Abstract
This research introduces a novel technology for reducing ordinary urea (OU) dissolution by developing double-coated urea (DCU) using phosphate rock (PR) as an outer layer to reduce its hydrolysis and sodium thiosulfate (STS) as an inner layer to inhibit the urease enzyme and nitrification process. Due to the double coating, the nitrogen content of DCU was lower than that of the OU (36.7% vs. 46.5%). The ultramorphological analysis using scanning electron microscopy (SEM) indicated that the controlled coating of urea, resulting from the outer layer of PR, was due to the adhesive effect of urea formaldehyde (UF), which was used as a glue. In addition, the transmission electron microscopy (TEM) analysis of the DCU revealed its high degree of agglomeration. The mechanical hardness of DCU was higher compared to that of OU (1.38 vs. 1.08 kgf). The seven-day dissolution rate test showed that OU reached 100% dissolution on the fifth day. The rate of DCU, however, was significantly lower (32% dissolution in the seventh day). Cumulative NO3− and NH4+ losses from a clay soil sample reached 68.3% and 7.6%, respectively, with OU measuring 40.5% compared to 4.9% for DCU 70 days after application. Field experiments showed a significant improvement in the marketable yield and agronomic nitrogen efficiency (ANE) of maize grains and zucchini fruits fertilized with DCU. Furthermore, the macro and micronutrient concentrations in maize grains and zucchini fruits showed an increase in the plants fertilized with DCU. In summary, double coating can be introduced as a novel technique to control urea dissolution in soil.
1. Introduction
Intensive agricultural systems have profoundly changed the global pedosphere, hydrosphere, and atmosphere [1,2,3]. In this regard, the increasing global mineral fertilizer consumption (108.744 million tonnes in 2020) has caused several deteriorative impacts on the ecosphere [4]. Due to its higher nitrogen content (46%), ordinary urea (OU) is the most commonly used nitrogen fertilizer worldwide [5]. The low nitrogen use efficiency of OU (20–35%) is associated with various potential losses: (i) leaching through high rainfall or irrigation, (ii) volatilization as ammonia and nitrous oxide emissions [6], (iii) denitrification to a variety of gaseous forms after the microbial reduction of NO3− [7], and (iv) microbial immobilization as microbial biomass nitrogen [8].
Due to their high dissolution rate, urea granules are rapidly hydrolyzed by urease enzymes into ammonium (NH4+), hydroxyl (OH−), and carbonate (CO32−) ions within two days after soil application [9]. Thereafter, the active nitrification process completely converts NH4+ to NO3− within a few weeks of application [10]. Consequently, the concentrations of NO3− in surface and groundwater resources have globally increased due to intensive urea fertilization, taking into consideration the negative charge of nitrate, which cannot be held by negatively charged soil particles [11]. Surface water contamination by NO3− (e.g., rivers, lakes, and estuaries) causes several negative effects on the aquatic ecosystem, including eutrophication, algae blooming, and fish poisoning [12]. In addition, NO3− in surface and groundwater can contaminate drinking water and cause harmful effects on human health [13]. In this regard, infants below one year of age are the most vulnerable to NO3− contamination due to the inhibition of oxygen transport in the blood, which causes methemoglobinemia [14]. On the other hand, the high dissolution rate of urea causes substantial increases in the emission of greenhouse gases (GHGs), including CO2, N2O, and CH4 [15]. Moreover, ammonia (NH3) emissions from terrestrial ecosystems can cause negative impacts on the atmosphere as well as on the rhizosphere (microbial diversity/activity, seed germination, and root architecture) [16,17].
Thus, there is an urgent need to maximize the efficiency of urea fertilizer either by reducing its dissolution rate or by reducing the activity of the urease enzyme and nitrification process. Slow-release coating technology has previously been widely investigated regarding controlling the rate of N-release to meet the nutritional demands of plants by coating urea granules with various materials capable of reducing their dissolution rate [18]. Various natural and synthetic materials have been tested for use as slow-release coatings. Among them, sulfur, resins, bio-composites, and other synthetic polymers have been widely investigated [19]. Most of these materials, however, are non-degradable, relatively expensive, and often not eco-friendly [20]. Additionally, most of these materials create non-uniform and easily breakable coating layers during the manufacturing process that cause shrinkage and cracks on the outer coating layer [5]. These cracks allow water to penetrate the fertilizer granules, which speeds up their dissolution.
Phosphate rock (PR) coatings have been credited with multiple benefits, including cost efficiency, simple processing methods, resistance against most environmental conditions, and the formation of a contiguous adherent layer [21]. Added to this, PR has several benefits following its application to soil, including a slow-release supply of nutrients other than phosphorus [22], the amelioration of soil acidity and lowering exchangeable Al3+ (Basak and Biswas, 2016), the immobilization of potentially toxic elements [23], and the enhancement of the soil microbial activity [24]. Another approach for retarding urea hydrolysis/transformation is to use urease and nitrification inhibitors that can reduce urease activity and retard NH4+ transformation to NO3− in soil. Thiosulfate compounds are among the most effective inhibitors and not only inhibit the nitrification process but also have an indirect effect on reducing the urease activity (Modolo et al., 2018). Thiosulfates (e.g., sodium and ammonium thiosulfate) are eco-friendly compounds with multiple agricultural applications, including sulfur nutrient supply [25], heavy metal stabilization [26,27], and the acid solubilization of phosphorus and micronutrients [28,29].
Despite the short-term effectiveness of urease and nitrification inhibitors in maximizing urea efficiency, their efficacy decreases significantly three weeks after application due to their rapid degradation in soil [30,31]. Thiosulfate compounds also show a high vulnerability to temperature and high rates of mineralization (one week under warming temperatures and three weeks under cooler temperatures) [32]. It is recommended, therefore, to identify a protective method for improving sodium thiosulfate (STS) resistance against soil microbial degradation, to maximize the inhibitory effect of the urease enzyme and nitrification process. The main objectives of this study were as follows: (1) studying the physicochemical properties of double-coated urea (DCU); (2) assessing its mechanical hardness and dissolution rate as compared with OU; (3) evaluating cumulative ammonium (NH4+) and nitrate (NO3−) losses from soil following DCU and OU application; and (4) evaluating the effect of OU and DCU fertilizers on marketable yield, nitrogen efficiency (ANE) and nutrient concentrations in maize grains and zucchini fruits in field investigations.
2. Materials and Methods
2.1. Materials
Ordinary urea (46.5% N), urea formaldehyde (UF), and PR were obtained from El-Delta Fertilizers Plant, Mansoura, Egypt. Sodium thiosulfate (ReagentPlus®, 99%) and all chemicals used of analytical grade were purchased from Sigma Aldrich Ltd. Alluvial soil (Vertic Torrifluvents) was obtained from the Agricultural Research Farm of Mansoura University, Egypt, in conjunction with starting the first field experiment.
2.2. DCU Preparation
OU (10 kg) was mixed with UF (2.5% w:w) in a fluidized bed granulator (Glatt GmbH, Binzen, Germany) before adding the first coating layer (Figure 1). The full description of the equipment is illustrated in Figure 1. The fluidized bed granulator was made from stainless steel for granulation, drying, and pellet coating. The delivery of the coating material was carried out via a dual-fluid spray nozzle inserted from the side of the coating/granulating chamber. A particle-free zone was made to prevent the over-wetting of particles using low pressure, which was supplied through a tube that links the coating chamber with the inlet air plenum.
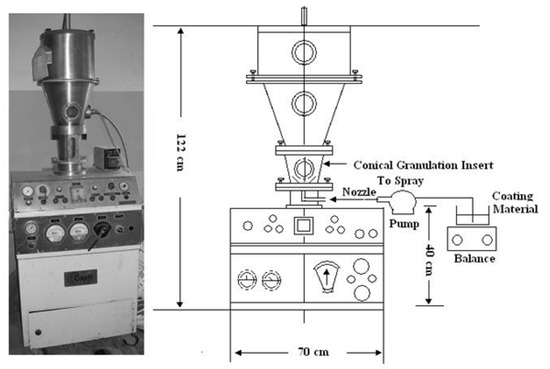
Figure 1.
A schematic of the fluidized bed granulator.
The first layer (STS at the rate of 2% w:w) was added using the nozzle of the sprayer pump. The coated urea was air-dried outside the fluidized bed granulator to obtain a better homogeneity. Thereafter, the air-dried product was reinserted inside the fluidized bed granulator and mixed with UF (2.5% w:w) before adding the second coating layer. Ground PR (<0.002 mm diameter) was added at a rate of 17.5% (w:w) inside the fluidized bed granulator. The final product was then air-dried before packaging (Figure 2).

Figure 2.
The engineered fertilizers: (a) OU and (b) DCU.
2.3. DCU Characterization
The nitrogen concentration in the DCU was determined using a CN analyzer (model Thermo Scientific, Flash 2000, Milan, Italy). The K-factor calibration curve was performed using 90–100 mg of urea standard (46.65% N). Then, subsamples (90–100 mg) from the DCU were analyzed 10 times to optimize the repeatability. To investigate the surface characteristics of DCU and OU, the samples were sputter-coated with gold before surface characterization using a SEM (JEOL JSM 6510 lv). The surface elemental composition of DCU and OU was observed simultaneously using an energy dispersive X-ray spectroscopy (Oxford X-Max 20). Microstructure investigation was performed using a TEM (JEOL JEM-2100) after dispersion in warm DI water for 20 min. To study the crystallographic properties of the DCU and OU, digital selected-area electron diffraction (SAED) was performed with a scattering electron beam source equipped with a TEM.
Compression testing was performed to investigate the mechanical hardness of DCU as compared with OU. As the engineered urea granules were not of a uniform diameter or shape, 24 granules were subjected to force using Instron equipment to obtain data for the crushing strength (kgf) vs. granule diameter. Granule diameter was obtained using a micrometer device. Granules were placed on a flat metal plate and the downward pressure of the Instron equipment was increased gradually until the granule fractured. To evaluate the efficacy of the double coating technique on urea release, a seven-day dissolution rate test was performed by adding 50 g of OU or DCU to 250 mL of distilled water without shaking. The temperature was adjusted to 25 °C using a water bath and the refractive index was measured daily in the fertilizer solution using a digital refractometer at a wavelength of 589 nm, as compared with distilled water [33]. The amount of urea released in the water was calculated using the standard calibration curve between the refractive index and standard urea concentrations.
2.4. Cumulative Nitrate and Ammonium Losses
To assess the protective effect of the double coating technology against nitrate and ammonium losses from the soil matrix, PVC columns (7.0 cm diameter and 40.0 cm depth) were uniformly packed with 2.0 kg of air-dried clay soil (Vertic Torrifluvents). Some physical and chemical analyses of the tested soils are presented in Table 1. An acid-washed sand layer (0.2–2.0 mm average diameter and 5.0 cm height) was inserted at the end of the PVC columns to ensure a uniform flow of leachates. A filter paper (Whatman® Ashless, Grade 42) was inserted between the soil and sand layers, and the bottom of each column was tightly sealed with silicone adhesive. A glass tube (5.0 mm internal diameter) was inserted inside the bottom of the columns to collect leachates inside closed-glass bottles using rubber stoppers with a hole for inserting into the other side of the glass tube. Water was applied to the soil columns at a 70% saturation percentage. The average values of temperature and relative humidity during the experiment were 25.0 ± 0.5 °C and 65.0 ± 0.5%, respectively. Columns were weighed every two days, and the loss of water was compensated by volume, assuming that water specific gravity was 1.0 g cm−3. Nitrogen was applied to the soil columns in the form of DCU or OU at the rate of 2.0 g column−1. Control treatments (without nitrogen addition) were performed to distinguish between the NO3− and NH4+ derived from the soil and those derived from fertilizers. Cumulative nitrate (CNP) and ammonium (CAP) percentages in leachates were calculated according to the following equations:

Table 1.
Some physical and chemical analyses of the experimental soils.
2.5. Field Experiments
Two field experiments were carried out in the Agricultural Research Farm of Mansoura University in a clay soil (Vertic Torrifluvents). Representative surface soil samples were collected from the experimental field before starting the field experiments. Collected samples were air-dried, crushed, and passed through a 2 mm sieve (ISO 11464). Maize seeds (Zea Maize L. cv Single Hybrid 10) were cultivated in the summer season to present a cereal crop. Thereafter, zucchini (Cucurbita Pepo L. cv Eskandrani) was cultivated as a successive planting to investigate the residual effect of the developed fertilizer. In a completely randomized block design, three fertilization treatments, OU, DCU, and a control (without nitrogen application), were arranged in three replicates. Nitrogen was applied at the rates of 285 and 225 kg ha−1 for maize and zucchini, respectively. Plants were irrigated following 50% depletion of the available soil water (the difference between field capacity and the permanent wilting point). Soil water content was gravimetrically measured according to the difference in mass of soil samples before and after drying at 105 °C ± 5 (ISO 11465). All agricultural practices were carried out according to the recommendations for maize and zucchini crops. Plants were harvested on August 30 and October 30 for maize and zucchini, respectively. Maize grains and zucchini fruits yield was measured, and the agronomic nitrogen efficiency (ANE) was calculated as additional economic yield per applied fertilizer unit [34].
where Yield (F) and Yield (C) are the obtained yields of fertilized and unfertilized (control) treatments, respectively.
ANE (kg kg−1) = Yield (F), kg − Yield (C), kg/Quantity of N applied, kg
Representative soil samples were collected following maize harvesting to identify the effect of DCU on residual nitrogen retained in the soil for the zucchini crop.
2.6. Soil, Leachate and Plant Analyses
Soil analyses were carried out using the standard methods: particle size distribution by the pipette method [35], soil reaction (pH) in soil paste and soil electrical conductivity in soil paste extract [36], total carbonate as the gasometric determination of released CO2 [37], NH4+ and NO3− concentrations in soil column leachates, and the soil availability of nitrogen using the Kjeldahl method [38]. Grain and fruit samples were oven-dried at 70 °C ± until reaching a constant weight. The total nitrogen in grain and fruit yields was determined using the automatic Kjeldahl method (model Raypa, DNP-2000) after digestion using sulfuric (H2SO4) and perchloric (HClO4) acids (1:1). Total phosphorus was determined colorimetrically at a wavelength of 680 nm using a spectrophotometer (Spekol), and total potassium was determined using a Gallen Kamp flame photometer and micronutrients (Fe, Zn and Mn) with an atomic absorption spectrophotometer (PerkinElmer model 5000).
2.7. Statistical Analysis
At a significance level of (0.05), data were statistically analyzed using CoStat (Version 6.303, CoHort, Berkeley, CA, USA, 1998–2004) based on a one-way ANOVA [39]. Standard deviation values (represented in error bars) were found using the Microsoft Excel Software (version 2010, Microsoft Corporation, Redmond, WA, USA). Data was further presented using the OriginPro 9.1. software.
3. Results
3.1. Fertilizer Characterization
Nitrogen concentration in the DCU decreased by about 21% as compared to the OU (36.7% vs. 46.5%, Figure 1). The incorporation of inner and outer coating layers in the modern urea fertilizer led to a reduction in the final concentration of nitrogen as compared with the initial concentration in the ordinary form. The ultramorphological analysis of the DCU indicated the presence of a spherical geometry outer layer with rough surfaces (Figure 3c), as compared with the soft, dense, compact, and rigid surface structures of OU (Figure 3a). The EDS spectra showed a lowering in the N peak in the DCU compared with the OU, which confirmed the data obtained by the CN analyzer. Moreover, a high peak of Ca was observed in the DCU with the disappearance of other peaks observed in the OU (e.g., Zn and Cu), which indicated the controlled coating of urea by the outer layer of rock phosphate (Figure 3d).
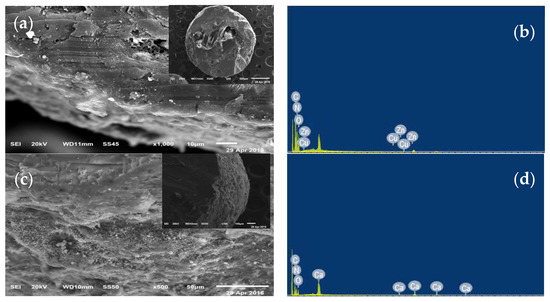
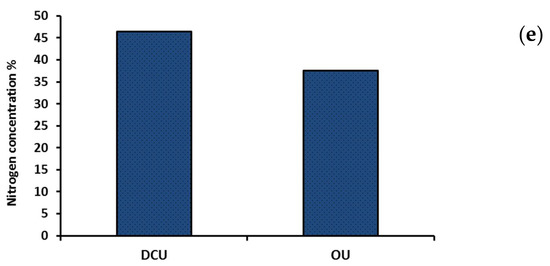
Figure 3.
SEM-EDS spectra of the OU and DCU: (a) SEM of OU, (b) EDS of OU, (c) SEM of DCU, (d) EDS of DCU, and (e) total N concentration in OU and DCU fertilizers.
As observed in the TEM micrographs, DCU also presented a spherical morphology with dark spots and a high degree of agglomeration, given the presence of an outer coating layer (Figure 4c). Added to this, the colloidal nature of urea formaldehyde with its adhesive properties might increase the agglomeration of DCU [40]. In contrast, OU appeared separated with a smaller particle size and non-uniform shape as compared with the DCU (Figure 4a). Although urea is one of the few organic compounds that possesses a highly crystallographic structure, its high solubility decreased its crystalline appearance in the SAED micrographs (Figure 4b). In contrast, the SAED pattern of DCU (Figure 4d) showed spots and ring patterns in a random orientation, illustrating the crystalline nature of the existing compounds: phosphate-bearing minerals with various degrees of crystallinity [41], the original urea granules [42] UF [43], STS [44], and the potential formation of crystalline thiourea following the reaction between urea and sodium thiosulfate [45].
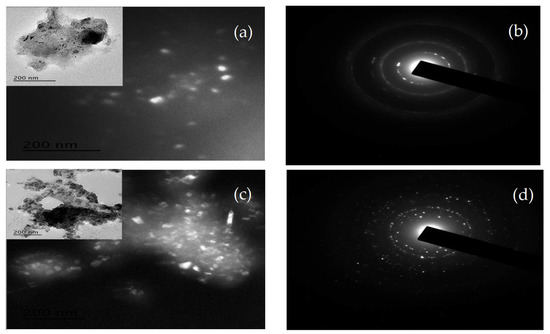
Figure 4.
TEM and SAED images of OU and DCU: (a) TEM micrographs of OU, (b) SAED pattern of OU, (c) TEM micrographs of DCU, and (d) SAED pattern of DCU.
A significant increase in the particle diameter was observed following the double coating (1.79 vs. 2.40 mm average diameter for OU and DCU, respectively). The mechanical hardness test showed that the mechanical crush strength increased with an increasing particle diameter of fertilizer granules (1.08 vs. 1.38 kgf average strength for OU and DCU, respectively), and this relation is expressed as a linear relationship between granule diameter (mm) and crushing strength (kgf) in Figure 5. Furthermore, various physical properties (e.g., hardness) could be enhanced after coating with PR. This increase in mechanical hardness could improve the ability of DCU to withstand fracture.
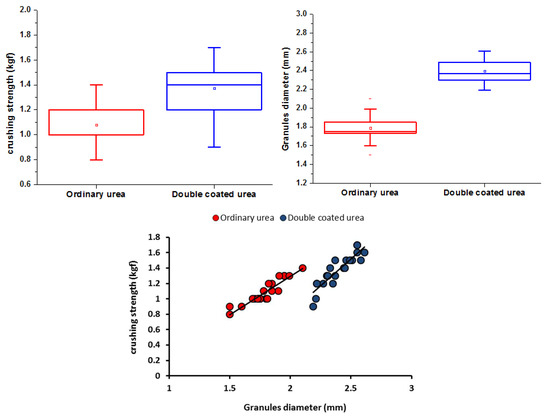
Figure 5.
Crushing strength test of OU and DCU.
The seven-day dissolution rate test showed that the dissolution rate of OU was 22% in the first day compared with only 3% for the DCU (Figure 6). This dissolution rate increased gradually as OU reached 100% in the fifth day. This rate, however, was relatively slow compared with the DCU, which reached the final day test with only 32% dissolution. The low dissolution rate of DCU was mainly attributed to the low solubility of PR, which served as the outer coating layer [46]. This dissolution rate is expected to be higher under wet soil conditions, given the supporting bio-leaching from soil microorganisms [47].
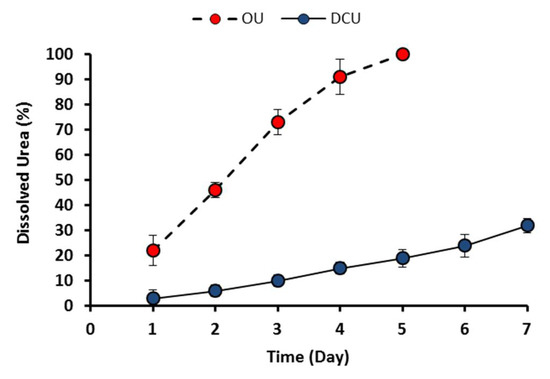
Figure 6.
Seven-day dissolution rate test of OU and DCU.
3.2. Cumulative Nitrate and Ammonium Losses
This experiment simulated the potential losses of available nitrogen forms from the soil matrix to groundwater following urea dissolution. In general, NO3− losses in leachates were more pronounced than those of NH4+ losses. This is mainly attributed to the fast transformation of NH4+ to NO3− following the nitrification process, and to the binding force of NH4+ to the negatively charged soil surface compared with leaching from the soil matrix, given the positive charge of ammonium ions, whereas nitrate is negatively charged. Cumulative nitrate and ammonium losses decreased significantly with the DCU as compared with the OU (Figure 7). In the first week, approximately 4.4% and 0.8% were lost from the total OU in forms of NO3− and NH4+, respectively. Meanwhile, the NO3− and NH4+ losses were significantly lower following the addition of DCU, with losses of only 0.1% and 0.05%, respectively. After reaching the fifth week, approximately 50% and 6.2% of the total applied OU was lost in the form of NO3− and NH4+, respectively, but only 20.7% and 2.3% was lost from the DCU. At the end of the experiment (70 days after fertilizer addition), about 68.3% and 7.6% were lost from the OU vs. 40.5% and 4.9% from the DCU, in the forms of NO3− and NH4+, respectively.
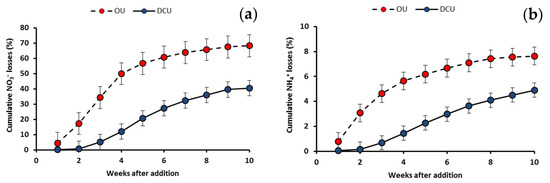
Figure 7.
Cumulative NO3− (a) and NH4+ (b) losses percentage of OU and DCU.
As previously discussed, in the seven-day dissolution rate test, the low solubility of PR (the outer coating layer) reduced the dissolution of DCU as compared with a relatively fast dissolution of OU. On the other hand, STS (the inner layer) reduced the NH4+ transformation to NO3− due to its inhibitory effect on the urease enzyme and nitrification process. Few studies have discussed the potential role of STS as a urease inhibitor. Thiosulfate does not have a direct effect on urease enzyme. However, there are several mechanisms proposed for its inhibition of the urease enzyme: (i) the abiotic and rapid reaction with soil, forming tetrathionate and liberating Fe2+ and Mn2+ ions, which are able to inactivate urease enzyme by binding to sulfhydryl groups [29]; (ii) the formation of tetrathionate following oxidation, which has an inhibitory effect on the urease enzyme [48]; and (iii) the potential formation of thiourea, which is considered to be a urease inhibitor following the reaction between urea and STS [49]. Besides its role as a urease inhibitor, STS has a remarkable effect as a nitrification inhibitor, with a 64% inhibition of nitrification and a 41% increase in apparent nitrogen recovery as compared with a sole urea application [50]. The mechanisms responsible for nitrification inhibition by STS are not fully understood;. However, a few reports have suggested the following inhibition mechanisms: (i) liberating Fe2+ and Mn2+ ions following the conversion of thiosulfate to tetrathionate can retard the nitrification process [51,52]; (ii) tetrathionate itself can inhibit the microbial or enzymatic activities responsible for nitrification [48,53]; (iii) the formation of carbonyl sulfide, which has the ability to inhibit nitrification [54]; and (iv) the direct inhibitory effect of thiosulfate on Nitrobacter sp. and other NO2− oxidizing microorganisms [55].
3.3. Field Experimentation
Significant increases in the grain and fruit yields of maize and zucchini were recorded with DCU fertilization as compared with the OU and control treatments (Table 2). The yield increase achieved by DCU application was 19.7% and 35.4% for maize and zucchini, respectively. This obtained yield increase resulted in maximizing the ANE of DCU as compared with OU (19.8 vs. 11.9, and 8.7 vs. 21.5 kg kg−1 for maize and zucchini, respectively). It is also clear that the increase in ANE with zucchini was more pronounced than with maize. This is mainly due to the residual effect of N retained in the soil after maize harvesting (Table 1), which might improve zucchini growth in its first growth stage. Several mechanisms could be responsible for improving the yield productivity of plants: (i) the outer coating layer (PR) led to the release of nitrogen in a controlled manner in synchrony with the sequential needs of the plants until the final growth stage [19,56]; (ii) the partial contribution of PR in phosphorus nutrition and improving soil quality properties [57,58]; (iii) the contribution of the inner coating layer (STS) to sulfur nutrition, and its inhibitory effect on the urease enzyme [48]; (iv) the role of STS as a nitrification inhibitor through retarding the biological oxidation of NH4+—N to NO3−—N [50,59]; and (v) the beneficial effect of urea formaldehyde as a slow-release N source and biological soil activator [60].

Table 2.
Total marketable yield and nutrients concentration in maize grains and zucchini fruits.
Due to the beneficial effect of the double coating technology on retarding urea dissolution/hydrolysis and inhibiting the nitrification process, the final concentration of nitrogen in maize grains and zucchini fruits was higher with DCU (1.88% and 4.69%, respectively) as compared with the OU (1.65% and 2.45%, respectively) and the control (1.53% and 2.02%, respectively) treatments. Phosphorus concentration was significantly higher in plants fertilized with DUC due to the slow-release supply of phosphorus derived from phosphate rock, which was reflected in an increasing available phosphorus concentration by about 40.1% following the DCU application. The increase in phosphorus concentration in maize grains and zucchini fruits was, respectively, 1.40 and 1.14-fold greater than with OU. Likewise, the available potassium concentration in the soil showed a significant increase following DCU application (227 mg vs. 335 mg kg−1), which led to increased potassium concentrations in the grains and fruits. Additionally, micronutrient concentrations in the maize grains and zucchini fruits also increased with DCU application. The slow-release supply of nitrogen might have activated the enzymes involved in N metabolism, thereby forcing the plants to absorb higher amounts of micronutrients (Ai et al., 2017).
4. Conclusions
Controlling urea dissolution is urgently needed to meet the nitrogen requirements of plants in all growth stages. The double coating technology created a controlled outer layer of PR with a high degree of agglomeration given the sticky effect of UF, and an inner layer of STS protected from fast degradation in soil. Research results concluded that the double coating technology improved urea resistance against fast dissolution, maximized the mechanical hardness of fertilizer granules, reduced the cumulative NO3− and NH4+ leaching from the soil matrix, maximized the ANE of maize and zucchini crops, and increased N concentration in grains and fruits. Furthermore, DCU application modulated the concentrations of macro and micronutrients in maize grains and zucchini fruits. The findings of this study highlight the applicability of double coating as a novel technology to improve urea efficacy in terrestrial ecosystems. Further investigations should be undertaken using different application rates to improve the economic revenues of DCU fertilizers.
Author Contributions
Conceptualization, A.E.-G. and A.M.; software, Z.A.; validation, E.-S.E.-N., A.M.E. and B.G.; formal analysis, Z.A. and A.M.E.; resources, E.-S.E.-N.; data curation, A.E.-G. and A.M.; writing—original draft preparation, A.E.-G. and E.-S.E.-N.; writing—review and editing, A.M.E., B.G., Z.A. and A.M.; visualization, A.M.E. and Z.A.; supervision, B.G. and A.M.; project administration, A.E.-G., E.-S.E.-N. and A.M.; funding acquisition, A.E.-G., E.-S.E.-N. and A.M.E. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support of this research from Mansoura University and El-Delta Fertilizers Plant, Egypt, is gratefully acknowledged. The authors extend their appreciation to the researchers supporting project number (RSP-2021/56), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Mansoura University, El-Delta Fertilizers Plant and researchers supporting project number (RSP-2021/56), King Saud University, Riyadh, Saudi Arabia for financial support. Authors also would like to acknowledge both Soil Fertility and Fertilizers Quality Control Lab. and Electron Microscopy Unit, Mansoura University, for the soil and fertilizer analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yin, X.; Yu, L.; Luo, X.; Zhang, Z.; Sun, H.; Mosa, A.A.; Wang, N. Sorption of Pb (II) onto< 1 μm effective diameter clay minerals extracted from different soils of the Loess Plateau, China. Geoderma 2019, 337, 1058–1066. [Google Scholar]
- Moghanm, F.S.; El-Banna, A.; El-Esawi, M.A.; Abdel-Daim, M.M.; Mosa, A.; Abdelaal, K.A.A. Genotoxic and anatomical deteriorations associated with potentially toxic elements accumulation in water hyacinth grown in drainage water resources. Sustainability 2020, 12, 2147. [Google Scholar] [CrossRef] [Green Version]
- Mosa, A.; Taha, A.A.; Elsaeid, M. In-situ and ex-situ remediation of potentially toxic elements by humic acid extracted from different feedstocks: Experimental observations on a contaminated soil subjected to long-term irrigation with sewage effluents. Environ. Technol. Innov. 2021, 23, 101599. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mosa, A.; Zhan, L.; Gao, B. Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: A critical review. Chemosphere 2021, 278, 130378. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Motasim, A.M.; Samsuri, A.W.; Abdul Sukor, A.S.; Adibah, A.M. Gaseous Nitrogen Losses from Tropical Soils with Liquid or Granular Urea Fertilizer Application. Sustainability 2021, 13, 3128. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Pervaiz, E.; Abbas Shah, G.; Ul Haq, M.; Zafar, M.I.; Zia, M. Slow-release urea prills developed using organic and inorganic blends in fluidized bed coater and their effect on spinach productivity. Sustainability 2020, 12, 5944. [Google Scholar] [CrossRef]
- Choi, W.-J.; Jin, S.-A.; Lee, S.-M.; Ro, H.-M.; Yoo, S.-H. Corn uptake and microbial immobilization of 15N-labeled urea-N in soil as affected by composted pig manure. Plant Soil 2001, 235, 1–9. [Google Scholar] [CrossRef]
- Zhengping, W.; Van Cleemput, O.; Demeyer, P.; Baert, L. Effect of urease inhibitors on urea hydrolysis and ammonia volatilization. Biol. Fertil. Soils 1991, 11, 43–47. [Google Scholar] [CrossRef]
- Watson, C.J.; Miller, H. Short-term effects of urea amended with the urease inhibitor N-(n-butyl) thiophosphoric triamide on perennial ryegrass. Plant Soil 1996, 184, 33–45. [Google Scholar] [CrossRef]
- Plaster, E. Soil Science and Management; Cengage Learning: Southbank, Australia, 2013. [Google Scholar]
- Gaidajis, G.; Kakanis, I. Life cycle assessment of nitrate and compound fertilizers production—A case study. Sustainability 2021, 13, 148. [Google Scholar] [CrossRef]
- Stayner, L.T.; Schullehner, J.; Semark, B.D.; Jensen, A.S.; Trabjerg, B.B.; Pedersen, M.; Olsen, J.; Hansen, B.; Ward, M.H.; Jones, R.R.; et al. Exposure to nitrate from drinking water and the risk of childhood cancer in Denmark. Environ. Int. 2021, 155, 106613. [Google Scholar] [CrossRef] [PubMed]
- Fossen Johnson, S. Methemoglobinemia: Infants at risk. Curr. Probl. Pediatric Adolesc. Health Care 2019, 49, 57–67. [Google Scholar] [CrossRef]
- Chatterjee, D.; Mohanty, S.; Guru, P.K.; Swain, C.K.; Tripathi, R.; Shahid, M.; Kumar, U.; Kumar, A.; Bhattacharyya, P.; Gautam, P.; et al. Comparative assessment of urea briquette applicators on greenhouse gas emission, nitrogen loss and soil enzymatic activities in tropical lowland rice. Agric. Ecosyst. Environ. 2018, 252, 178–190. [Google Scholar] [CrossRef]
- Wan, X.; Wu, W.; Li, C.; Liu, Y.; Wen, X.; Liao, Y. Soil ammonia volatilization following urea application suppresses root hair formation and reduces seed germination in six wheat varieties. Environ. Exp. Bot. 2016, 132, 130–139. [Google Scholar] [CrossRef]
- Klimczyk, M.; Siczek, A.; Schimmelpfennig, L. Improving the efficiency of urea-based fertilization leading to reduction in ammonia emission. Sci. Total Environ. 2021, 771, 145483. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [PubMed]
- Saleh, K.; Steinmetz, D.; Hemati, M. Experimental study and modeling of fluidized bed coating and agglomeration. Powder Technol. 2003, 130, 116–123. [Google Scholar] [CrossRef]
- Hiromoto, S.; Yamazaki, T. Micromorphological effect of calcium phosphate coating on compatibility of magnesium alloy with osteoblast. Sci. Technol. Adv. Mater. 2017, 18, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Szilas, C.; Semoka, J.M.R.; Borggaard, O.K. Can local Minjingu phosphate rock replace superphosphate on acid soils in Tanzania? Nutr. Cycl. Agroecosyst. 2007, 77, 257–268. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Li, R.; Zhang, Z. Immobilization of Lead and Cadmium in Contaminated Soil Using Amendments: A Review. Pedosphere 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, Q.; Feng, B.; Xu, G.; Chi, R. Biosolubilization of rock phosphates in a bioreactor using a microbial consortium from rhizospheric soils: An analysis of the microbial community and technical feasibility. Min. Metall. Explor. 2018, 35, 184–191. [Google Scholar]
- Graziano, P.L.; Parente, G. Response of irrigated maize to urea-ammonium nitrate and ammonium thiosulphate solutions on a sulphur deficient soil. Nutr. Cycl. Agroecosyst. 1996, 46, 91–95. [Google Scholar] [CrossRef]
- He, L.; Wang, M.; Zhang, G.; Qiu, G.; Cai, D.; Wu, Z.; Zhang, X. Remediation of Cr(VI) contaminated soil using long-duration sodium thiosulfate supported by micro–nano networks. J. Hazard. Mater. 2015, 294, 64–69. [Google Scholar] [CrossRef]
- Issaro, N.; Besancon, S.; Bermond, A. Thermodynamic and kinetic study of the single extraction of mercury from soil using sodium-thiosulfate. Talanta 2010, 82, 1659–1667. [Google Scholar] [CrossRef]
- Morden, G.; Soper, R.; Huzel, V.; Swan, M. The effect of thiosulfate on phosphorus availability and uptake by plants. J. Plant Nutr. 1986, 9, 1315–1321. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Havlin, J.L. Soil and Environmental Effects on Urease Inhibition by Ammonium Thiosulfate. Soil Sci. Soc. Am. J. 1992, 56, 950–956. [Google Scholar] [CrossRef]
- Byrnes, B.H.; Amberger, A. Fate of broadcast urea in a flooded soil when treated with N-(n-butyl) thiophosphoric triamide, a urease inhibitor. Fertil. Res. 1988, 18, 221–231. [Google Scholar] [CrossRef]
- McGeough, K.L.; Watson, C.J.; Müller, C.; Laughlin, R.J.; Chadwick, D.R. Evidence that the efficacy of the nitrification inhibitor dicyandiamide (DCD) is affected by soil properties in UK soils. Soil Biol. Biochem. 2016, 94, 222–232. [Google Scholar] [CrossRef]
- Goos, R.J.; Johnson, B.E. Thiosulfate oxidation by three soils as influenced by temperature. Commun. Soil Sci. Plant Anal. 2001, 32, 2841–2849. [Google Scholar] [CrossRef]
- Blouin, G.M.; Rindt, D.W.; Moore, O.E. Sulfur-coated fertilizers for controlled release. Pilot-plant production. J. Agric. Food Chem. 1971, 19, 801–808. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient Use Efficiency in Plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Singh, R.A. Soil Physical Analysis; Kalyani Publishers: New Delhi, India, 1989. [Google Scholar]
- RICHARDS, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Dewis, J.; Freitas, F. Food and Agriculture Organization of the United Nations. Physical and Chemical Methods of Soil and Water Analysis; Food and Agriculture Organization of the United Nations: Rome, Italy, 1970. [Google Scholar]
- Hesse, P.R. A Textbook of Soil Chemical Analysis; Chemical Pub. Co.: New York, NY, USA, 1971. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Ferra, J.M.M.; Mendes, A.M.; Costa, M.R.N.; Carvalho, L.H.; Magalhães, F.D. A study on the colloidal nature of urea-formaldehyde resins and its relation with adhesive performance. J. Appl. Polym. Sci. 2010, 118, 1956–1968. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Li, Y.C.; Harris, W.G. Phosphate minerals and solubility in native and agricultural calcareous soils. Geoderma 2014, 232–234, 164–171. [Google Scholar] [CrossRef]
- Hendricks, S.B. The crystal structure of urea and the molecular symmetry of thiourea. J. Am. Chem. Soc. 1928, 50, 2455–2464. [Google Scholar] [CrossRef]
- Park, B.-D.; Causin, V. Crystallinity and domain size of cured urea–formaldehyde resin adhesives with different formaldehyde/urea mole ratios. Eur. Polym. J. 2013, 49, 532–537. [Google Scholar] [CrossRef]
- Sekkina, M.A.; El-Shereafy, E.-S.; Mashaly, A.; El-Ashry, M. γ-Pyrolysis of crystalline sodium thiosulphate penta hydrate. J. Radioanal. Nucl. Chem. 1998, 237, 113–119. [Google Scholar] [CrossRef]
- Ezhil Vizhi, R.; Kalainathan, S.; Baghavan Narayana, G. Structural and microhardness studies of pure and thiourea doped glycine phosphite single crystal. Cryst. Res. Technol. 2008, 43, 778–782. [Google Scholar] [CrossRef]
- Chien, S.H. Solubility assessment for fertilizer containing phosphate rock. Fertil. Res. 1993, 35, 93–99. [Google Scholar] [CrossRef]
- Li, L.; Lv, Z.; Zuo, Z.; Yang, Z.; Yuan, X. Effect of energy source and leaching method on bio-leaching of rock phosphates by Acidithiobacillus ferrooxidans. Hydrometallurgy 2016, 164, 238–247. [Google Scholar] [CrossRef]
- McCarty, G.W.; Bremner, J.M.; Krogmeier, M.J. Effects of thiosulfate and tetrathionate on urease activity and seedling growth. Commun. Soil Sci. Plant Anal. 1991, 22, 755–768. [Google Scholar] [CrossRef]
- Khan, K.M.; Naz, F.; Taha, M.; Khan, A.; Perveen, S.; Choudhary, M.I.; Voelter, W. Synthesis and in vitro urease inhibitory activity of N,N′-disubstituted thioureas. Eur. J. Med. Chem. 2014, 74, 314–323. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Hina, M.; Tahir, M.M. Effect of Azadirachta indica (neem), sodium thiosulphate and calcium chloride on changes in nitrogen transformations and inhibition of nitrification in soil incubated under laboratory conditions. Chemosphere 2011, 82, 1629–1635. [Google Scholar] [CrossRef]
- Jiang, X.; Xin, X.; Li, S.; Zhou, J.; Zhu, T.; Müller, C.; Cai, Z.; Wright, A.L. Effects of Fe oxide on N transformations in subtropical acid soils. Sci. Rep. 2015, 5, 8615. [Google Scholar] [CrossRef]
- Xin, X.; Jiang, X.; Su, J.; Yan, X.; Ni, J.; Faeflen, S.J.; Huang, X.; Wright, A.L. Manganese oxide affects nitrification and ammonia oxidizers in subtropical and temperate acid forest soils. CATENA 2016, 137, 24–30. [Google Scholar] [CrossRef]
- Goos, R.J.; Ahrens, W.H. Ammonium Thiosulfate Effect on Herbicide Longevity in Soil. Agron. J. 1992, 84, 459–463. [Google Scholar] [CrossRef]
- Saad, O.A.L.O.; Lehmann, S.; Conrad, R. Influence of thiosulfate on nitrification, denitrification, and production of nitric oxide and nitrous oxide in soil. Biol. Fertil. Soils 1996, 21, 152–159. [Google Scholar] [CrossRef]
- Janzen, H.H.; Bettany, J.R. Influence of Thiosulfate on Nitrification of Ammonium in Soil1. Soil Sci. Soc. Am. J. 1986, 50, 803–806. [Google Scholar] [CrossRef]
- Patel, S.K.; Panda, D.; Mohanty, S.K. Relative ammonia loss from urea-based fertilizers applied to rice under different hydrological situations. Fertil. Res. 1989, 19, 113–119. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Ceglie, F.G.; Aly, A.; Mihreteab, H.T.; Ciaccia, C.; Tittarelli, F. Phosphorus availability from rock phosphate: Combined effect of green waste composting and sulfur addition. J. Environ. Manag. 2016, 182, 557–563. [Google Scholar] [CrossRef]
- Mechri, B.; Attia, F.; Tekaya, M.; Cheheb, H.; Hammami, M. Agronomic application of olive mill wastewaters with rock phosphate increase the 10Me18:0 fatty acid marker of actinomycetes and change rhizosphere microbial functional groups under long-term field conditions. Soil Biol. Biochem. 2014, 70, 62–65. [Google Scholar] [CrossRef]
- Sallade, Y.E.; Sims, J.T. Evaluation of thiosulfate as a nitrification inhibitor for manures and fertilizers. Plant Soil 1992, 147, 283–291. [Google Scholar] [CrossRef]
- Ikeda, S.; Suzuki, K.; Kawahara, M.; Noshiro, M.; Takahashi, N. An Assessment of Urea-Formaldehyde Fertilizer on the Diversity of Bacterial Communities in Onion and Sugar Beet. Microbes Environ. 2014, 29, 231–234. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).