Effects of Contact Time and Flow Configuration on the Acid Mine Drainage Remediation Capabilities of Pervious Concrete
Abstract
1. Introduction
2. Methodology
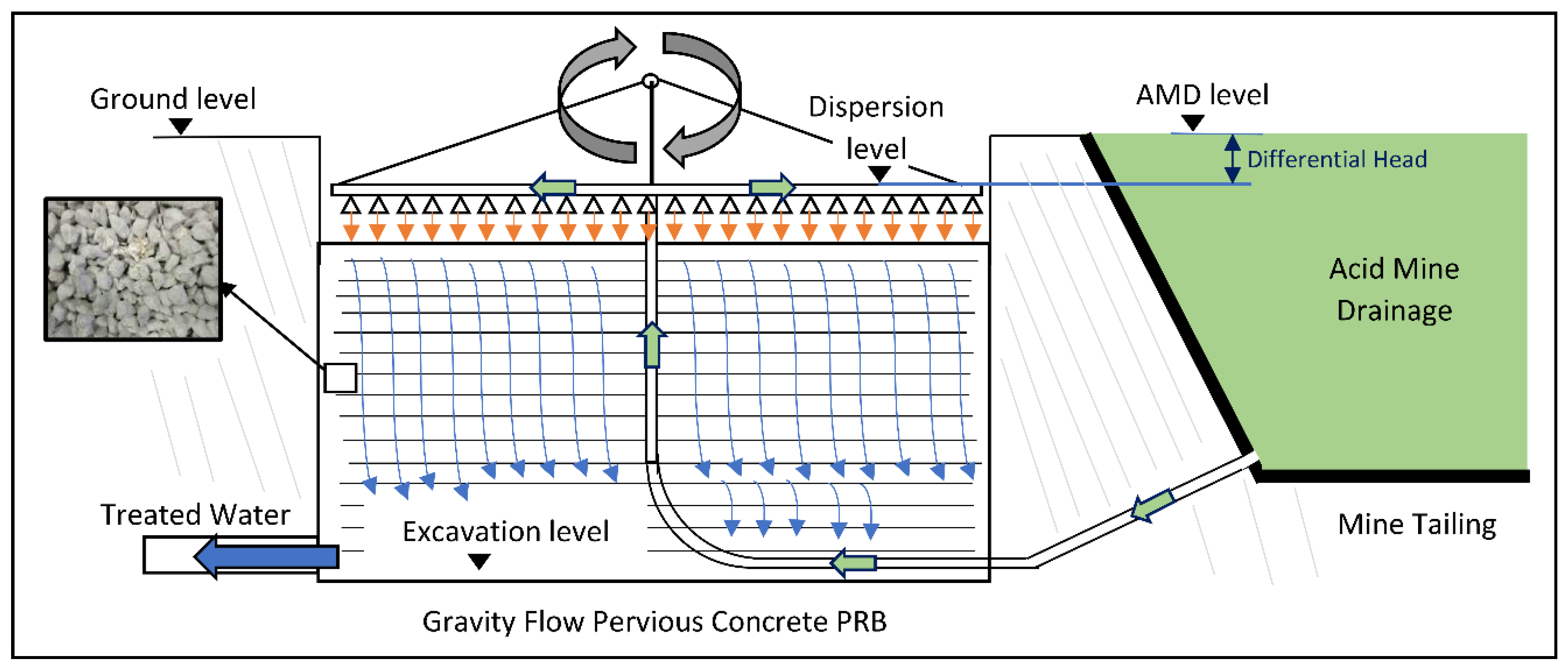
2.1. Equipment and Materials
2.2. Acid Mine Drainage Collection and Storage
2.3. Experimental Methods
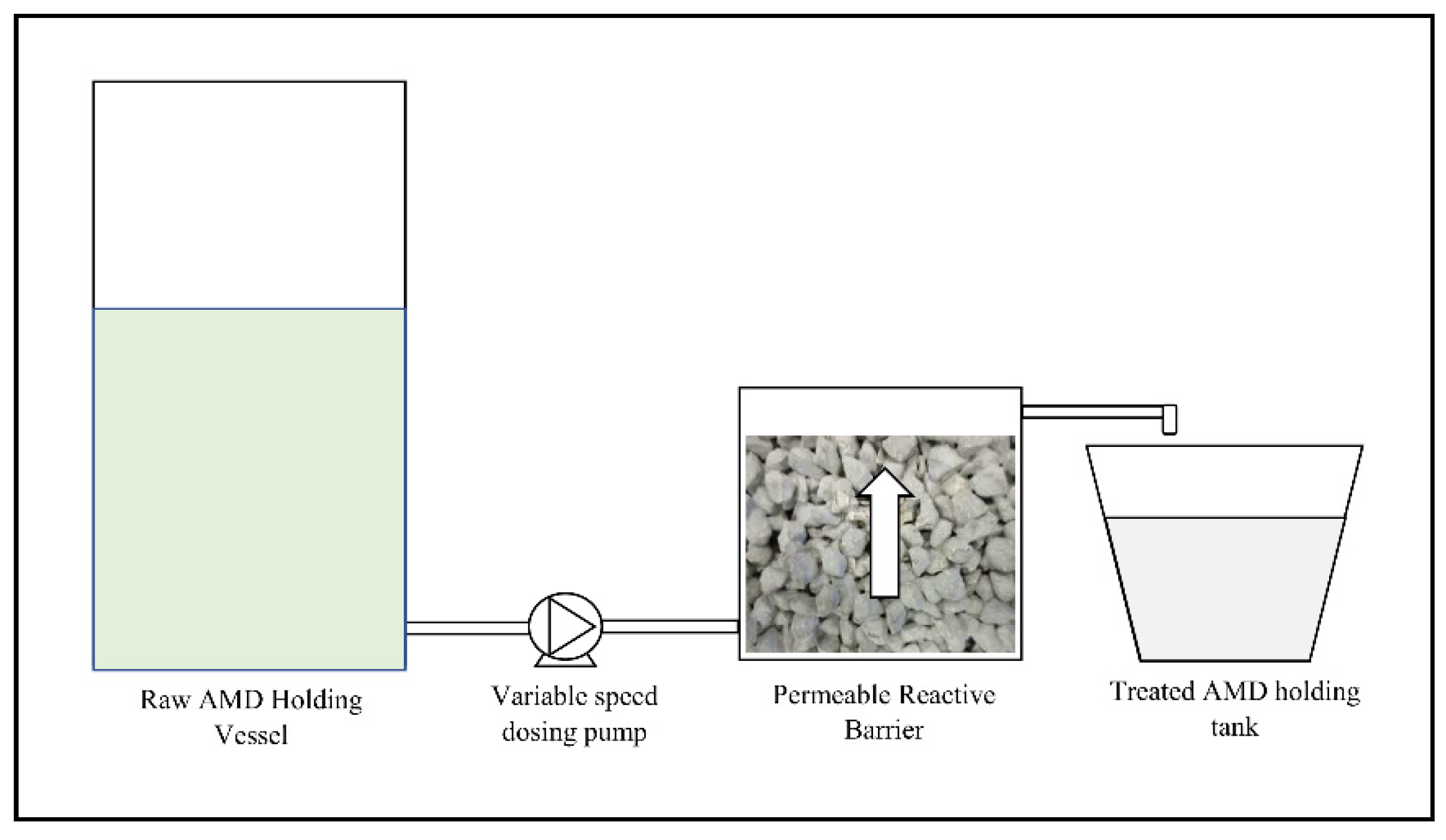
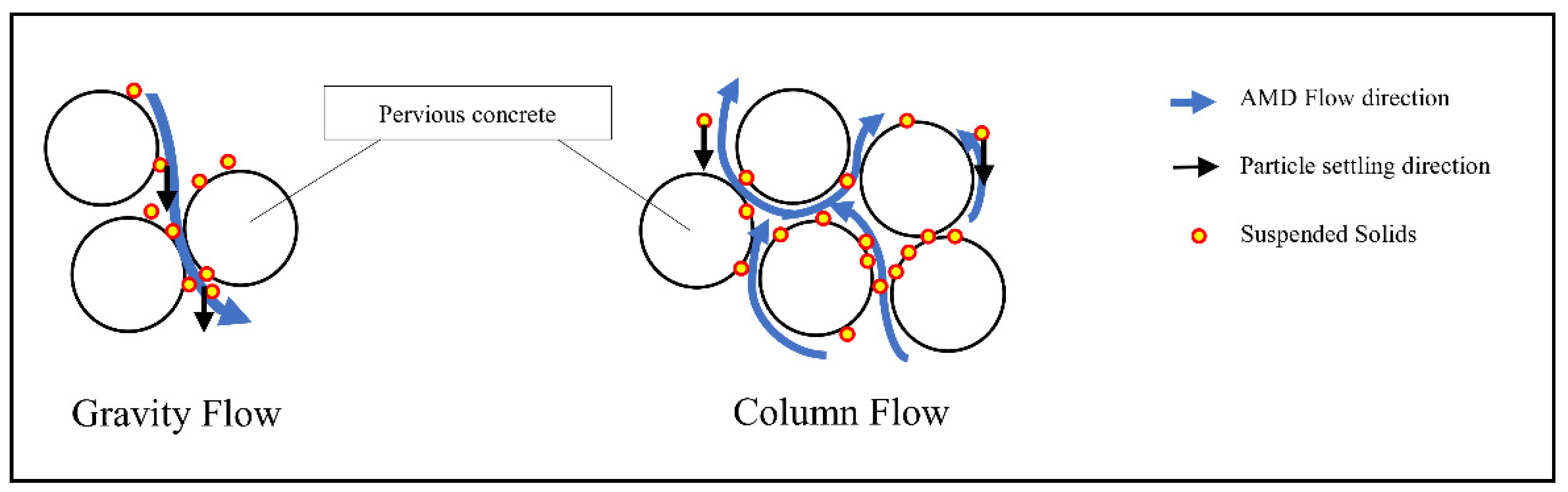
2.3.1. Column Flow Test Procedure
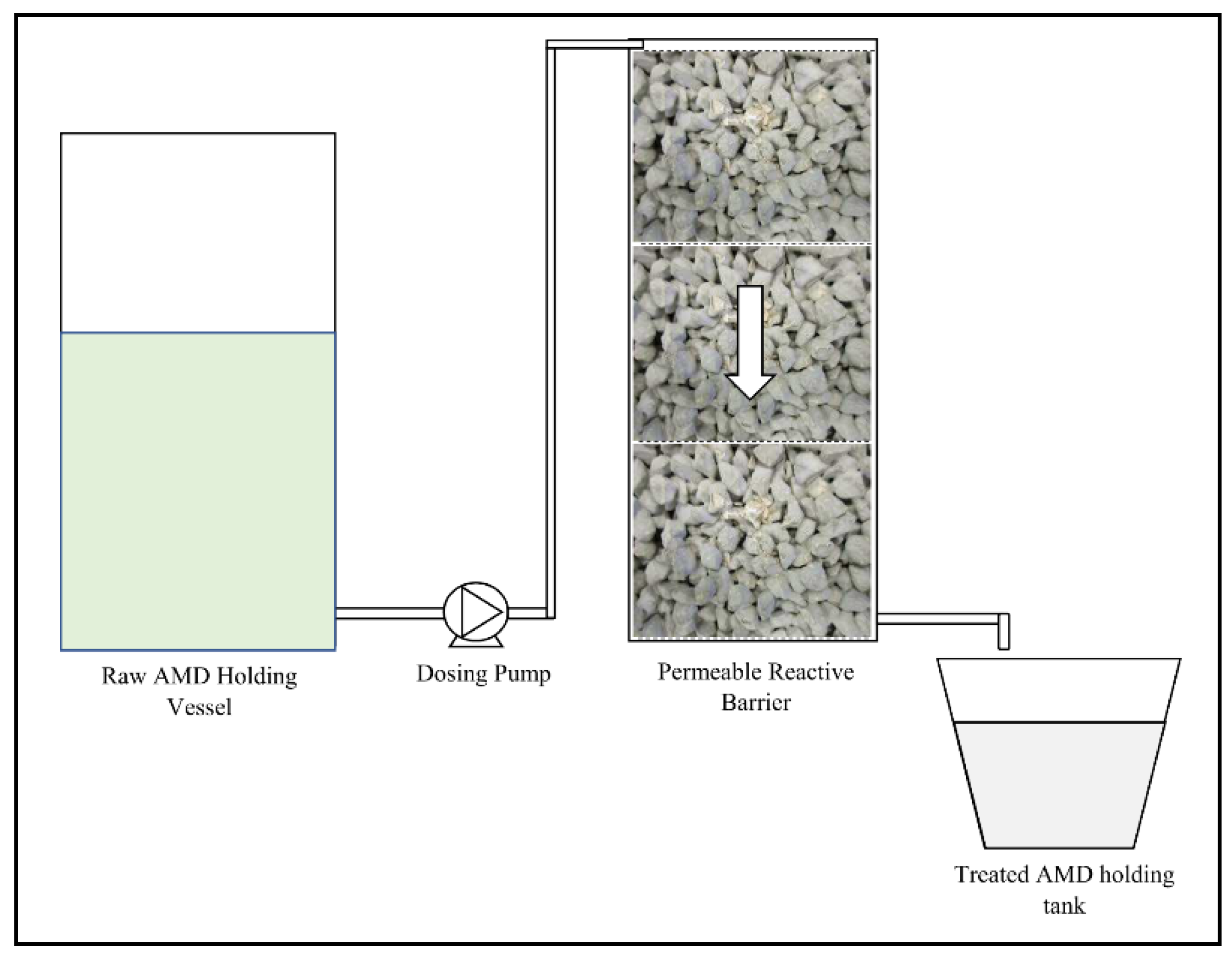
2.3.2. Gravity Flow Test Procedure
2.4. Chemical Analysis Methods
2.4.1. Dissolved Metals
2.4.2. Sulphate
2.4.3. pH
2.4.4. Total Suspended Solids
2.4.5. Statistical Methods
3. Results and Discussions
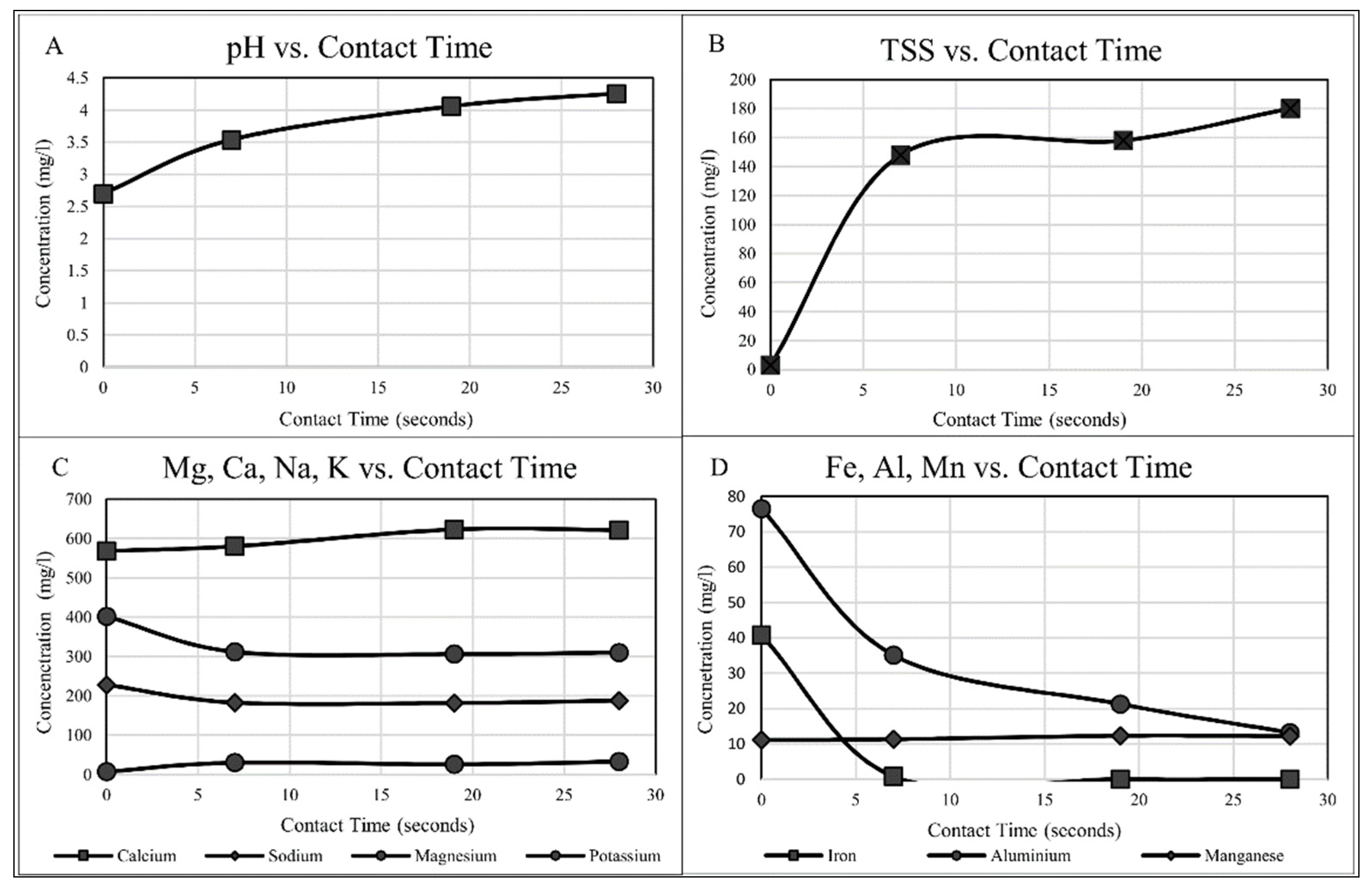
3.1. Column Flow Experimental Results
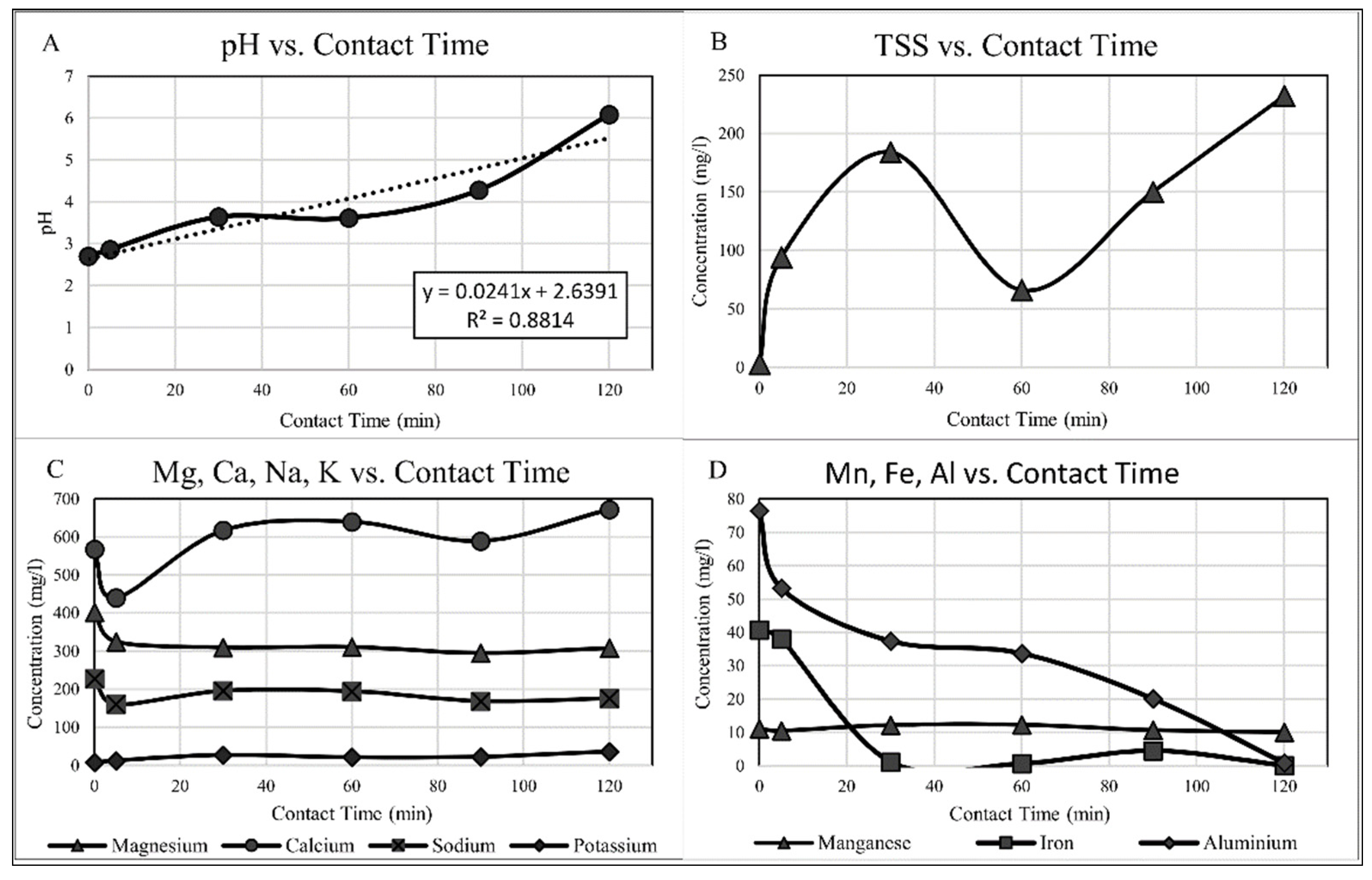
3.1.1. pH Correction
3.1.2. Aluminium
3.1.3. Iron
3.1.4. Magnesium and Manganese
3.1.5. Sodium and Potassium
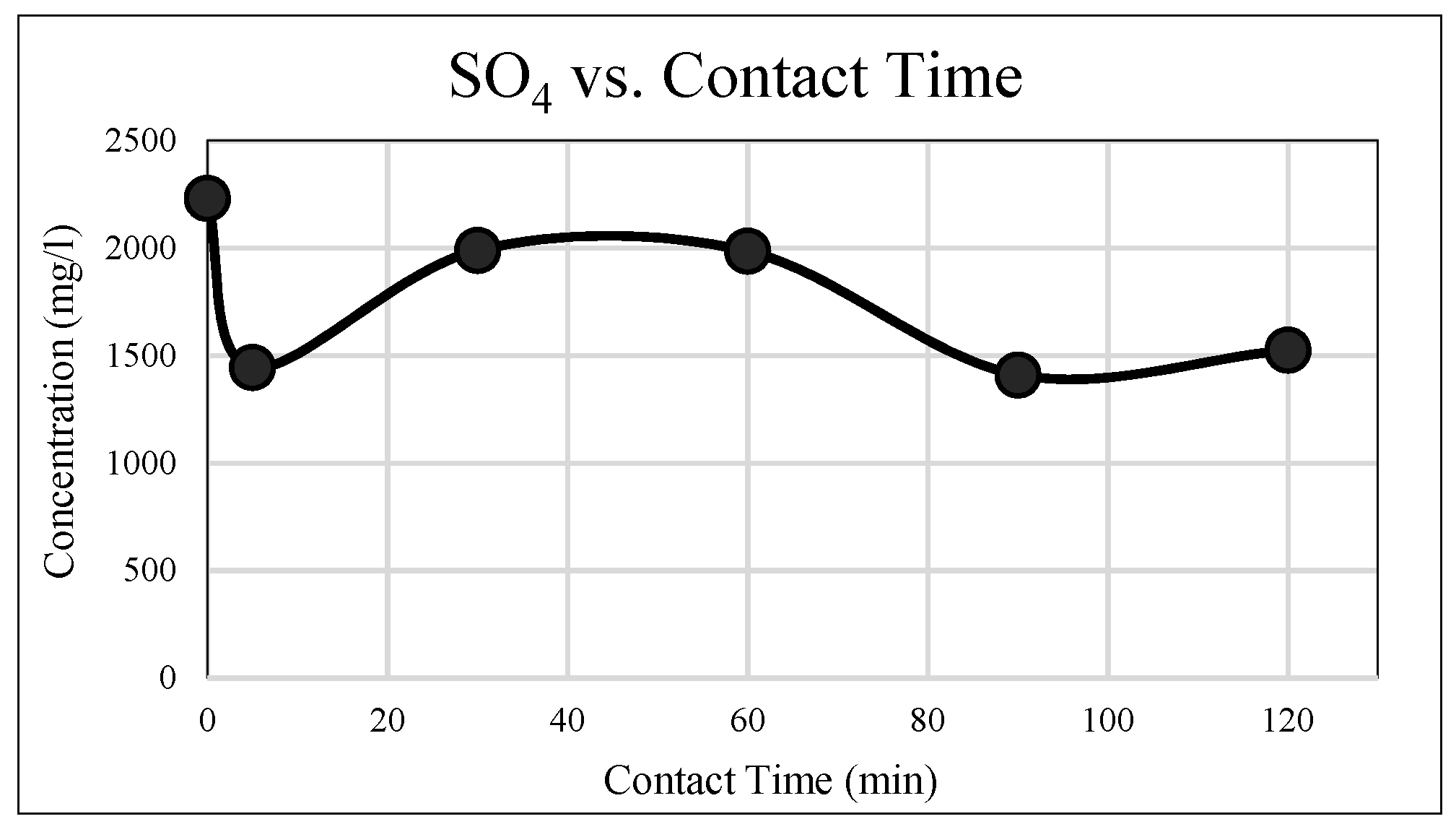
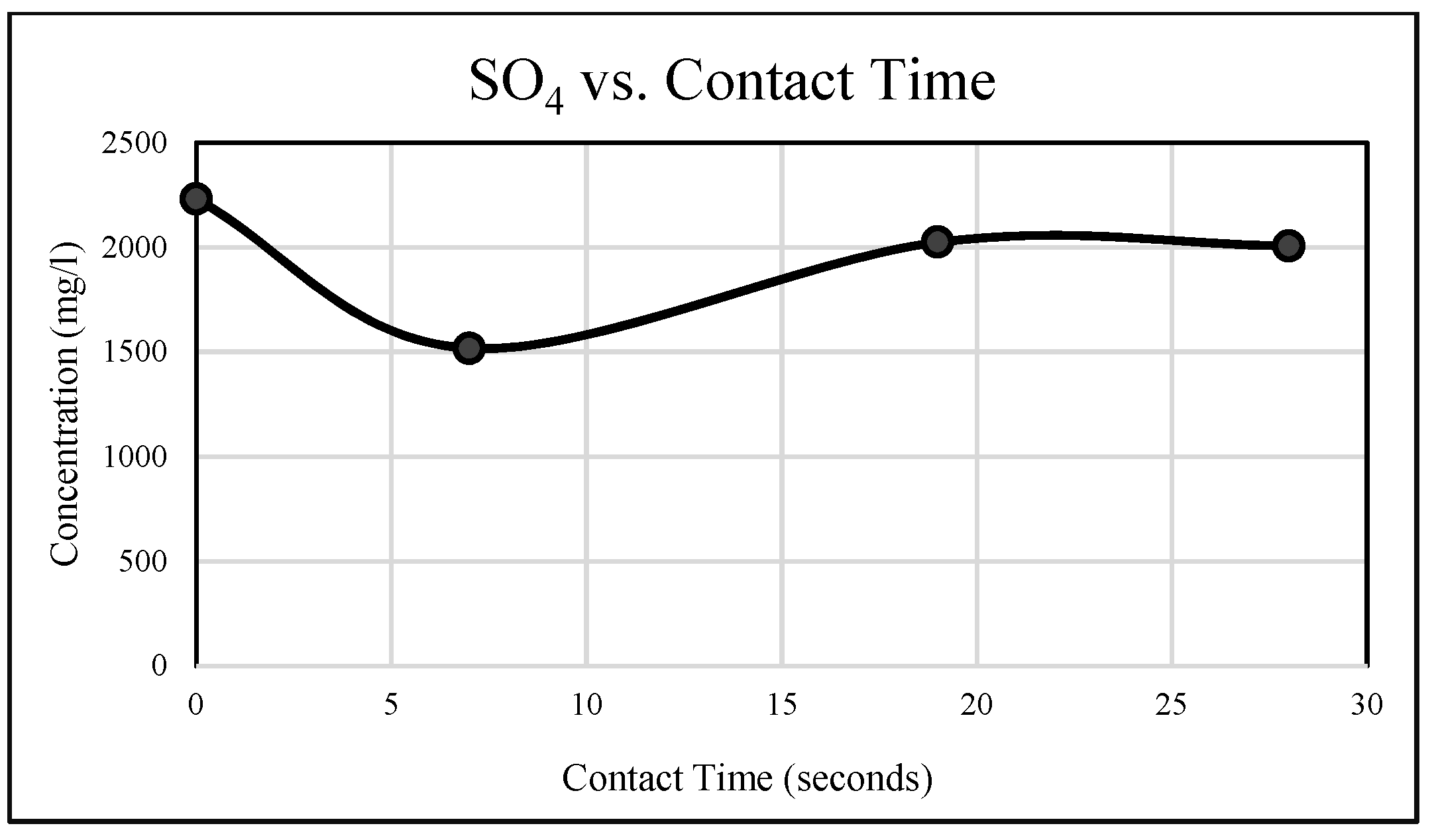
3.1.6. Sulphate
3.1.7. Total Suspended Solids
3.2. Gravity Flow Experimental Results
3.2.1. pH Correction
3.2.2. Aluminium
3.2.3. Iron
3.2.4. Magnesium and Manganese
3.2.5. Sodium and Potassium
3.2.6. Sulphate
3.2.7. Total Suspended Solids and Clogging
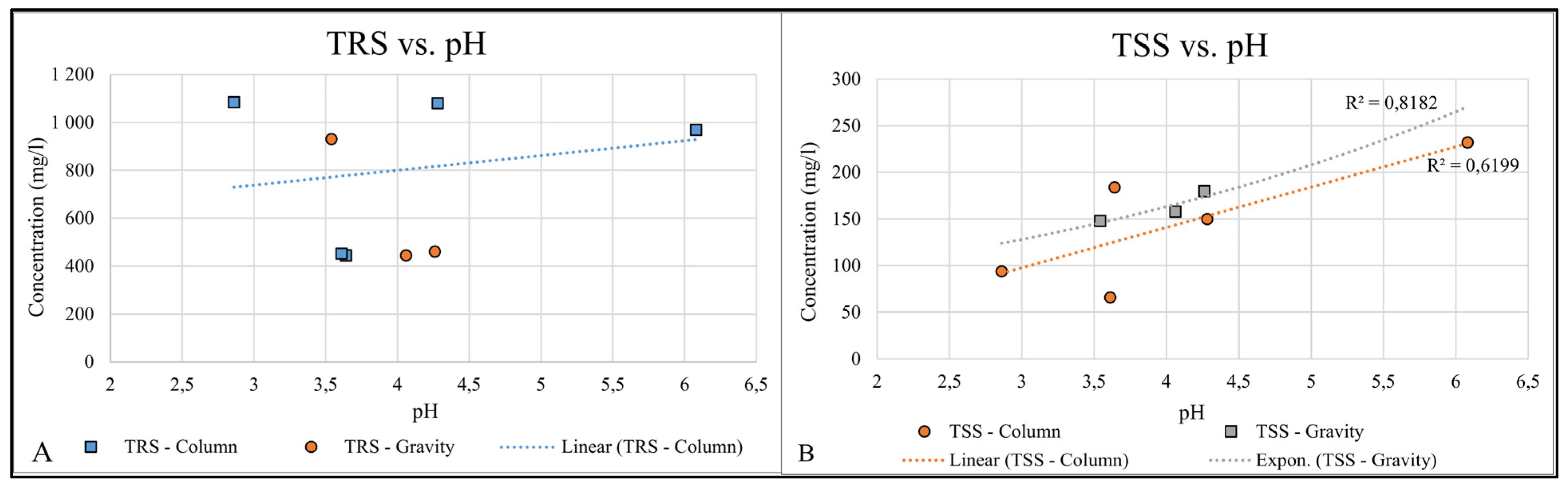
3.3. Suspended Solids Settleability
4. Implications
5. Conclusions
- Initial concentrations of Fe were reduced to concentrations of less than 1 mg/L at pH values of less than 4 under both configurations, suggesting that the remediation mechanisms of pozzolanic pervious concrete PRB involve the oxidation of Fe2+ to Fe3+ leading to precipitation as Fe(OH)3.
- Both gravity flow and column flow configurations at the tested contact times are highly effective for heavy metals Fe and Al removal, with 99% and over 80% removal efficiency, respectively; are limited for SO4, Mg and Na removal with up to 17%, 22% and 20% removal rates, respectively; and are ineffective at reducing Mn concentrations.
- The dissolved concentrations of Ca and K under gravity flow and column flow configuration increased by up to 16% and more than threefold, respectively, due to the leaching of the minerals from the pervious concrete resulting in alkalinity and raising the pH.
- The pervious concrete PRBs under the gravity flow configuration captured 8% fewer precipitated solids on the reactive surface when compared to the column flow configuration. This finding suggests that slower clogging of the PRB could be attained when operating under the gravity flow configuration leading to longer productive lifespan.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water: Hydrology, Pollution, Remediation; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Braungardt, C.B.; Achterberg, E.P.; Elbaz-Poulichet, F.; Morley, N.H. Metal geochemistry in a mine-polluted estuarine system in Spain. Appl. Geochem. 2003, 18, 1757–1771. [Google Scholar] [CrossRef]
- Msagati, T.A.; Nkambule, T.I.; Kefeni, K.; Mamba, B. Synthesis and application of hematite nanoparticles for acid mine drainage treatment. J. Environ. Chem. Eng. 2018, 6, 1865–1874. [Google Scholar]
- Thisani, S.K.; Kalllon, D.V.V.; Byrne, P. Geochemical Classification of Global Mine Water Drainage. Sustainability 2020, 12, 10244. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Rose, P. Long-term sustainability in the management of acid mine drainage wastewaters—Development of the Rhodes BioSURE Process. Water S. Afr. 2013, 39, 583–592. [Google Scholar] [CrossRef][Green Version]
- Johnson, B.D.; Hallberg, K.B. Acid Mine Drainage Remediation Options: A Review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Thisani, S.K.; Kallon, D.V.V. Review of Remediation Solutions for Acid Mine Drainage Using the Modified Hill Framework. Sustainability 2021, 13, 8118. [Google Scholar] [CrossRef]
- Shabalala, A.N.; Ekolu, S.O.; Diop, S. Permeable Reactive Barriers for Acid Mine Drainage Treatment: A Review. In Construction Materials and Structures; IOS Press: Amsterdam, The Netherlands, 2014; pp. 1416–1425. [Google Scholar]
- Waybrant, K.R.; Blowes, D.W.; Ptacek, C.J. Selection of Reactive Mixtures for use in Permeable Reactive Walls for Treatment of Mine Drainage. Environ. Sci. Technol. 1998, 32, 1972–1979. [Google Scholar] [CrossRef]
- Perez, N.; Schwarz, A.O.; Barahona, E.; Sanhueza, P.; Diaz, I.; Urrutia, H. Performance of two differently designed permeable reactive barriers with sulfate and zinc solutions. Sci. Total Environ. 2018, 642, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.A.; Fretwell, B.A.; Mosley, N.G.; Smith, J.W. Guidence on the Use of Permeable Reactive Bariers for Remediating Contaminated Groundwater; National Groundwater Contaminated Land Centre Report NC/01/51; UK Environmental Agency: Bristol, UK, 2002.
- Henderson, A.D.; Demond, A.H. Long-Term Performance of Zero-Valent Iron Permeable Reactive Barriers: A Critical Review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Moodley, I.; Sheridan, C.M.; Kappelmeyer, U.; Akcil, A. Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by-products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Ekolu, S.O.; Azane, F.Z.; Diop, S. A concrete reactive barrier for acid mine drainage treatment. In Proceedings of the Institution of Civil Engineers—Water Management; Thomas Telford Ltd.: London, UK, 2015; Volume 167, pp. 373–380. [Google Scholar]
- Shabalala, A.N.; Ekolu, S.O.; Diop, S.; Solomon, F. Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage—Column study. J. Hazard. Mater. 2017, 353, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Mafanya, L.; Kallon, D.V.V.; Simelane, S.P. Chemical Analysis of AMD Properties Based on Factorial Method; OIC: Jeddah, Saudi Arabia, 2019; pp. 399–403. [Google Scholar]
- Zaal, S.M. Passive Treatment of Acid Mine Drainage Through Permeable Concrete and Organic Filtration. Ph.D. Thesis, University of Witwatersrand, Johannesburg, South Africa, 2016. [Google Scholar]
- Mafanya, L.; Kallon, D.V.V.; Simelane, S.P. Flow Properties Upon Treatment of Acid Mine Drainage Using Pervious Concrete. In Proceedings of the SAIIE NeXXXt 21, Gqeberha, South Africa, 30 September–2 October 2019; pp. 399–403. [Google Scholar]
- Mafanya, L. Flow properties Upon Treatment of Acid Mine Drainage Using Pervious Concrete. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2020. [Google Scholar]
- Ekolu, S.O.; Bitandi, L.K. Prediction of Longevities of ZVI and Pervious Concrete Reactive Barriers Using the Transport Simulation Model. J. Environ. Eng. 2018, 144, 1–10. [Google Scholar] [CrossRef]
- Thisani, S.K.; Kallon, D.V.V.; Byrne, P. A fixed bed pervious concrete anaerobic bioreactor for biological sulphate remediation of acid mine drainage using simple organic matter. Sustainability 2021, 13, 6529. [Google Scholar] [CrossRef]
- Cement & Concrete Institute. Cementitious Material for Concrete Standards, Selection and Properties; Cement & Concrete Institute: Midrand, South Africa, 2009. [Google Scholar]
- Neville, A.M. Properties of Concrete, 4th ed.; John Wiley and Sons Inc.: New York, NY, USA, 1996. [Google Scholar]
- Golab, A.N.; Indraratna, B.; Peterson, M. Selection of permeable reactive barrier materials for treating acid mine groundwater in sulphate soil terrains based on laboratory column tests. Environ. Earth Sci. 2009, 59, 241–254. [Google Scholar] [CrossRef]
- Ekstrom, T. Leaching of Concrete: Experiments and Modelling. Division of Building Materials. Ph.D. Thesis, LTH, Lund University, Lund, Sweden, 2001. [Google Scholar]
- Hoglund, L.O. Project SAFE. Modelling of Long-Term Concrete Degradation Processes in the Swedish SFR Repository; SKB Rapport R-01-08; Svensk Kärnbränslehantering AB: Solna, Sweden, 2001. [Google Scholar]
- Lu, H.; Wei, F.; Tang, J.; Giespy, J.P. Leaching of metals from cement under simulated environmental conditions. J. Environ. Manag. 2016, 169, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Monhemius, J. Precipitation diagrams for metal hydroxides, sulphates, arsenates and phosphates. Trans. Inst. Min. Metall. 1977, 86, C202–C206. [Google Scholar]
- Stewart, D.R. Water Filtration Innovation to Optimize Recovery and Lower TCO. J. Int. Mine Water Assoc. 2013, 1, 735–740. [Google Scholar]
- Rose, A.W. Advances in Passive Treatment of Coal Mine Drainage 1998–2009. In Proceedings of the 27th ASMR, Pittsburgh, PA, USA, 5–10 June 2010; pp. 847–887. [Google Scholar]
- Komnitsas, K.; Bartzas, G.; Paspaliaris, I. Efficiency of limestone and red mud barriers: Laboratory column studies. Miner. Eng. 2004, 17, 183–194. [Google Scholar] [CrossRef]
- Takahide, S. Kurita Water Treatment Handbook, 2nd ed.; Kurita Water Industries, Ltd.: Tokyo, Japan, 1999. [Google Scholar]
- Kirby, C.S.; Dennis, S.; Kahler, A. Aeration to degas CO2, increase pH, and increase iron oxidation rates for efficient treatment of net alkaline mine drainage. Appl. Geochem. 2009, 24, 1175–1184. [Google Scholar] [CrossRef]
- Zang, C.; Huang, S.; Wu, M.; Du, S.; Scholz, M.; Geo, F.; Lin, C.; Guo, Y.; Dong, Y. Comparison of relationships between pH, dissolved oxygen and chlorophyll a for aquaculture and non-aquaculture waters. Water Air Soil Pollut. 2011, 219, 157–174. [Google Scholar] [CrossRef]
- Farage, R.M.P.; Quina, M.J.; Gando-Ferreira, L.; Silva, C.M.; de Souza, J.L.L.; Torres, C.M.M.E. Kraft pulp mill dregs and grits as permeable reactive barrier for removal of copper and sulfate in acid mine drainage. Sci. Rep. 2020, 10, 4083. [Google Scholar] [CrossRef] [PubMed]
- Shabalala, A.N. Assessment of locally available reactive materials for use in permeable reactive barriers (PRBs) in remediating acid mine drainage. Water SA 2013, 39, 251–256. [Google Scholar] [CrossRef][Green Version]
- AFCEE. Technical Protocol for Enhanced Anaerobic Bioremediation Using Permeable Mulch Biowalls and Bioreactors; Air Force Center for Engineering and the Environment: Denver, CO, USA, 2008. [Google Scholar]
- Liang, L.; Sullivan, A.B.; West, O.R.; Moline, G.R.; Kamolpornwijit, W. Predicting the precipitation of mineral phases in permeable reactive barriers. Environ. Eng. Sci. 2003, 20, 635–653. [Google Scholar] [CrossRef]
- Wantanaphong, J.; Mooney, S.J.; Bailey, E.H. Quantification of pore clogging characteristics in potential permeable reactive barrier (PRB) substrates using image analysis. J. Contam. Hydrol. 2006, 86, 299–320. [Google Scholar] [CrossRef]



| Concrete ID | Mix Proportion (dry)—kg per m3 | % Extender | Porosity | |||||
|---|---|---|---|---|---|---|---|---|
| CEM 1 | Fly Ash | Silica Fume | 9.5 mm Granite | Optima 175 | Water | |||
| CEM VI A | 197 | 18 | 9 | 1462 | 1.75 | 75 | 12.1% | 21.5% |
| Determinand | Concentration | Units |
|---|---|---|
| Sulphate (SO4) | 2232 | mg/L |
| Total Suspended Solids (TSS) | <3 | mg/L |
| Arsenic (As) | 1.3 | µg/L |
| Calcium (Ca) | 568 | mg/L |
| Sodium (Na) | 228 | mg/L |
| Ferrous Iron (Fe2+) | 40.7 | mg/L |
| Total Iron (Total Fe) | 43.6 | mg/L |
| Manganese (Mn) | 11,124 | µg/L |
| Magnesium (Mg) | 402 | mg/L |
| Aluminium (Al) | 76,587 | µg/L |
| Potassium (K) | 7.59 | mg/L |
| Copper (Cu) | 1.02 | µg/L |
| Zinc (Zn) | 84 | µg/L |
| Boron (B) | 51 | µg/L |
| Nickel (Ni) | 54 | µg/L |
| Lead (Pb) | 3.05 | µg/L |
| pH | 2.7 | |
| Turbidity | 0.9 | NTU |
| Gravity Flow | Column Flow | Units | |
|---|---|---|---|
| Flow Type | Unsaturated | Saturated | - |
| PRB Reactive Volume | 0.010125 | 0.003375 | m3 |
| AMD Volume Treated | 25 | 25 | L |
| Time to Treat Volume | 2.3 | 37.5 | min |
| Treatment Rate | 1066.667 | 197.333 | L/m3/min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thisani, S.K.; Kallon, D.V.V.; Byrne, P. Effects of Contact Time and Flow Configuration on the Acid Mine Drainage Remediation Capabilities of Pervious Concrete. Sustainability 2021, 13, 10847. https://doi.org/10.3390/su131910847
Thisani SK, Kallon DVV, Byrne P. Effects of Contact Time and Flow Configuration on the Acid Mine Drainage Remediation Capabilities of Pervious Concrete. Sustainability. 2021; 13(19):10847. https://doi.org/10.3390/su131910847
Chicago/Turabian StyleThisani, Sandisiwe Khanyisa, Daramy Vandi Von Kallon, and Patrick Byrne. 2021. "Effects of Contact Time and Flow Configuration on the Acid Mine Drainage Remediation Capabilities of Pervious Concrete" Sustainability 13, no. 19: 10847. https://doi.org/10.3390/su131910847
APA StyleThisani, S. K., Kallon, D. V. V., & Byrne, P. (2021). Effects of Contact Time and Flow Configuration on the Acid Mine Drainage Remediation Capabilities of Pervious Concrete. Sustainability, 13(19), 10847. https://doi.org/10.3390/su131910847







