic Frameworks. Inorg. Chem. 2016, 55, 9250–9257. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, M.; Li, K. Synthesis of Fe/M (M=Mn, Co, Ni) bimetallic metal organic frameworks and their catalytic activity for phenol degradation at mild conditions. Inorg. Chem. Front. 2017, 4, 144–153. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Rykov, A.I.; Han, H.; Jin, C.; Liu, X.; Wang, J. Excellent Photo-Fenton Catalysts of Fe-Co Prussian Blue Analogues and Their Reaction Mechanism Study. Appl. Catal. B Environ. 2015, 179, 196–205. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Qi, H.; Liu, X.; Yang, L. Free radical behaviours during methylene blue degradation in Fe2+/H2O2 system. Environ. Technol. 2017, 40, 1–25. [Google Scholar] [CrossRef] [PubMed]
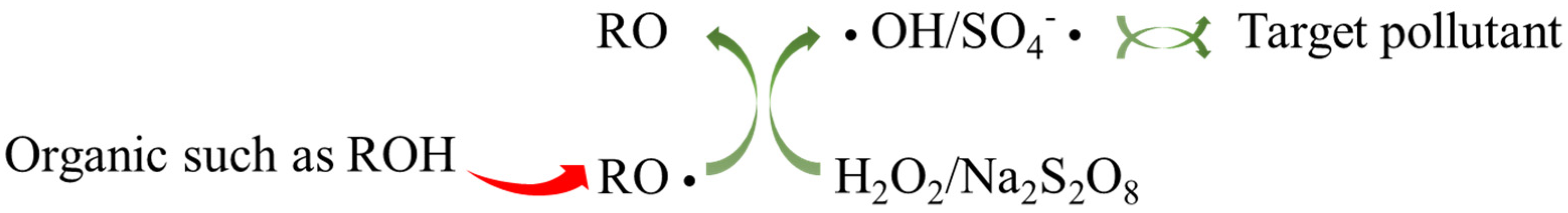
| Chelator | Merit | Demerit |
|---|---|---|
| Humus | Ubiquitous in nature | No obvious influence and even inhibition when pH is low |
| Carboxylic acid | Suitable for acid to neutral range; Chelating agent can effectively degrade | Affected by pH and molar ratio to transition metal |
| Amino carboxylic acid | The time of Fe2+ participation in Fenton reaction can be extended; It can activate the dissolved oxygen in the system and produce H2O2 spontaneously; The effect is better under neutral neutral or alkaline conditions | The molar ratio of transition metal has influence on the promoting effect; Environmental degradation is poor |
| Organics Class | Organic Substance | Structure | Oxidant | Reaction pH | Target Pollutant | Source |
|---|---|---|---|---|---|---|
| Phenols | Hydroquinone | 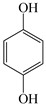 | H2O2 (10 mmol L−1), Fe3+ (0.5 mmol L−1) and hydroquinone (0.1 mmol L−1) | 3.1~3.2 | DMP (1 mmol L−1) | [22] |
| Pentachlorophenol | 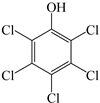 | Na2S2O8 (0.5 mol L−1) and pentachlorophenol (1 mmol L−1) | 6.5~10.5 | Nitrobenzene (1 mmol L−1) | [36] | |
| AA | 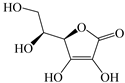 | H2O2 (1 mmol L−1), AA (0.5 mmol L−1) and Fe3O4 (1 g L−1) | 4 | Alachlor (20 mg L−1) | [24] | |
| Na2S2O8 (40 mmol L−1) and AA (1.0 mmol L−1) | 7.2 | Pentachlorophenol (10 mg L−1) | [35] | |||
| Humus | Humic Acid | / | H2O2 (130 mmol L−1), Fe2+ (30 μmol L−1) and HA (50~100 mg L−1) | 5~7 | Benzene (25 μmol L−1) | [42] |
| H2O2 (50 mmol L−1), Fe2+ (5 mmol L−1) and HA (10 mg L−1) | 6.5 | 15 organic compounds | [48] | |||
| Carboxylic Acid Compounds | CA |  | H2O2 (50 mmol L−1), Fe2+ (10 mmol L−1) and CA (10 mmol L−1) | 5~7 | 2,4,6–trichlorophenol (1.5 mmol L−1) | [60] |
| Gallic Acid |  | H2O2 (8 mmol L−1) and Fe3+ (0.1 mmol L−1) | 3.6 | gallic acid (0.11 mmol L−1) | [62] | |
| Amino Carboxylic Acids | EDTA | 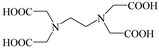 | Na2S2O8 (5 mmol L−1), Fe0(1.0 g L−1) and EDTA (1 mmol L−1) | 6.0 | Reactive Green 19 (0.05 mmol L−1) | [69] |
| EDDS | a mixture consisted of different configurations | H2O2 (5 mol L−1) and Fe3+-EDDS (1 mol L−1) | 6.2 | bisphenol A (20 μmol L−1) | [10] | |
| NTA | 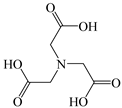 | H2O2 (100 mmol L−1), Fe3O4 (1.0 g L−1) and NTA (0.5 mmol L−1) | 7 | Carbamazepine (63.5 μmol L−1) | [73] | |
| Others | Hydroxylamine | 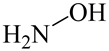 | H2O2 (0.4 mmol L−1), Fe2+ (10.0 μmol L−1) and NH2OH (0.4 mmol L−1) | 2.0~5.7 | benzoic acid (40.0 μmol L−1) | [14] |
| 1,4-benzoquinone |  | Persulfate (5 mmol L−1) | 7.4 | PCB 28 (0.5 mg L−1) | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Shi, J.; Yang, H.; Huang, J.; Sheng, F. How Organic Substances Promote the Chemical Oxidative Degradation of Pollutants: A Mini Review. Sustainability 2021, 13, 10993. https://doi.org/10.3390/su131910993
Hu Z, Shi J, Yang H, Huang J, Sheng F. How Organic Substances Promote the Chemical Oxidative Degradation of Pollutants: A Mini Review. Sustainability. 2021; 13(19):10993. https://doi.org/10.3390/su131910993
Chicago/Turabian StyleHu, Zhewei, Jiaqi Shi, Hao Yang, Jianbo Huang, and Feng Sheng. 2021. "How Organic Substances Promote the Chemical Oxidative Degradation of Pollutants: A Mini Review" Sustainability 13, no. 19: 10993. https://doi.org/10.3390/su131910993
APA StyleHu, Z., Shi, J., Yang, H., Huang, J., & Sheng, F. (2021). How Organic Substances Promote the Chemical Oxidative Degradation of Pollutants: A Mini Review. Sustainability, 13(19), 10993. https://doi.org/10.3390/su131910993





