Coffee (Coffea arabica L.): Methods, Objectives, and Future Strategies of Breeding in Ethiopia—Review
Abstract
1. Introduction
- Assess the different breeding methods applied, identify the research gaps, and highlight future strategies for coffee breeding in Ethiopia.
2. Coffee Growth, Floral Biology, and Fruit Characters
2.1. Coffee Growth
2.2. Floral Biology and Fruit Characters
3. Production and Productivity of Coffee in Ethiopia
4. Methods and Objectives of Coffee Breeding in Ethiopia
4.1. Breeding Methods
4.1.1. Selection and Stability Analysis
Genetic Variability
Selection Procedures
- Step 1: Mother tree selected with desirable traits from the forest coffee. However, selections are made from the genetically variable original population;
- Step 2: Evaluation of mother trees, seedling transplanting, and field management;
- Step 3: Replicated yield trials with the remaining lines, especially comparing them with established commercial varieties;
- Step 4: Multilocation trials for many years (seasons), usually at 3–5 locations for 3–5 years.
- Step 5: Variety release, seed multiplication, and distribution.
4.1.2. Hybridization Methods
Development of Hybrid Coffee Varieties
Coffee Heterosis in Ethiopia
Combining Ability
Development of Pure Line Varieties
4.1.3. Biotechnology in Breeding
4.2. Main Breeding Objectives
4.2.1. Breeding for Productivity/Yield
4.2.2. Breeding for Diseases Resistance
Breeding for Resistance to Coffee Leaf Rust (CLR)
Breeding for Coffee Berry Disease Resistance
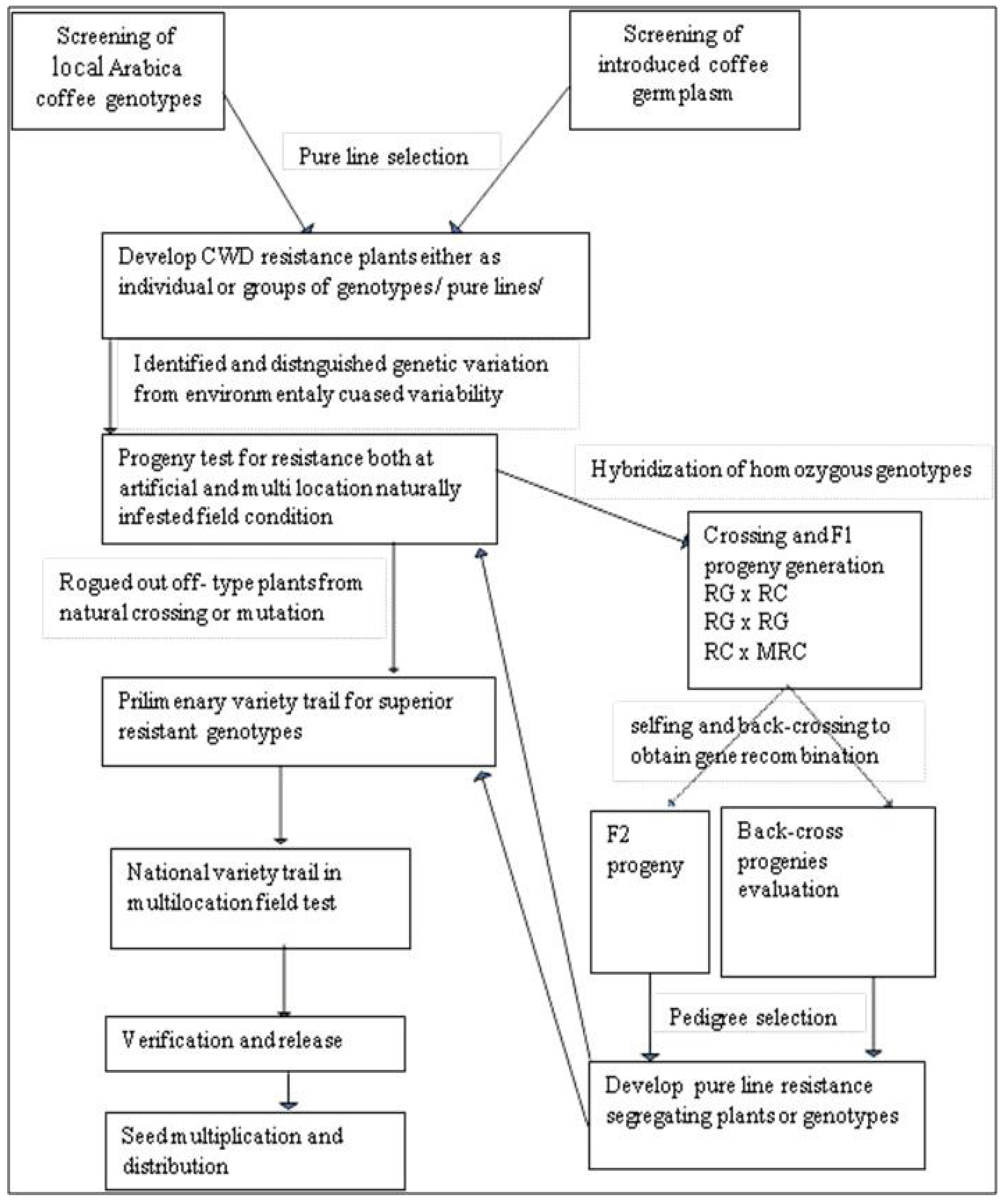
Breeding for Coffee Wilt Disease Resistance
4.2.3. Breeding for Quality
5. Coffee Research Achievements in Ethiopia
6. Future Strategies for Coffee Breeding
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berthaud, J.; Charrier, A. Genetic resources of Coffea. In Coffee Agronomy; Clarke, R.J., Macrae, R., Eds.; Elsevier Applied Science: London, UK, 1988; pp. 1–41. [Google Scholar]
- Wrigley, G. Coffee; Longman Scientific and Technical: London, UK, 1988; p. 639. [Google Scholar]
- Davis, A.P. Psilanthusmannii, the Type Species of Psilanthus, Transferred to Coffea. Nord. J. Bot. 2011, 29, 471–472. [Google Scholar] [CrossRef]
- Charrier, A.; Berthaud, J. Botanical classification of coffee. In Coffee, Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Wilson, K.C., Eds.; Croom Helm: London, UK, 1985; pp. 13–47. [Google Scholar]
- Thomas, A.S. The Wild Arabica coffee on the Boma Plateau of Anglo-Egyptian Sudan. Empir. J. Exp. Agric. 1942, 10, 207–212. [Google Scholar]
- Sylvain, P.G. Ethiopian coffee-its significance for the world coffee problems. Econ. Bot. 1958, 12, 111–139. [Google Scholar] [CrossRef]
- Anthony, F.; Berthaud, J.; Guillaumet, J.L.; Lourd, M. Collecting wild coffee species in Kenya and Tanzania. Plant Genet. Resour. Newsl. 1987, 69, 23–29. [Google Scholar]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- Tadesse, W.; Demel, T. The Forest Coffee Ecosystems: On Going Crices, Problems and Apportunities for Gene Conservation and Utilization. In Imperative Problems Associated with Forestry in Ethiopia; Biological Society of Ethiopia: Addis Ababa, Ethiopia, 2001; pp. 131–142. [Google Scholar]
- Githae, E.W.; Chuah-Petiot, M.; Mworia, J.K.; Odee, D.W. A botanical inventory and diversity assessment of Mt. Marsabit forest, a sub-humid montane forest in the arid lands of Northern Kenya. Afr. J. Ecol. 2008, 46, 39–45. [Google Scholar] [CrossRef]
- Conway, J. Export Volumes of Coffee-Producing Countries December. Available online: https://www.statista.com/statistics/268135/ranking-of-coffee-exporting-countries (accessed on 31 August 2021).
- Philippe, L.; Benoít, B.; Hervé, E. Breeding coffee (Coffea arabica) for Sustainable production. In Breeding Plantation Tree Crops: Tropical Species; Springer: New York, NY, USA, 2009; pp. 525–543. [Google Scholar]
- Mishra, M.K.; Slater, A. Recent Advances in the Genetic Transformation of Coffee. Biotechnol. Res. Int. 2012, 2012, 580857. [Google Scholar] [CrossRef] [PubMed]
- ITC. Coffee: An Exporter’s Guide; International Trade Centre: Geneva, Switzerland, 2002. [Google Scholar]
- ICO. International Coffee Organization. Coffee Market Report 2004. Available online: https://www.ico.org/show_news.asp?id=14 (accessed on 1 August 2021).
- ICO. Annual Review. 2010. Available online: http://www.ico.org (accessed on 8 June 2021).
- Food and Agricultural Organization of United Nations (FAO). Analysis of Price Incentives for Coffee in Ethiopia; Technical Notes Series; FAO: Rome, Italy, 2004; p. 49. [Google Scholar]
- CGM (Coffee Geography Magazine). The Evolution of Coffee Production in Brazil. 2020. Available online: https://coffeegeography.com/2020/09/14/the-evolution-in-coffee-production-in-brazil/ (accessed on 30 August 2021).
- Alemseged, A.; Getaneh, A. Ethiopian Coffee Exporters Association ECEA: Abraham Tegegn Woldesenbet Value Chain Analysis of Vegetables: The Case of Habro and Kombolcha Woredas in Oromia Region; Ethiopian Coffee Exporters Association (ECEA): Addis Ababa, Ethiopia, 2013. [Google Scholar]
- CSA (Central Statistical Agency). Agricultural Sample Survey: Report on Area and Production of Major Crops of Private Peasant Holdings for Meher Season of 2017; CSA: Addis Ababa, Ethiopia, 2017.
- Tesfaye, T.; Bizuayehu, T.; Girma, A. Coffee Production Constraints and Opportunities at Major Growing Districts of Southern Ethiopia. Cogent Food Agric. 2020, 6, 1741982. [Google Scholar]
- CSA (Central Statistical Agency). Agricultural Sample Survey: Report on Area and Production of Major Crops of Private Peasant Holdings for Meher Season of 2018/19. 58; Central statistical Agency: Addis Ababa, Ethiopia, 2019.
- MoA. Plant Variety Release, Protection and Seed Quality Control Directorate. Crop Variety Register; Issue No. 22; MoA: Addis Ababa, Ethiopia, 2019. [Google Scholar]
- Tefera, A.; Bickford, R. Coffee Annual Country: Ethiopia. USDA and GAIN Report Number: ET2021-0013; United States Department of Agriculture: Washington, DC, USA, 2020. [Google Scholar]
- Abebaw, Y.M.; Tobiaw, D.C.; Abate, B.A.; Eshete, B.K.; Seymour, S.K.; Tesfaye, K. Plant Tissue Culture Research and Development in Ethiopia: A Case Study on Current Status, Opportunities, and Challenges. Adv. Agric. 2021, 2021, 9979549. [Google Scholar]
- Bayetta, B.; Pierre, J.L. Arabica coffee (Coffea arabica L.) Landrace Variety Development Strategy in Its Center of Origin and Diversity. In Proceedings of the 21th International Scientific Colloquium on Coffee, Montpelier, France, 11 September 2006. [Google Scholar]
- Bayetta, B. Arabica Coffee Breeding for Yield and Resistance to Coffee Berry Disease (Colletotrichum kahawae Sp. nov.). Ph.D. Thesis, University of London, London, UK, 2001. [Google Scholar]
- Fekede, G.T.; Gosa, A.G. Opportunities and constraints of coffee production in West Hararghe, Ethiopia. J. Agric. Econ. Rural. Dev. 2015, 2, 54–59. [Google Scholar]
- Gezahagn, K. Factors Influencing Coffee Productivity in Jimma Zone, Ethiopia. World J. Agric. Sci. 2019, 15, 228–234. [Google Scholar]
- Geneti, D. Progress of Coffee (Coffea arabica L) Hybridization Development Study in Ethiopia: A Review. Food Sci. Qual. Manag. 2019, 92, 22–28. [Google Scholar] [CrossRef]
- CSA (Central Statistical Agency). Statistical Abstract 2007; CSA: Addis Ababa, Ethiopia, 2008.
- Meyer, F.G.; Fernie, L.M.; Narasimhaswamy, R.L.; Monaco, L.C.; Greathead, D.J. FAO Coffee Mission to Ethiopia 1964–1965; FAO: Rome, Italy, 1968. [Google Scholar]
- Wintgens, J.N. Coffee: Growing, Processing, Sustainable Production. A Guidebook for Growers, Processors, Traders and Researchers; Wiley-Vch Verlag Gmbh and Co. Kgaa: Hoboken, NJ, USA, 2009; p. 996. [Google Scholar]
- Thiago, F.; Joel, S.; Rubens, G.; Adriana, F. Introduction to Coffee Plant and Genetics. In Coffee: Production, Quality and Chemistry; Royal Society of Chemistry: London, UK, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Obso, T.K. Ecology and Development Series No. 46. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2006. [Google Scholar]
- Carvalho, A. Principles and Practice of Coffee Plant Breeding for Productivity and Quality factors: Coffea arabica. In Coffee: Agronomy; Clarke, R.J., Macrae, R., Eds.; Elsevier Applied Sciences Publishers Ltd.: London, UK, 1988; Volume 4, pp. 129–165. [Google Scholar]
- Walyaro, D.J.; Van Der Vossen, H.A.M. Pollen longevity and artificial cross-pollination in Coffea arabica L. Euphytica 1977, 26, 225–231. [Google Scholar] [CrossRef]
- Carvalho, A.; Ferwerda, F.P.; Frahm-lelived, J.A.; Medina, D.M.; Monaco, L.C. Coffee (C. arabica L. and C. canephora Pierre ex Froehner); IITA: Ibadan, Nigeria, 1969; pp. 186–244. [Google Scholar]
- Bossolasco, L. A Study Case on Coffee (Coffea arabica L.) Limu Coffee. Sous la Dir. de François Verdeaux 2009. Available online: https://core.ac.uk/download/pdf/39837832.pdf (accessed on 17 April 2021).
- Cannell, M.G.R. Physiology of the Coffee Crop; Clifford, M.N., Willson, K.C., Eds.; AVI Publishing Co.: Westport, CT, USA, 1985; p. 108. [Google Scholar]
- Carvalho, A.; Monaco, L.C. Natural cross pollination in Coffea arabica L. Int. Soc. Hort. Sci. 1962, 4, 447–449. [Google Scholar]
- Van der Vossen, H.A.M. Agronomy I: Coffee breeding practices. In Coffee; Clark, R.J., Vitzthum, Eds.; Recent Development Black Well Science Ltd.: London, UK, 2001; pp. 184–189. [Google Scholar]
- William, H.U. All about Coffee; Benediction Classic: London, UK, 2009; ISBN 1849029121. [Google Scholar]
- Prado, S.G.; Collazo, J.A.; Stevenson, P.C.; Irwin, R.E. A Comparison of Coffee Floral Ttraits under Two Different Agricultural Practices. Sci. Rep. 2019, 9, 7331. [Google Scholar] [CrossRef]
- Orwa, C.A.; Mutua, K.R.; Jamnadass, R.S.; Anthony. Agroforestree Database. 2009. Available online: http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp (accessed on 2 May 2021).
- Aerts, R.; Gazahegni, B.; Gijbels, P.; Kittessa, H.; Van Glabeke, S.; Vandepitte, K.; Muys, B.; Roldan-Ruiz, I.; Honnay, O. Genetic Variation and Risks of Introgression in the Wild Coffea arabica gene pool in South-Western Ethiopian Montane Rain Rorests. Evol. Appl. 2013, 6, 243–252. [Google Scholar] [CrossRef]
- Habtamu, G.; Gizachew, A.; Meseret, D.; Ashenafi, A. Arabica Coffee (Coffea arabica L.) Hybrid Genotypes evaluation for Growth Characteristics and Yield Performance under Southern Ethiopian Growing Condition. Acad. Res. J. Agri. Sci. Res. 2018, 6, 89–96. [Google Scholar]
- FAO. Major Food and Agricultural Commodities and Producers-Countries by Commodity. 2012. Available online: http://www.fao.org (accessed on 10 June 2020).
- CSA (Central Statistical Agency). Reports on Area and Production of Crops (Private Peasant Holdings, Meher Season); CSA: Addis Ababa, Ethiopia, 2017.
- Jima, D. Review on Coffee Production and Marketing in Ethiopia. Ethiop. J. Mark. Consum. Res. 2020, 67, 7–15. [Google Scholar]
- Labouisse, J.P.; Kotecha, S. Preserving diversity for specialty coffees. A focus on production systems and genetic resources of arabica coffee in Ethiopia. In Proceedings of the SCAA 20th Annual Conference, Minneapolis, MN, USA, 2–5 May 2008. [Google Scholar]
- Labouisse, J.P.; Bayetta, B.; Kotecha, S.; Bertrand, B. Current status of coffee (Coffea arabica L.) genetic resources in Ethiopia: Implications for conservation. Genet. Resour. Crop Evol. 2008, 55, 1079–1093. [Google Scholar] [CrossRef]
- Medina-Filho, H.P.; Bordignon, R.; Guerreiro-Filho, O.; Maluf, M.P.; Fazuoli, L.C.C. Breeding of Arabica Coffee at IAC, Brazil: Objectives, Problems and Prospects. In Proceedings of the VIth International Solanaceae Conference, Madison, WI, USA, 23–27 July 2006. [Google Scholar]
- EIAR. Research on Coffee and Tea. 2020. Available online: http://www.eiar.gov.et/jarc/index.php/jarc-research/coffee-and-tea (accessed on 16 March 2021).
- Clarke, R.; Vitzthum, O.G. Coffee: Recent Developments; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sharma, J.Q. Statistical and Biometrical Techniques in Plant Breeding; New Age International Pvt Ltd.: Delhi, India, 1998; p. 67. [Google Scholar]
- Yeshitila, K. Prospects of forest genetic resources conservation. In Proceedings of the Public Meeting on Integrated Forest Policy Dervelopment in Ethiopia, Forumfor Environment (FfE), Addis Ababa, Ethiopia, 27–29 November 2002; Institute of Biodiversity: Addis Ababa, Ethiopia, 2004. [Google Scholar]
- Walyaro, D.J.A. Consideration in Breeding of Improved Yield and Quality in Arabica Coffee (Coffee arabica L.); Walyaro: Wagengen, The Netherlands, 1983. [Google Scholar]
- Mekuria, T.; Neuhoff, D.; Kopke, U. The Status of Coffee Production and the Potential for Organic Conversion in Ethiopia. In Proceedings of the Conference on International Agricultural Research for Development, Deutscher, Tropentag, Berlin, Germany, 5–7 October 2004. [Google Scholar]
- Mesfin, K.; Bayetta, B. Phenotypic Diversity in the Hararge Coffee (Coffea arabica L.) Germplasm for Quantitative Traits. East Afr. J. Sci. 2008, 2, 13–18. [Google Scholar]
- Olika, K.; Sentayehu, A.; Taye, K.; Weyessa, G. Variability of Quantitative Traits in Limmu Coffee (Coffea arabica L.) in Ethiopia. Int. J. Agric. Res. 2011, 6, 482–493. [Google Scholar]
- Gizachew, A.; Hussein, M. Association and path coefficient analysis of yield and yield attributes of coffee (Coffea arabica L.) under Sidama specialty coffee growing area, Awada, Southern Ethiopia. Adv. Crop Sci. Tech. 2017, 5, 2. [Google Scholar]
- Getachew, A.; Sintayehu, A.; Kufa, T.; Benti, T. Genetic Diversity Analysis of Some Ethiopian Specialty Coffee (Coffea arabica L.) Germplasm Accessions Based on Morphological Traits. Time J. Agric. Vet. Sci. 2013, 1, 47–54. [Google Scholar]
- Desalegn, A. Review on Genetic Diversity of Coffee (Coffea Arabica L.) in Ethiopia. Int. J. For. Hortic. 2017, 3, 18–27. [Google Scholar]
- Sant’Ana, G.C.; Pereira, L.F.P.; Pot, D.; Ivamoto, S.T.; Domingues, D.S.; Ferreira, R.V. Genome-wide association study reveals candidate genes influencing lipids and diterpenes contents in Coffea arabica L. Sci. Rep. 2018, 8, 465. [Google Scholar] [CrossRef]
- Abdi, A.; Hussein, M.; Amsalu, A. Phenotypic Diversity for Quantitative Characters in Arabica Coffee Landraces from Eastern Ethiopia. Pelagia Research Library. Asian J. Plant Sci. Res. 2020, 10, 51–57. [Google Scholar]
- Lemi, B.; Ashenafi, A.; Sentayew, A. Genotype × Environment Interaction and Yield Stability of Arabica coffee (Coffea arabica L.) Genotypes. Insights Aquac. Biotechnol. 2019, 3, 1–2. [Google Scholar]
- Lashermes, P.; Trouslot, P.; Anthony, F.; Combes, M.C.; Charrier, A. Genetic Diversity for RAPD Markers between Cultivated and Wild Accessions of Coffea arabica. Euphytica 1996, 87, 59–64. [Google Scholar] [CrossRef]
- Steiger, D.L.; Nagai, C.P.H.; Moore, C.W.; Morden, R.V.O.; Ming, R. AFLP Analysis of Genetic Diversity within and among Coffea arabica Cultivars. Theor. Appl. Genet. 2002, 105, 209–215. [Google Scholar] [CrossRef]
- Fekadu, T.; Melaku, A.; Bayetta, B.; Behailu, A.; Tadesse, B.; Ashenafi, A. Developing Improved Pure Line Coffee Varieties for Different Coffee Growing Areas of Ethiopia. In Proceedings on Four Decades of Coffee Research and Development in Ethiopia, A National Workshop, Addis Ababa, Ethiopia, 14–17 August 2007; pp. 14–17. [Google Scholar]
- JARC; EIAR. Four Decades of Coffee Research and Development in Ethiopia. In Program and Book of Abstracts. A National Work Shop; Ethiopian Institute of Agricultural Research: Addis Ababa, Ethiopia, 2007. [Google Scholar]
- Falconer, D.S.; Mackay, F.C. Introduction to Quantitative Genetics; Longman: New York, NY, USA, 1996; p. 464. [Google Scholar]
- Cilas, C.; Bouharmont, P.; Boccara, M.; Eskes, A.B.; Baradat, P.H. Prediction of Genetic value for Coffee Production in Coffea arabica from a Half diallel with Lines and Hybrids. Euphytica 1998, 104, 49–59. [Google Scholar] [CrossRef]
- Ameha, M. Hetrosis and arabica coffee breeding in Ethiopia. Plant Breed. Abst. 1990, 60, 594–598. [Google Scholar]
- Ballechew, B.; Behailu, A.; Gibramu, T. Description and Production Recommendations for New Cultivars of Arabica Coffee; Report, No 34; IAR Research: Addis Abeba, Ethiopia, 1998; p. 7. [Google Scholar]
- Mohammed, W. Heterosis and Combining Ability Analysis for Coffee Quality in Diallel Crosses of Diverse Coffee (Coffea arabica L.) Parents in Origin. East Afr. J. Sci. 2011, 5, 12–21. [Google Scholar]
- Ayano, A.; Sentayehu, A.; Abush, T. Combining Ability for Yield and Morphological Characters in Southwestern Ethiopian Origin Coffee Hybrids. Sky J. Agric. Res. 2014, 3, 128–136. [Google Scholar]
- Dula, G.; Bayetta, B.; Ermias, H. Heterosis and Combining Ability Analysis for Morphological Characters in Western Ethiopian Origin Coffee (Coffea arabica L.). Master’s Thesis, Ambo University College of Agriculture and Veterinary Science, Ambo, Ethiopia, 2018. [Google Scholar]
- Tadesse, B. Progress in Arabica Coffee Breeding in Ethiopia: Achievements, Challenges and Prospects. Int. J. Sci. Basic Appl. Res. 2017, 33, 15–25. [Google Scholar]
- Kumar, V.; Naidu, M.M.; Ravishankar, G.A. Developments in coffee biotechnology-in vitro plant propagation and crop improvement. Plant Cell Tissue Organ. Cult. 2006, 87, 49–65. [Google Scholar] [CrossRef]
- Adane, A. Agricultural biotechnology research and development in Ethiopia. Afr. J. Biotechnol. 2009, 8, 7196–7204. [Google Scholar]
- Workia, A.; Tileye, F.; Tesfaye, D. Somatic embryogenesis of a coffee (Coffea arabica L.) hybrid using leaf explants. J. Hortic. Sci. Biotechnol. 2013, 88, 469–475. [Google Scholar]
- Alemayehu, T.; Crouzillat, D.; Petiard, V.; Brouhan, P. Genetic diversity of Arabica coffee (Coffea arabica L.) collections. Eur. J. Appl. Sci. Technol. 2010, 1, 63–79. [Google Scholar]
- Kassahun, T.; Govers, K.; Endashew, B. ISSR fingerprinting of Coffea arabica throughout Ethiopia reveals high variability in wild populations and distinguishes them from landraces. Plant Syst. Evol. 2014, 300, 881–897. [Google Scholar]
- Lashermes, P.; Combes, M.C.; Cros, J.; Trouslot, P.; Anthony, F.; Charrier, A. Origin and Genetic Diversity of Coffea arabica L. based on DNA Molecular Markers. In Proceedings of the16th Conference of ASIC, Kyoto, Japan, 4–9 December 1995; pp. 528–535. [Google Scholar]
- Amsalu, A.; Endeshaw, B. Multivariate analysis of morphological variation in sorghum (Sorghum bicolor (L.) Moench) germplasm from Ethiopia and Eritrea. Genet. Resour. Crop. Evol. 1999, 46, 273–284. [Google Scholar]
- Motta, L.B.; Soares, T.C.B.; Ferrao, M.A.G.; Caixeta, E.T.; Lorenzoni, R.M.; Souza Neto, J.D.D. Molecular characterization of arabica and Conilon coffee plants genotypes by SSR and ISSR markers. Braz. Arch. Biol. Technol. 2014, 57, 728–735. [Google Scholar] [CrossRef][Green Version]
- Sousa, T.V.; Caixeta, E.T.; Alkimim, E.R.; De Oliveira, A.C.B.; Pereira, A.A.; Zambolim, L.; Sakiyama, N.S. Molecular markers useful to discriminate Coffea arabica cultivars with high genetic similarity. Euphytica 2017, 213, 75. [Google Scholar] [CrossRef]
- Da Silva, B.S.R.; Sant’Ana, G.C.; Chaves, C.L.; Androcioli, L.G.; Ferreira, R.V.; Sera, G.H.; Charmetant, P.; Leroy, T.; Pot, D.; Domingues, D.S.; et al. Population Structure and Genetic Relationships between Ethiopian and Brazilian Coffea arabica genotypes revealed by SSR markers. Genetica 2019, 147, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.S.N.; von Pinho, E.V.R.; Carvalho, M.G.; Esselink, G.D.; Vosman, B. Development of Microsatellite Markers for the Identification of Brazilian Coffea arabica Varieties. Genet. Mol. Biol. 2010, 33, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, B.; Endale, G.; Kassahun, T.; Gezahegn, B.; Philippe, L.; Martina, K.; Nasser, K.Y. Genetic Diversity among Commercial Arabica coffee (Coffeaarabica, L.) Varieties in Ethiopia using SSRM. J. Crop. Improv. 2020, 35, 147–168. [Google Scholar]
- Dessalegn, Y.; Herselman, L.; Labuschagne, M.T. AFLP Analysis Among Ethiopian Arabica coffee Genotypes. Afr. J. Biotechnol. 2008, 7, 3193–3199. [Google Scholar]
- Gudeta, D. Genetic Diversity of Ethiopian Coffee (Coffea arabica L.) Collection Using SSR Marker. Master’s Thesis, Jimma University, Jimma, Ethiopia, 2020. [Google Scholar]
- Vitzthum, O.G.; Leroy, C.R.J. Coffee: Recent Developments; Blackwell Science: Hoboken, NJ, USA, 2001. [Google Scholar]
- Labouisse, J.P. Summary of Passport Data of Coffee Germplasm Maintained at JARC; Ethiopian Institute of Agricultural Research: Jimma, Ethiopia, 2006. [Google Scholar]
- Mesfin, K.G. Summary of a Decade of South Ethiopian Coffee Improvement Activities at Awada Coffee Research Center: Fruit of the Landrace Arabica Coffee Variety Development Strategy. In Horticultural Crops; Baimey, H.K., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Bertrand, B.; Etienne, H.; Eskes, A. Growth, production and bean quality of Coffea arabica as affected by inter-specific grafting: Consequences for rootstock breeding. HortScience 2001, 36, 269–273. [Google Scholar] [CrossRef]
- Guzzo, S.D. Aspectos bioquímicos moleculares da resistência sistêmica adquirida. Int. J. Res. Agric. For. 2004, 7, 32. [Google Scholar]
- Van der Graaff, N.A.; Pieters, R. Resistance levels in Coffea arabica to G. xylarioides and distribution pattern of the disease. Neth. J. Plant Pathol. 1978, 84, 117–120. [Google Scholar] [CrossRef]
- Merga, J. Review on Resistance Breeding Methods of Coffee Leaf Rust in Ethiopia. Int. J. Res. Agric. For. 2020, 7, 32–41. [Google Scholar]
- Robinson, R.A. Terminal Report of the FAO Coffee Pathologist to the Government of Ethiopia; FAO AGO/74/443; FAO: Rome, Italy, 1974; p. 17. [Google Scholar]
- Admikew, G. Review Article: Genetics and Breeding Overview for Coffee Wilt Disease Resistance. J. Biol. Agric. Healthc. 2019, 9, 7. [Google Scholar] [CrossRef]
- Beckman, H. The Nature of Wilt Diseases of Plants; APS Press: St. Paul, MN, USA, 1987. [Google Scholar]
- Strange, R.N. Plant Disease Control: Towards Environmentally Acceptable Methods; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Admikew, G. Mode of Inheritance of Resistance to Coffee Wilt Disease (G. xylarioides Heim and Saccas) in Arabica Coffee (Coffea arabica L.) Genotypes. Master’s Thesis, Jimma University, Jimma, Ethiopia, 2017. [Google Scholar]
- Demelash, T.; Kifle, B. Evaluation of Released Arabica Coffee Varieties (Coffea aabica L.) for Major Coffee Diseases with Special Emphasis to Coffee Wilt Disease (G. xylarioides) at Jimma, Ethiopia. J. Biol. Agric. Healthc. 2015, 5, 81–86. [Google Scholar]
- Chala, J.; Girma, A.; Demelash, T.; Arega, Z.; Shine, B.; Adem, A. Development and Release of Coffee Berry Disease Resistant Varieties to Specialty Coffee Producing Regions in Ethiopia; Jimma Agricultural Research Centre: Jimma, Ethiopia, 2012. [Google Scholar]
- Demelash, T. Evaluation of Arabica Coffee (Coffea arabica L.) Germplasm for Major Coffee Disease with Special Emphasis to Coffee Wilt Disease (Gibberella xylarioides) at Jimma, Ethiopia. Master’s Thesis, Jimma University, Jimma, Ethiopia, 2013. [Google Scholar]
- Girma, A. Diversity in Pathogencity and Genetics of Gibberella xyilarioides (Fusarium xylarioides) Population and Resistance of Coffee spp. in Ethiopia. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2004. [Google Scholar]
- Leroy, T.; Ribeyre, F.; Bertrand, B.; Charmetant, P.; Dufour, M.; Montagnon, C.; Marraccini, P.; Pot, D. Genetics of Coffee Quality. Braz. J. Plant Physiol. 2006, 18, 229–242. [Google Scholar] [CrossRef]
- Abeyot, T.; Sentayehu, A.; Taye, K.; Weyessa, G. Variability and association of quality and biochemical attributes in some promising Coffea arabica germplasm collections in southwestern Ethiopia. Int. J. Plant Breed. Genet. 2011, 5, 302–316. [Google Scholar]
- MoA. Plant Variety Release, Protection and Seed Quality Control Directorate; Crop Variety Register: Addis Ababa, Ethiopia, 2004. [Google Scholar]
- MoA. Plant Variety Release, Protection and Seed Quality Control Directorate; Crop Variety Register: Addis Ababa, Ethiopia, 2016. [Google Scholar]
- Demelash, T. Achievements and Prospects of Coffee Research in Ethiopia: A Review. Int. J. Res. Stud. Agric. Sci. 2019, 5, 41–51. [Google Scholar]
- Afework, L. Assessment of Coffee (Coffea Arabica L.) Genetic Erosion and Genetic Resources Management in Ethiopia. Int. J. Agr. Ext. 2019, 7, 223–229. [Google Scholar]
- Watts, C.A. Brewing Storm: The Climate Change Risks to Coffee; The Climate Institute, Fairtrade Australia & New Zealand: Auckland, New Zealand, 2016; ISBN 978-1-921611-35-3. [Google Scholar]
- Killeen, J.T.; Harper, G. Coffee in the 21st Century. In Will Climate Change and Increased Demand Lead to New Deforestation? Conservation International: New York, NY, USA, 2016. [Google Scholar]
- Justin, M.; Jenny, W.; Susana, B.; Timothy, W.; Tadesse, W.; Zeleke, K.; Sebsebe, D.; Davis, A. Resilience potential of the Ethiopian coffee sector under climate change. Nat. Plants 2017, 3. [Google Scholar] [CrossRef]





| Year | Coffee Growers | Area in ha | Percentage Change in Area | Production in Quintal | Percentage Change in Production | Yield q/ha | % Change in Yield |
|---|---|---|---|---|---|---|---|
| 2012/13 | 4,217,961.00 | 528,751.11 | - | 373,940.642 | - | 0.707 | - |
| 2013/14 | 4,546,785.00 | 538,466.80 | 2.00 | 392,006.222 | 5.00 | 0.728 | 3.00 |
| 2014/15 | 4,723,483.00 | 561,761.82 | 4.00 | 419,980.156 | 7.00 | 0.748 | 3.00 |
| 2015/16 | 5,270,777.00 | 653,909.76 | 16.00 | 414,596.455 | −1.00 | 0.634 | −15.00 |
| 2016/17 | 6,455,194.00 | 700,474.69 | 7.00 | 469,091.124 | 13.00 | 0.67 | 6.00 |
| 2017/18 | 5,019,513.00 | 725,961.24 | 4.00 | 449,229.808 | −4.00 | 0.619 | 8.00 |
| No. | Method | Source Population | Breeding System | Output | Propagation by | Example |
|---|---|---|---|---|---|---|
| 1 | Arabica Pure line selection | Variety | Selfing | Line | Seed | Caturra (Brazil), Kent (India) SL 28 (Kenya) java (Cameroon) |
| 2 | Intraspecific F1 hybrid | Varieties/accession, the pedigree of crosses | Crossing and selfing | Composite hybrid, F1 hybrid F1 clones | Seed (hand-pollination) Somatic embryogenesis | Ruiro II (Kenya) Ababuna (Ethiopia) in progress Catimor × Et (C. America) |
| 3 | Pedigree selection after hybridization (sometimes also backcrossing) | Varieties | Crossing and selfing | Line | Seed | Catuai, Tupi (Brazil),catimor Sarchimor (costa Rica), S795(India) Colombia (Colombia) |
| 4 | Interspecific hybridization (Arebica × Robusta) backcrossing and pedigree selection | Arebica varieties tetraploid/diploid robusta genotype | Crossing and selfing | Line | Seed | Lacatu Brazil, S2828 (India) |
| Variety Group | Number of Varieties | Year of Release | Yield Range (q/ha) | |
|---|---|---|---|---|
| On Station | On Farm | |||
| CBD Resistant Varieties | 16 | 1978–2018 | 12.2–23.8 | 6.0–10.0 |
| Low and Midland Varieties | 5 | 1997–2002 | 16.6–23.4 | 9.0–20.0 |
| Hybrid Varieties | 4 | 1997–2018 | 24.0–26.0 | 13.0–20.0 |
| Highland Varieties | 4 | 2006 | 16.4–23.5 | 15.2–16.2 |
| Land-race Varieties | 12 | 2006, 2010 | 11.9–20.4 | 7.2–16.2 |
| Total | 41 | |||
| Origin | Variety Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| South Ethiopia | SR1(1) | 1.00 | |||||||||||
| SR2(2) | 0.18 | 1.00 | |||||||||||
| SR3(3) | 0.28 | 0.42 | 1.00 | ||||||||||
| SR4(4) | 0.37 | 0.34 | 0.39 | 1.00 | |||||||||
| East Ethiopia | HR1(5) | 0.29 | 0.43 | 0.54 | 0.40 | 1.00 | |||||||
| HR2(6) | 0.38 | 0.39 | 0.37 | 0.33 | 0.44 | 1.00 | |||||||
| HR3(7) | 0.37 | 0.24 | 0.33 | 0.35 | 0.4 | 0.6 | 1.00 | ||||||
| HR4(8) | 0.49 | 0.33 | 0.40 | 0.47 | 0.51 | 0.45 | 0.51 | 1.00 | |||||
| West Ethiopia | WR1(9) | 0.42 | 0.29 | 0.28 | 0.33 | 0.38 | 0.35 | 0.41 | 0.38 | 1.00 | |||
| WR2(10) | 0.48 | 0.32 | 0.30 | 0.36 | 0.31 | 0.31 | 0.33 | 0.37 | 0.57 | 1.00 | |||
| WR3(11) | 0.34 | 0.28 | 0.33 | 0.39 | 0.40 | 0.34 | 0.29 | 0.31 | 0.42 | 0.44 | 1.00 | ||
| WR4(12) | 0.31 | 0.32 | 0.30 | 0.30 | 0.37 | 0.34 | 0.27 | 0.31 | 0.38 | 0.54 | 0.47 | 1.00 |
| NO. | Variety/Cultivar | Year of Released | Yield(q/ha) | Canopy | Breeder/Maintainer | |
|---|---|---|---|---|---|---|
| On Station | On Farm | |||||
| 1 | 741 | 1977/78 | 12.2 | 6–7 | Open | JARC/EIAR |
| 2 | 744 | 1979/80 | 16.6 | 8–9 | Open | JARC/EIAR |
| 3 | 7440 | 1979/80 | 16.2 | 8–9 | Intermediate | JARC/EIAR |
| 4 | 7454 | 1980/81 | 18.3 | 8–9 | Intermediate | JARC/EIAR |
| 5 | 7487 | 1980/81 | 23.8 | 9–10 | Intermediate | JARC/EIAR |
| 6 | 74,110 | 1978/79 | 19.1 | 9–10 | Compact | JARC/EIAR |
| 7 | 74,112 | 1978/79 | 18.1 | 9–10 | Compact | JARC/EIAR |
| 8 | 74,140 | 1978/79 | 19.7 | 9–10 | Compact | JARC/EIAR |
| 9 | 74,148 | 1979/80 | 18.0 | 6–7 | Compact | JARC/EIAR |
| 10 | 74,158 | 1978/79 | 19.1 | 9–10 | Compact | JARC/EIAR |
| 11 | 74,165 | 1978/79 | 17.3 | 8–9 | Compact | JARC/EIAR |
| 12 | 754 | 1980/81 | 14.8 | 7–8 | Compact | JARC/EIAR |
| 13 | 75,227 | 1980/81 | 17.9 | 8–9 | Open | JARC/EIAR |
| 14 | Dessu | 1982 | 20.0 | Open | JARC/EIAR | |
| 15 | Ababuna (741 × Dessu) * | 1997 | 23.8 | 15.5 | Open | JARC/EIAR |
| 16 | Melko-CH2 (7395 × Dessu) * | 1997 | 24.0 | 13.1 | Intermediate | JARC/EIAR |
| 17 | Catimor J-19 | 1997 | JARC/EIAR | |||
| 18 | Catimor J-21 | 1997 | JARC/EIAR | |||
| 19 | Gesiha | 2002 | JARC/EIAR | |||
| 20 | Me’oftu | 2002 | JARC/EIAR | |||
| 21 | Gawe * | 2002 | JARC/EIAR | |||
| 22 | Angafa | 2006 | JARC/EIAR | |||
| 23 | Yachi | 2006 | JARC/EIAR | |||
| 24 | Buno-washi 2–05 (7416) | 2006 | JARC/EIAR | |||
| 25 | Mocha (H-739/98) | 2010 | JARC/EIAR/MCARC/OARI | |||
| 26 | Bultum (H-857/98) | 2010 | JARC/EIAR/MCARC, ORARI | |||
| 27 | Mocha (H-739/98) | 2010 | JARC/EIAR/MCARC/OARI | |||
| 28 | Mercha-1 (H-823/98) | 2010 | JARC/EIAR/MCARC, OARI | |||
| 29 | Harusa (H-674/98) | 2010 | EIAR/MCARC/OARI | |||
| 30 | Menesibu (W78/84) | 2010 | JARC/EIAR | |||
| 31 | Sende (W92/98) | 2010 | JARC/EIAR | |||
| 32 | Challa (W76/98) | 2010 | JARC/EIAR | |||
| 33 | Haru-1(W66/98) | 2010 | JARC/EIAR | |||
| 34 | Koti (85257) | 2010 | JARC/EIAR | |||
| 35 | Odicha (974) | 2010 | JARC/EIAR | |||
| 36 | Fayate (971) | 2010 | JARC/EIAR | |||
| 37 | Tepi HC5 | 2016 | 20.3 | JARC/EIAR | ||
| 38 | Melko-Ibsitu | 2016 | 19.3 | JARC/EIAR | ||
| 39 | EIAR50/CH | 2016 | 20.9 | JARC/EIAR | ||
| 40 | L55/01 (Limu 1) | 2018 | JARC/EIAR | |||
| 41 | 74,158 × 7530 (Gera coffee hybrid 1) * | 2018 | JARC/EIAR | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melese, Y.Y.; Kolech, S.A. Coffee (Coffea arabica L.): Methods, Objectives, and Future Strategies of Breeding in Ethiopia—Review. Sustainability 2021, 13, 10814. https://doi.org/10.3390/su131910814
Melese YY, Kolech SA. Coffee (Coffea arabica L.): Methods, Objectives, and Future Strategies of Breeding in Ethiopia—Review. Sustainability. 2021; 13(19):10814. https://doi.org/10.3390/su131910814
Chicago/Turabian StyleMelese, Yebirzaf Yeshiwas, and Semagn Asredie Kolech. 2021. "Coffee (Coffea arabica L.): Methods, Objectives, and Future Strategies of Breeding in Ethiopia—Review" Sustainability 13, no. 19: 10814. https://doi.org/10.3390/su131910814
APA StyleMelese, Y. Y., & Kolech, S. A. (2021). Coffee (Coffea arabica L.): Methods, Objectives, and Future Strategies of Breeding in Ethiopia—Review. Sustainability, 13(19), 10814. https://doi.org/10.3390/su131910814





