Potential Use of Native Yeasts to Produce Bioethanol and Other Byproducts from Black Sugarcane, an Alternative to Increment the Subsistence Farming in Northern Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Selection of Native Yeasts
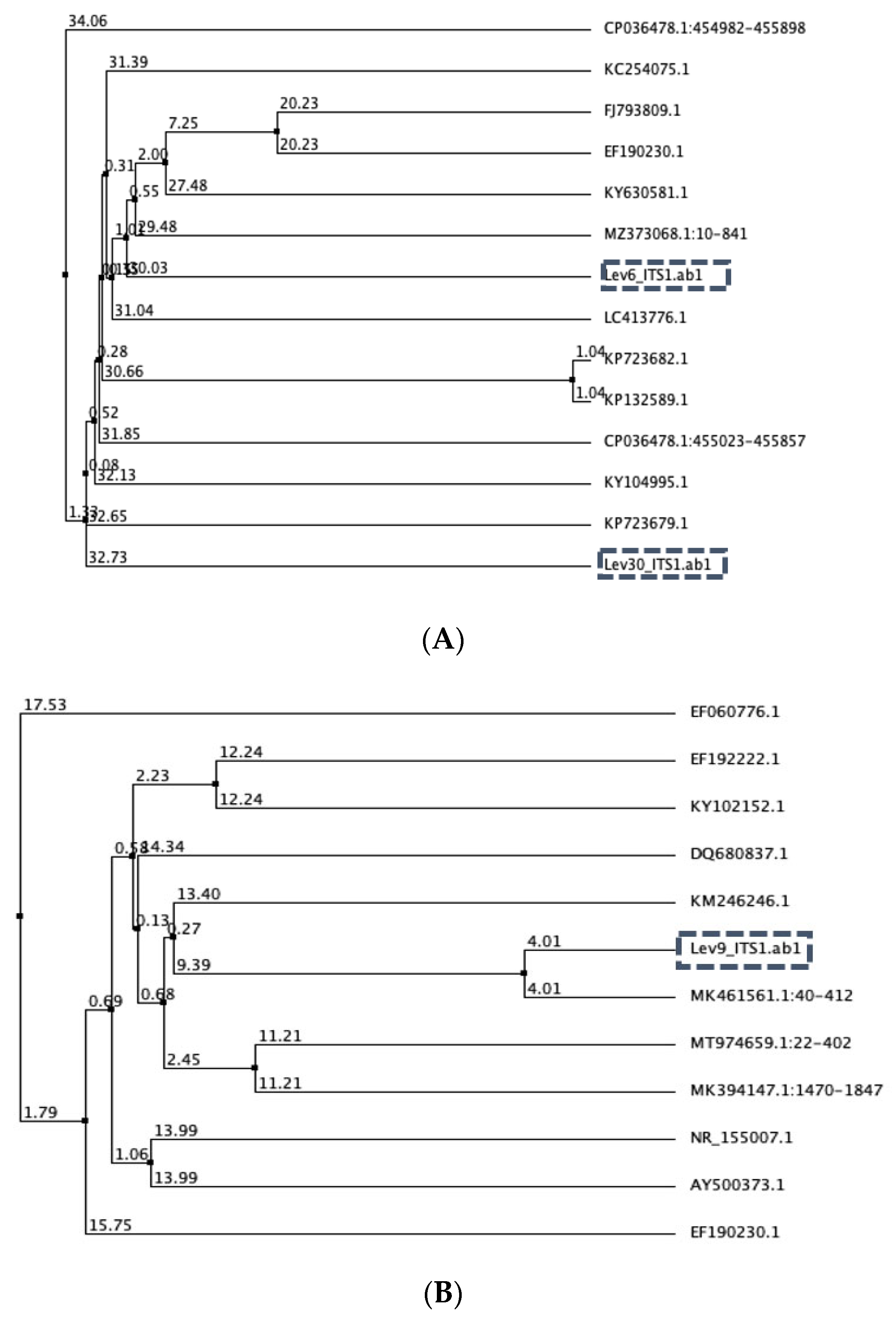
2.2. Identification of Selected Isolates
2.3. Evaluation of the Growth Kinetics of Native Yeasts In Vitro
2.4. Study of Fermentation Variables with Native Yeasts in Sugarcane Juice
2.5. Determination of Alcoholic Degree
2.6. Determination of the Yield of Alcohol, Biomass Production, Ethanol Concentration and Volumetric Productivity
2.7. GC-MS Spectrometry to Analyze the Production of Ethanol and Other Byproducts
2.8. Statistical Analysis
3. Results and Discussion
3.1. Yeast Identification
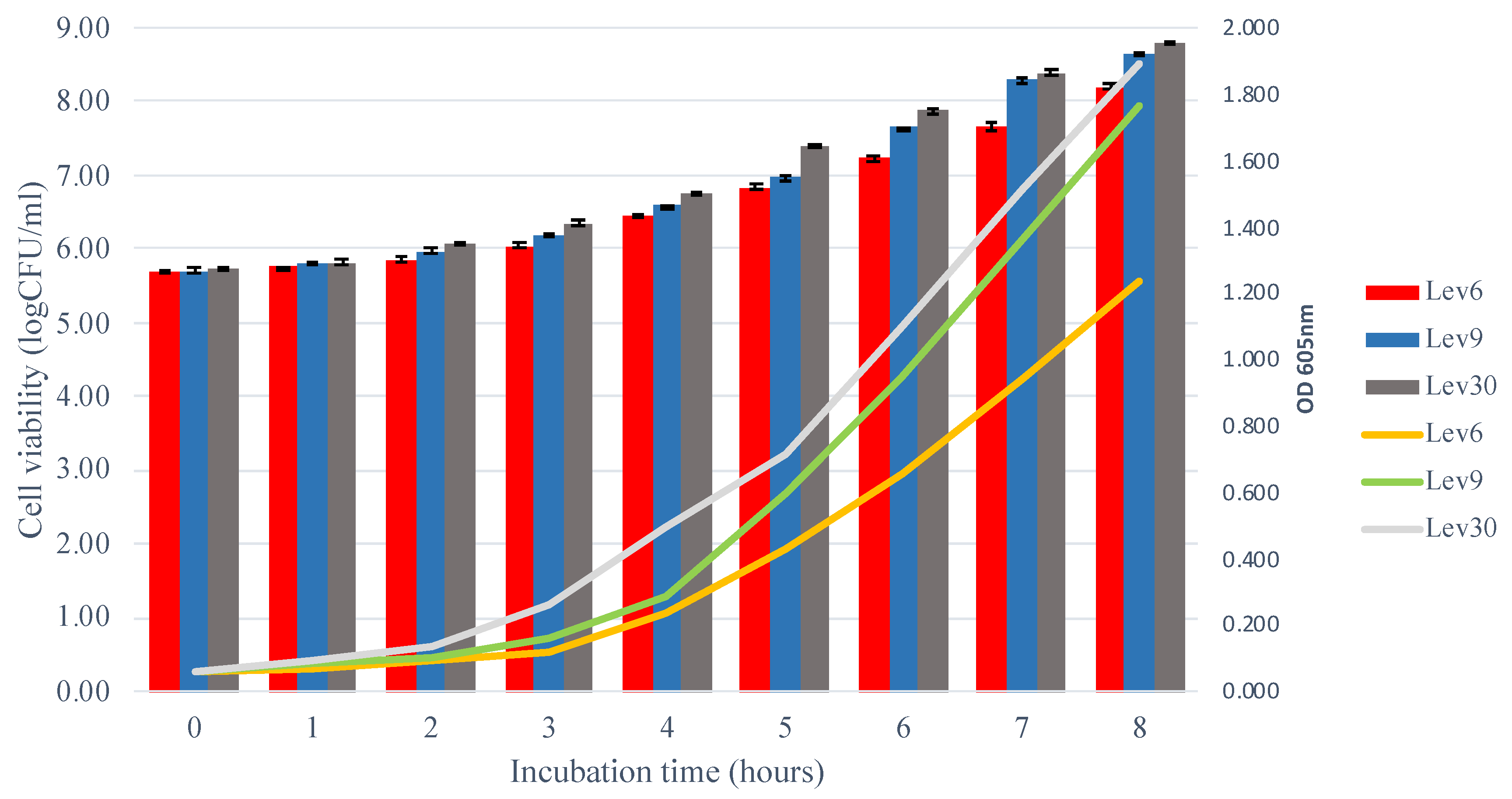
3.2. Growth Kinetics of Selected Yeasts
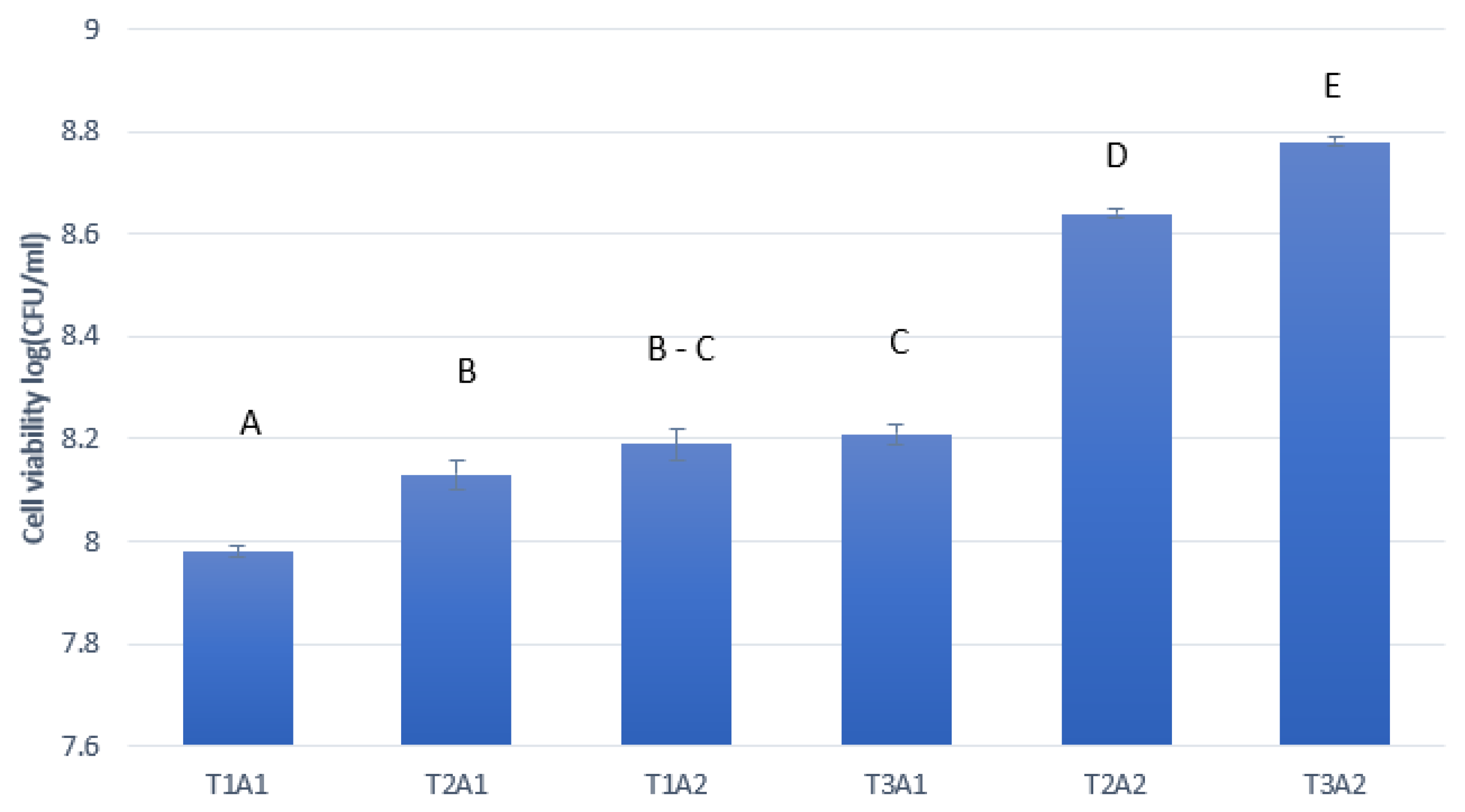
3.3. Fermentation and Ethanol Production from Sugarcane
3.4. Production of Ethanol and Byproducts Obtained from Alcoholic Fermentation
3.5. Analysis of Molecules Detected upon Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Antoni, D.; Zverlov, V.V.; Schwarz, W.H. Biofuels from microbes. Appl. Microb. Biotechnol. 2007, 77, 23–35. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Boyce, A.N. Bioethanol production from fermentable sugar juice. Sci. World. J. 2014, 2014, 957102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Energy Agency. Global CO2 Emissions in 2019. 2020. Available online: https://www.iea.org/articles/global-co2-emissions-in-2019 (accessed on 15 March 2020).
- Organización Latinoamericana de Energía. Bioetanol de Caña de Azúcar. Energía para el Desarrollo Sostenible. 2008. Available online: http://www.olade.org/sites/default/files/CIDA/Biocomustibles/CEPAL/bioetanol%20ca%C3%B1a%20de%20azucar.pdf (accessed on 20 June 2020).
- Halder, P.; Azad, K.; Shah, S.; Sarker, E. Prospects and technological advancement of cellulosic bioethanol ecofuel production. In Advances in Eco-Fuels for a Sustainable Environment; Azad, K., Ed.; Woodhead Publishing Series in Energy; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 211–236. [Google Scholar] [CrossRef]
- Susmozas, A.; Martín-Sampedro, R.; Ibarra, D.; Eugenio, M.E.; Iglesias, R.; Manzanares, P.; Moreno, A.D. Process strategies for the transition of 1G to sdvanced bioethanol production. Processes 2020, 8, 1310. [Google Scholar] [CrossRef]
- Filip, O.; Janda, K.; Kristoufek, L.; Zilberman, D. Food versus fuel: An updated and expanded evidence. Energy Econ. 2019, 82, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Environment and Water. Ecuador Impulsa la Gestión Adecuada de Residuos Orgánicos en las Ciudades. Available online: https://www.ambiente.gob.ec/ecuador-impulsa-la-gestion-adecuada-de-residuos-organicos-en-las-ciudades/ (accessed on 22 July 2020).
- Ecuador Sugar Cane Research Center. Report 2016. Available online: https://cincae.org/wp-content/uploads/2013/04/Informe-Anual-2016.pdf (accessed on 10 June 2020).
- Piedra, V.; Núñez, J. Importancia de la Destilación de Etanol para el Desarrollo de la Economía del Ecuador. Report 2016. Available online: https://es.scribd.com/document/339659614/Produccion-de-etanol-en-el-ecuador (accessed on 23 January 2020).
- Paucar, J.; Robalino, J. Modelo Estratégico para la Industrialización de la Caña de azucar en el Ecuador. 2009. Available online: http://bibdigital.epn.edu.ec/bitstream/15000/8489/3/CD-2144.pdf (accessed on 5 December 2019).
- Sánchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- National Institute of Statistics and Censuses. Estadísticas Agropecuarias. Report 2019. Available online: https://www.ecuadorencifras.gob.ec/estadisticas-agropecuarias-2/ (accessed on 22 July 2020).
- Jurado, C.N. La Industria de los Biocombustibles en Ecuador: Caso Producción y Comercialización de Etanol Anhidro en la Provincia de Bolívar. 2014. Available online: http://repositorio.puce.edu.ec/bitstream/handle/22000/6903/7.36.000576.pdf?sequence=4&isAllowed=y (accessed on 5 December 2019).
- Garzón, I. Biocombustibles: Conversión de Sistema de Alimentación de un Motor de Gasolina a Etanol. 2012. Available online: http://repositorio.usfq.edu.ec/bitstream/23000/2007/1/105526.pdf (accessed on 23 January 2020).
- Aguilar-Rivera, N. Bioetanol de la caña de azúcar. AIA 2007, 11, 25–39. [Google Scholar]
- Elfasakhany, A. State of art of using biofuels in spark ignition engines. Energies 2021, 14, 779. [Google Scholar] [CrossRef]
- Bezerra, T.L.; Ragauskas, A.J. A review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuel. Bioprod. Biorefin. 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Arshad, M.; Abbad, M.; Iqbal, M. Ethanol production from molasses: Environmental and socioeconomic prospects in Pakistan: Feasibility and economic analysis. Environ. Technol. Innov. 2019, 14. [Google Scholar] [CrossRef]
- Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Ethanol production at elevated temperatures using encapsulation of yeast. Biotechnol. J. 2011, 156, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Djelal, H.; Chniti, S.; Jemni, M.; Weill, A.; Sayed, W.; Amrane, A. Identification of strain isolated from dates (Phœnix dactylifera L.) for enhancing very high gravity ethanol production. Environ. Sci. Pollution Res. 2017, 24, 9886–9894. [Google Scholar] [CrossRef] [PubMed]
- Tenea, G.N.; Jarrin-V, P.; Yepez, L. Microbiota of wild fruits from the amazon region of Ecuador: Linking diversity and functional potential of lactic acid bacteria with their origin. In Ecosystem and Biodiversity of Amazonia; Mikkola, H.J., Ed.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/online-first/microbiota-of-wild-fruits-from-the-amazon-region-of-ecuador-linking-diversity-and-functional-potenti (accessed on 22 January 2021). [CrossRef]
- Gupthar, A.; Bhattacharya, S.; Basu, T. Evaluation of the maximum specific growth rate of a yeast indicating non-linear growth trends in batch culture. World J. Microbiol. Biotechnol. 2000, 16, 613–616. [Google Scholar] [CrossRef]
- van Dijken, J.P.; Weusthuis, R.A.; Pronk, J.T. Kinetics of growth and sugar consumption in yeasts. Antonie Leeuwenhoek 1993, 63, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Still Spirits. A Guide to Help you Choose the Right Yeast for Your at Home Distilling Needs. Available online: https://www.cannonhillbrewing.com.au/wp-content/uploads/2017/08/still-spirits-yeast-1.pdf (accessed on 18 November 2019).
- Stills Spirits. Turbo Yeast Best Practices. 2019. Available online: https://stillspirits.com/pages/turbo-yeast-best-practices (accessed on 18 November 2019).
- Hashsham, S.A.; Baushke, S.W. Energetics, stoichiometry, and kinetics of microbial growth. In Methods for General and Molecular Microbiology, 3rd ed.; Reddy, C.A., Ed.; American Society for Microbiology Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Da Silva, G.P.; de Araujo, E.F.; Silva, D.O.; Guimaraes, W.V. Ethanolic fermentation of sucrose, sugarcane juice and molasses by Escherichia coli strain ko11 and Klebsiella oxytoca strain P2. Braz. J. Microbiol. 2005, 36, 395–404. [Google Scholar] [CrossRef]
- Cordell, R.L.; Pandya, H.; Hubbard, M.; Turner, M.A.; Monks, P.S. GC-MS analysis of ethanol and other volatile compounds in micro-volume blood samples-quantifying neonatal exposure. Anal. Bioanal. Chem. 2013, 405, 4139–4147. [Google Scholar] [CrossRef]
- Tikka, C.; Osuru, H.P.; Atluri, N.; Raghavulu, P.C.; Yellapu, N.K.; Mannur, I.S.; Prasad, U.V.; Aluru, S.; Narashimba Varna, K.; Bhaskar, M. Isolation and characterization of ethanol tolerant yeast strains. Bioinformation 2013, 9, 421–425. [Google Scholar] [CrossRef]
- Moreno, A.D.; Tomás-Pejó, E.; Olsson, L.; Geijer, C. Candida intermedia CBS 141442: A Novel Glucose/Xylose Co-Fermenting Isolate for Lignocellulosic Bioethanol Production. Energies 2020, 13, 5363. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Khongsay, N.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. Optimization of agitation and aeration for very high gravity ethanol fermentation from sweet sorghum juice by Saccharomyces cerevisiae using an orthogonal array design. Energies 2012, 5, 561–576. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, J.; Espinoza, M.; Cabanillas, J.; Ávila, I.; García, A.; Julca, J.; Tacanga, D.; Zuta, I.; Linares, G. Evaluación de la cinética de crecimiento de Saccharomyces cerevisiae utilizando un medio de cultivo a base de melaza de caña y suero lácteo. Agroind. Sci. 2015, 5, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, D.I.; Chia, Y.C.; Tai, C.Y.; Ou, A. Investigation of chemical quality of sugarcane (Saccharum officinarum L.) wine during fermentation by Saccharomyces cerevisiae. J. Food Qual. 2010, 33, 248–267. [Google Scholar] [CrossRef]
- Maier, R. Bacterial Growth. In Environmental Microbiology; Pepper, I.L., Gerba, C.P., Gentry, T.J., Maier, R.M., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 37–54. [Google Scholar]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.; Butzke, C.E. Diagnosis and rectification of stuck and sluggish fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar]
- Vieira, E.; Stupiello, M.; Andrietta, S. Yeast biomass production: A new approach in glucose-limited feeding strategy. Braz. J. Microbiol. 2013, 44, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.L.; Choong, Y.M.; Su, N.W.; Lee, M.H. A rapid method for determination of ethanol in alcoholic beverages using capillary gas chromatography. J. Food Drug Anal. 2003, 11, 133–140. [Google Scholar] [CrossRef]
- Tiscione, N.; Alford, I.; Yeatman, D.; Shan, X. Ethanol analysis by headspace gas chromatography with simultaneous flame-Ionization and mass spectrometry detection. J. Anal. Toxicol. 2011, 35, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Bushra, R. Functionalized nanomaterials for chromatography. In Nanomaterials in Chromatography, 1st ed.; Hussain, C.M., Ed.; Elsevier: Newark, NJ, USA, 2018; pp. 403–414. [Google Scholar] [CrossRef]
- El-Moaty, H. Bioactive compounds of Moricandia nitens and its anticancer effect. Indo Am. J. Pharm. Sci. 2016, 3, 1283–1290. [Google Scholar]
- Nwaiwu, O.; Itumoh, M. Chemical contaminants associated with palm wine from Nigeria are potential food safety hazards. Beverages 2017, 3, 16. [Google Scholar] [CrossRef]
- Shubharani, R.; Sivaram, V.; Kishore, B.R. In Vitro cytotoxicity of Indian bee propolis on cancer cell lines. Int. J. Pharm. Biol. Sci. 2014, 5, 698–706. [Google Scholar]
- Dhanasekaran, D.; Latha, S.; Suganya, P.; Panneerselvam, A.; Kumar, T.; Alharbi, N.; Arunachalam, C.; Alharbi, S.A.; Thajuddin, N. Taxonomic identification and bioactive compounds characterization of Psilocybe cubensis DPT1 to probe its antibacterial and mosquito larvicidal competency. Microb. Pathog. 2020, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef] [Green Version]
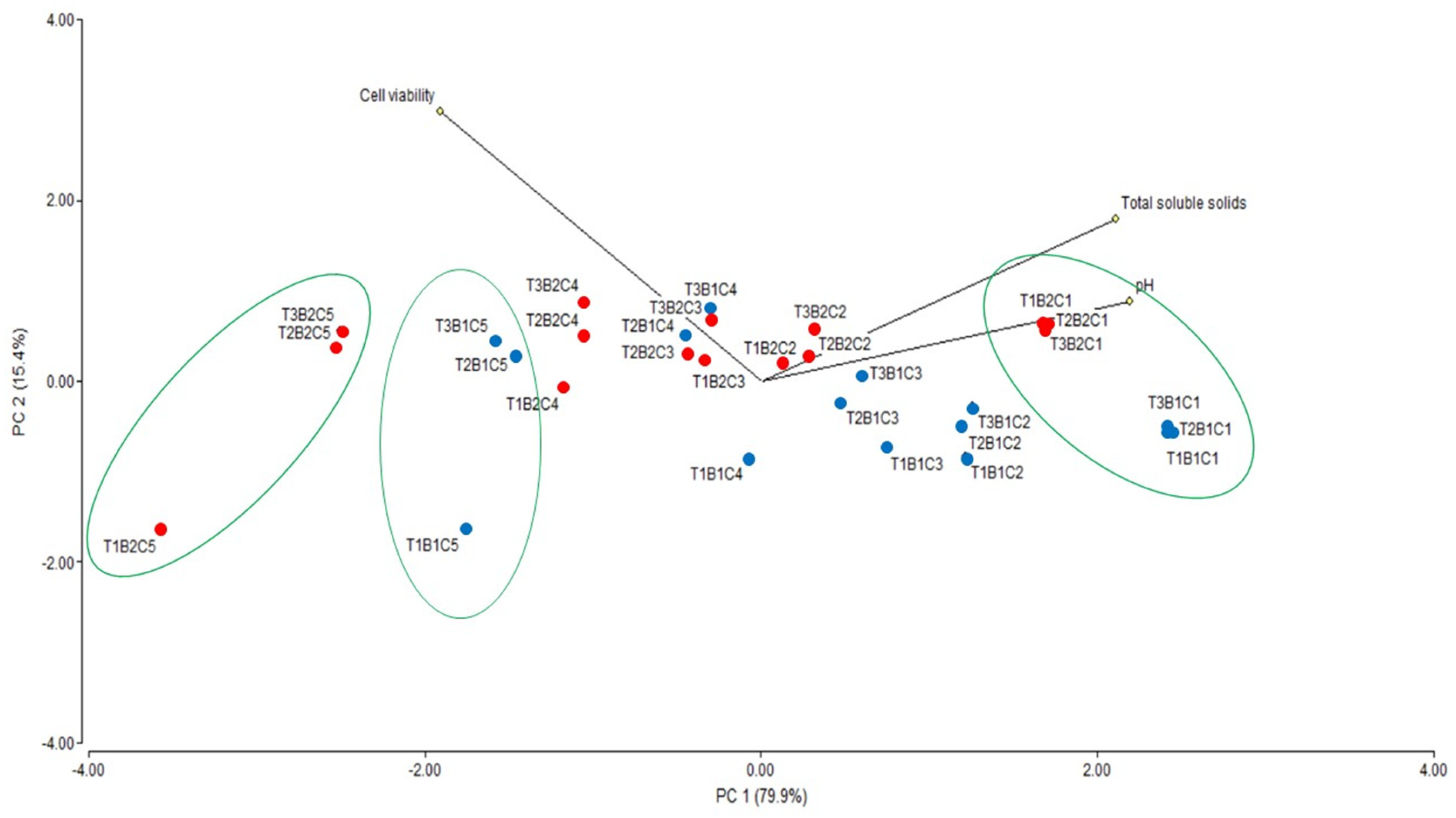
| Correlation of Variables | Cell Viability | Total Soluble Solids | pH | |
|---|---|---|---|---|
| Cell viability | Pearson correlation | 1 | −0.571 ** | −0.668 * |
| Sig. (bilateral) | 0.001 | 0.000 | ||
| N | 30 | 30 | 30 | |
| Total soluble solids | Pearson correlation | −0.571 ** | 1 | 0.849 ** |
| Sig. (bilateral) | 0.001 | 0.000 | ||
| N | 30 | 30 | 30 | |
| pH | Pearson correlation | −0.668 * | 0.849 ** | 1 |
| Sig. (bilateral) | 0.000 | 0.000 | ||
| N | 30 | 30 | 30 | |
| Ethanol (mL) | ∆S (g) | % ABV | Biomass (g) | Yp/s (%) | Yx/s (%) | Q (g/L) | Qp (g/L/h) | |
|---|---|---|---|---|---|---|---|---|
| Lev6 | 7.7 | 58 | 3.4 | 14.0 | 10.4 | 9.8 | 43.92 | 0.61 |
| Lev9 | 4.1 | 33 | 2.0 | 14.7 | 9.8 | 19.4 | 25.92 | 0.36 |
| Lev30 | 3.1 | 28 | 1.7 | 16.2 | 8.8 | 28.2 | 24.48 | 0.34 |
| PTY | 11.6 | 119 | 7.1 | 12.4 | 7.7 | 3.4 | 47.52 | 0.66 |
| CTY | 10.0 | 111 | 6.6 | 13.2 | 7.1 | 4.5 | 48.96 | 0.68 |
| Samples | Retention Time (min) | Molecule | Relative (%) |
|---|---|---|---|
| Lev6 | 1.387 | 2-aziridinylethyl amine | 0.306 |
| 1.542 | Ethanol | 99.712 | |
| Lev9 | 1.389 | 2-aziridinylethyl amine | 0.347 |
| 1.489 | Ethanol | 99.653 | |
| Lev30 | 1.395 | 2-aziridinylethyl amine | 0.758 |
| 1.444 | nitrile tetraacetyl-d-xylonic | 0.507 | |
| 1.486 | Ethanol | 98.735 | |
| PTY | 1.390 | 2-aziridinylethyl amine | 0.204 |
| 1.487 | Ethanol | 99.796 | |
| CTY | 1.390 | Carbon dioxide | 0.035 |
| 1.489 | Ethanol | 99.965 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenea, G.N.; Veintimilla, F. Potential Use of Native Yeasts to Produce Bioethanol and Other Byproducts from Black Sugarcane, an Alternative to Increment the Subsistence Farming in Northern Ecuador. Sustainability 2021, 13, 10924. https://doi.org/10.3390/su131910924
Tenea GN, Veintimilla F. Potential Use of Native Yeasts to Produce Bioethanol and Other Byproducts from Black Sugarcane, an Alternative to Increment the Subsistence Farming in Northern Ecuador. Sustainability. 2021; 13(19):10924. https://doi.org/10.3390/su131910924
Chicago/Turabian StyleTenea, Gabriela N., and Fabricio Veintimilla. 2021. "Potential Use of Native Yeasts to Produce Bioethanol and Other Byproducts from Black Sugarcane, an Alternative to Increment the Subsistence Farming in Northern Ecuador" Sustainability 13, no. 19: 10924. https://doi.org/10.3390/su131910924
APA StyleTenea, G. N., & Veintimilla, F. (2021). Potential Use of Native Yeasts to Produce Bioethanol and Other Byproducts from Black Sugarcane, an Alternative to Increment the Subsistence Farming in Northern Ecuador. Sustainability, 13(19), 10924. https://doi.org/10.3390/su131910924







