Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review
Abstract
:1. Introduction
2. Plastics Recycling
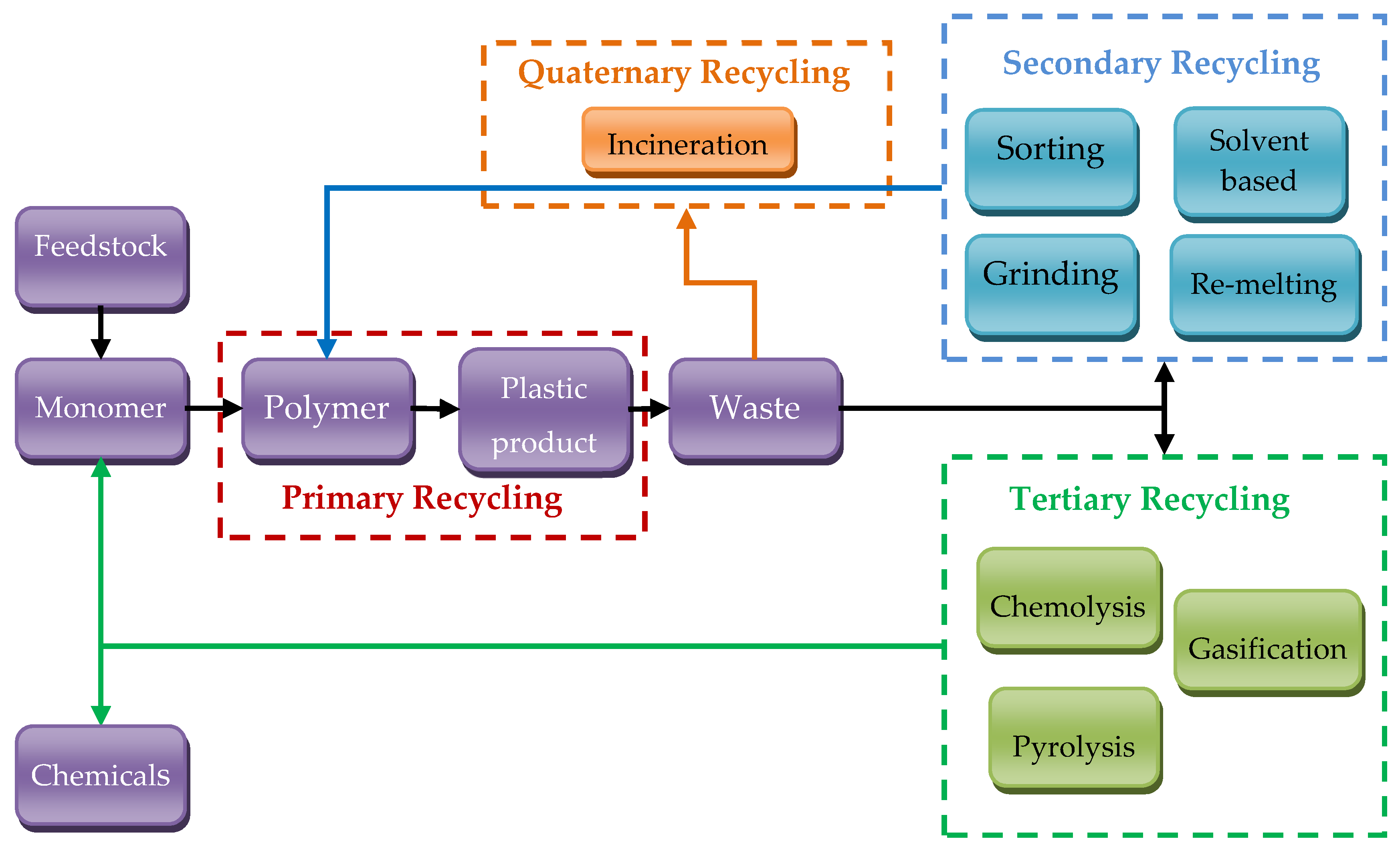
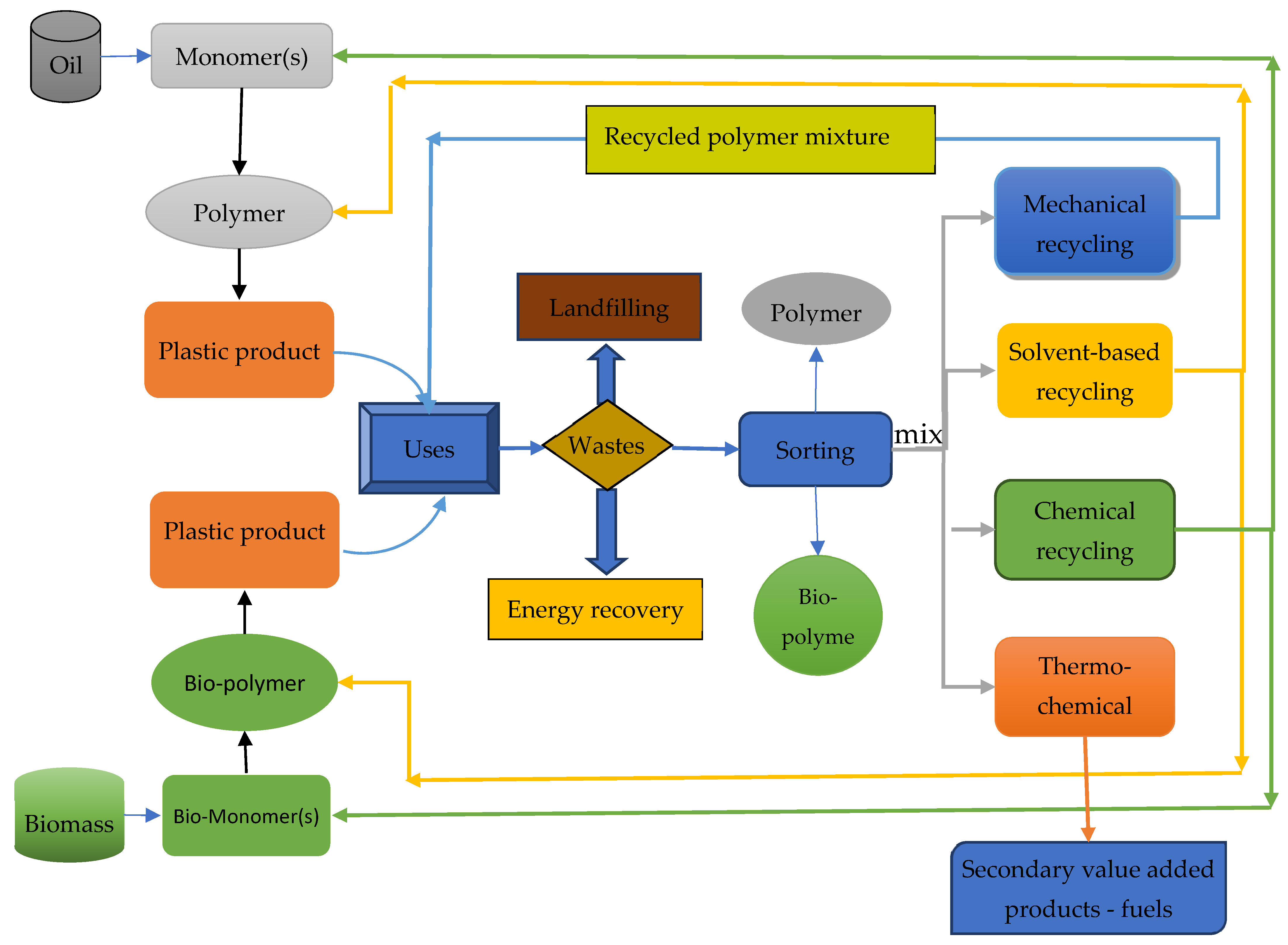
2.1. General Recycling Routes in Plastic Wastes
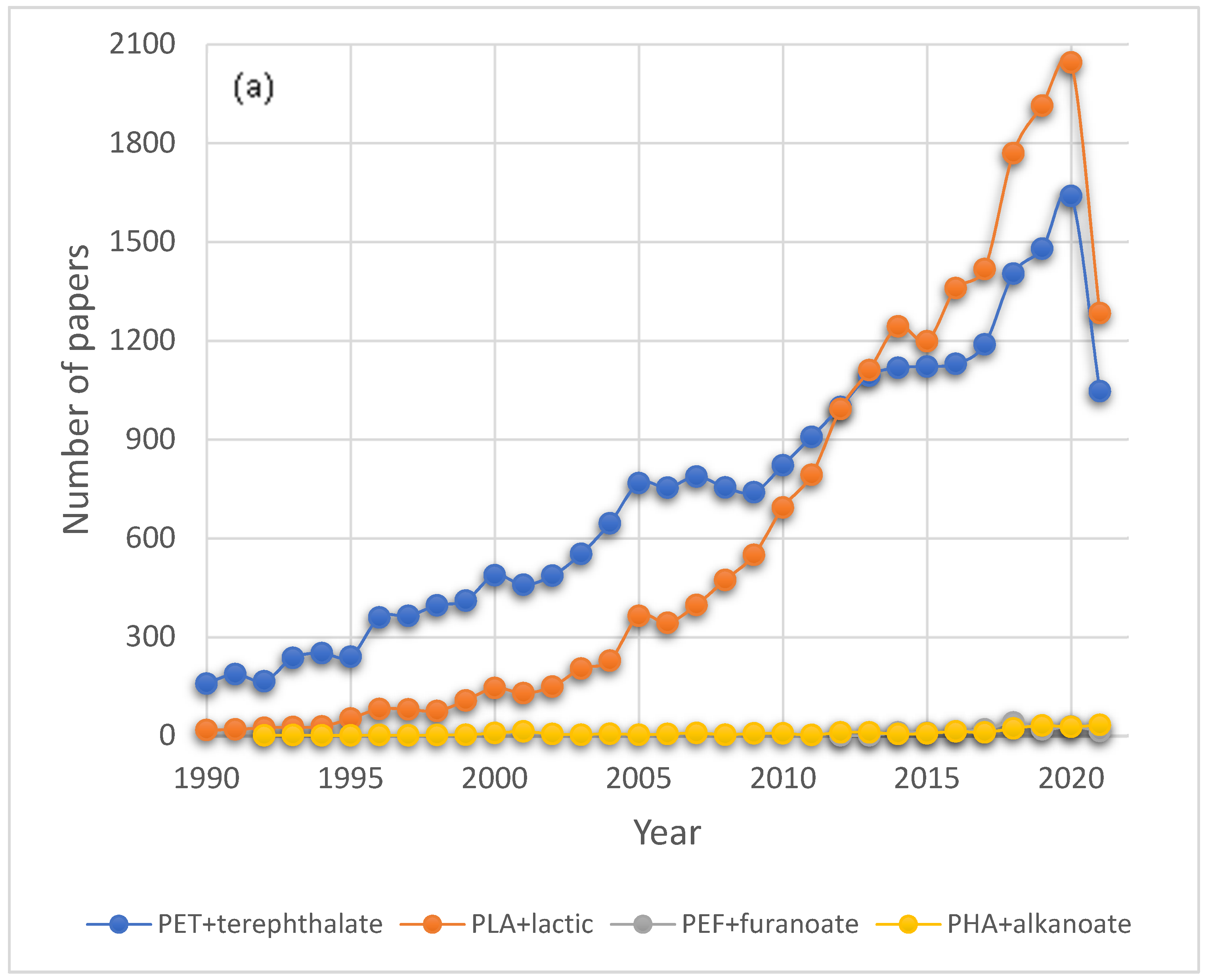
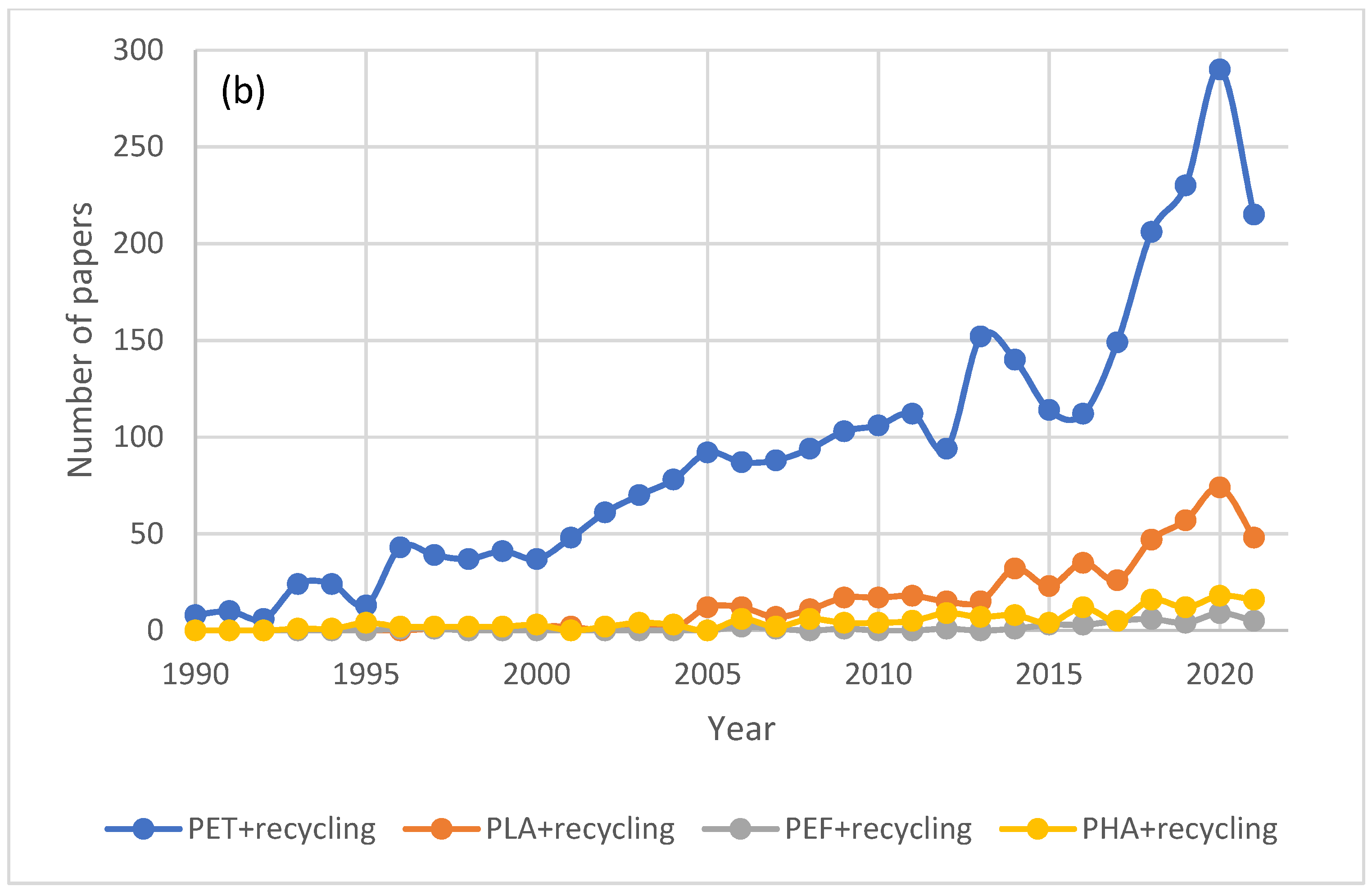
2.2. Recycling of Petroleum-Based Plastics in the Presence of Bio-Based Plastics
| Density (kg/L) | Tg (°C) | Tm (°C) | Onset of Degradation (°C) | Processing Temperature | |
|---|---|---|---|---|---|
| PET | 1.35–1.39 [11] | 69 [26] | 255 [11] | 410 [36] | 270 [26] |
| PLA | 1.20–1.45 [11] | 45–60 [35] | 155–165 [11] | 320 [37] | 180 [38] |
| PHB | 1.25–1.30 [11,39] | 2–5 [35] | 180 [11] | 220–250 [40,41] | 170 [42] |
| PEF | 1.40–1.55 [11] | 84.3 [16] | 225 [11] | 325 [16] |
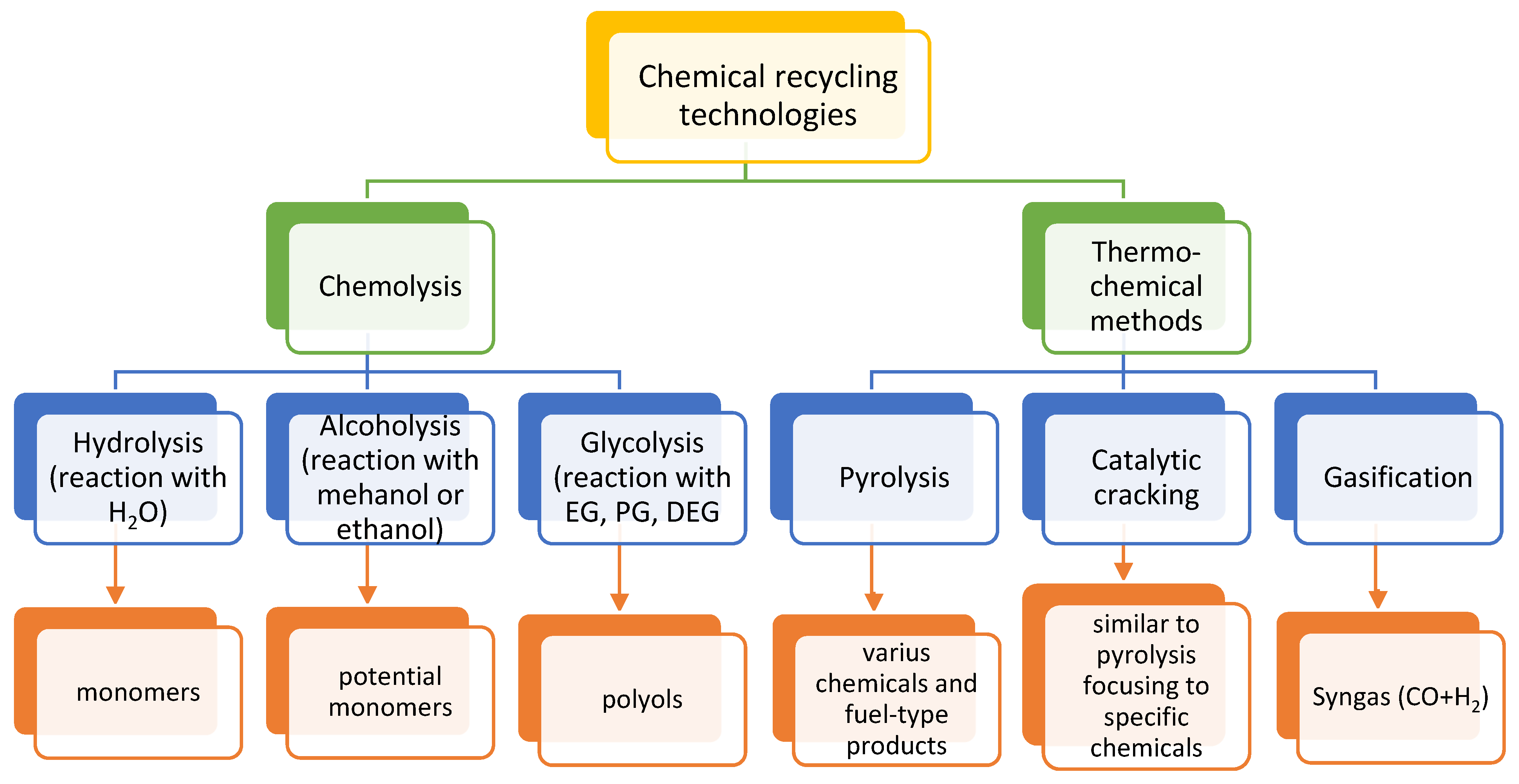
3. Chemical Recycling of Plastics
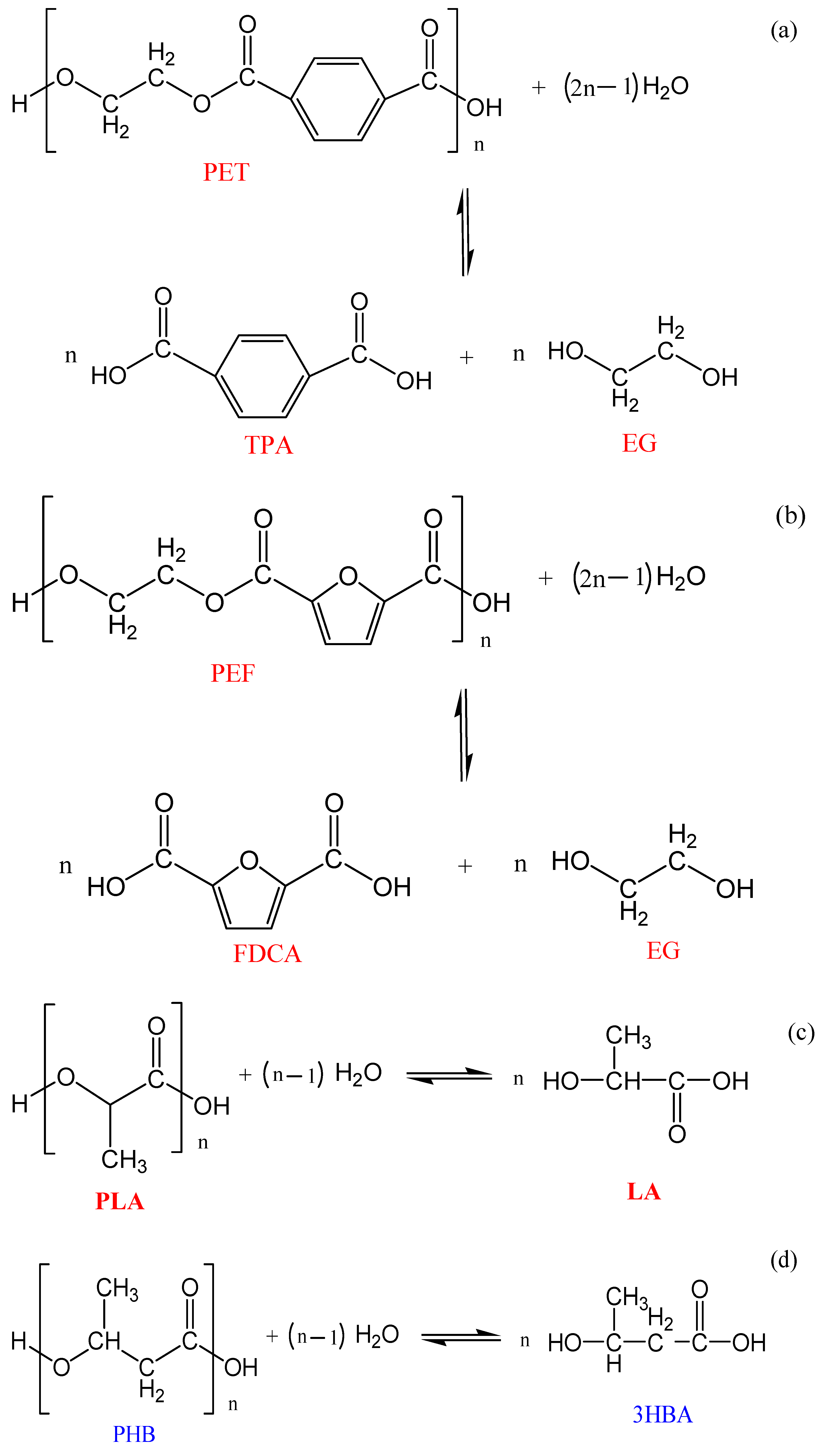
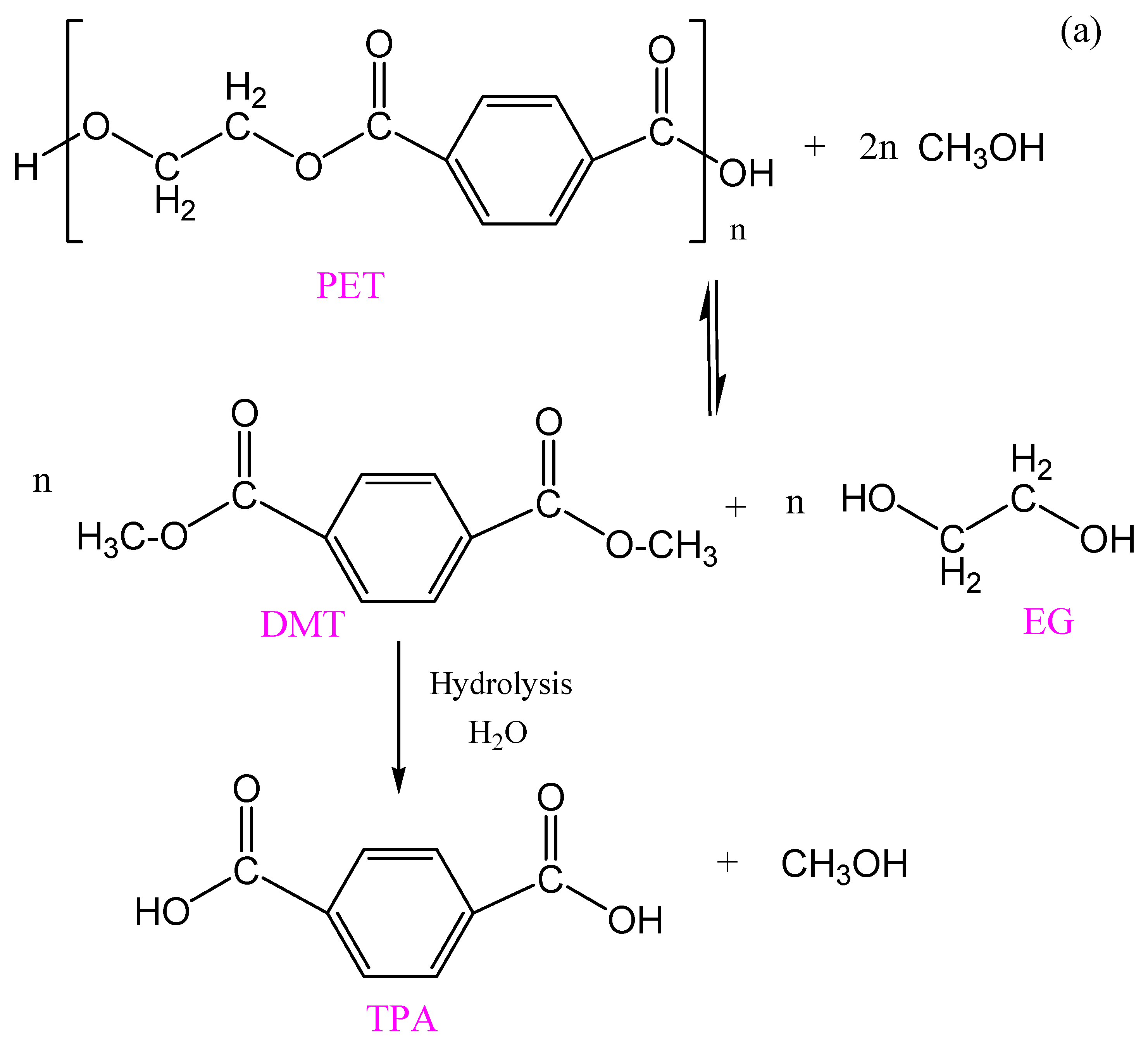
3.1. Chemolysis by Hydrolysis
3.1.1. Neutral Hydrolysis
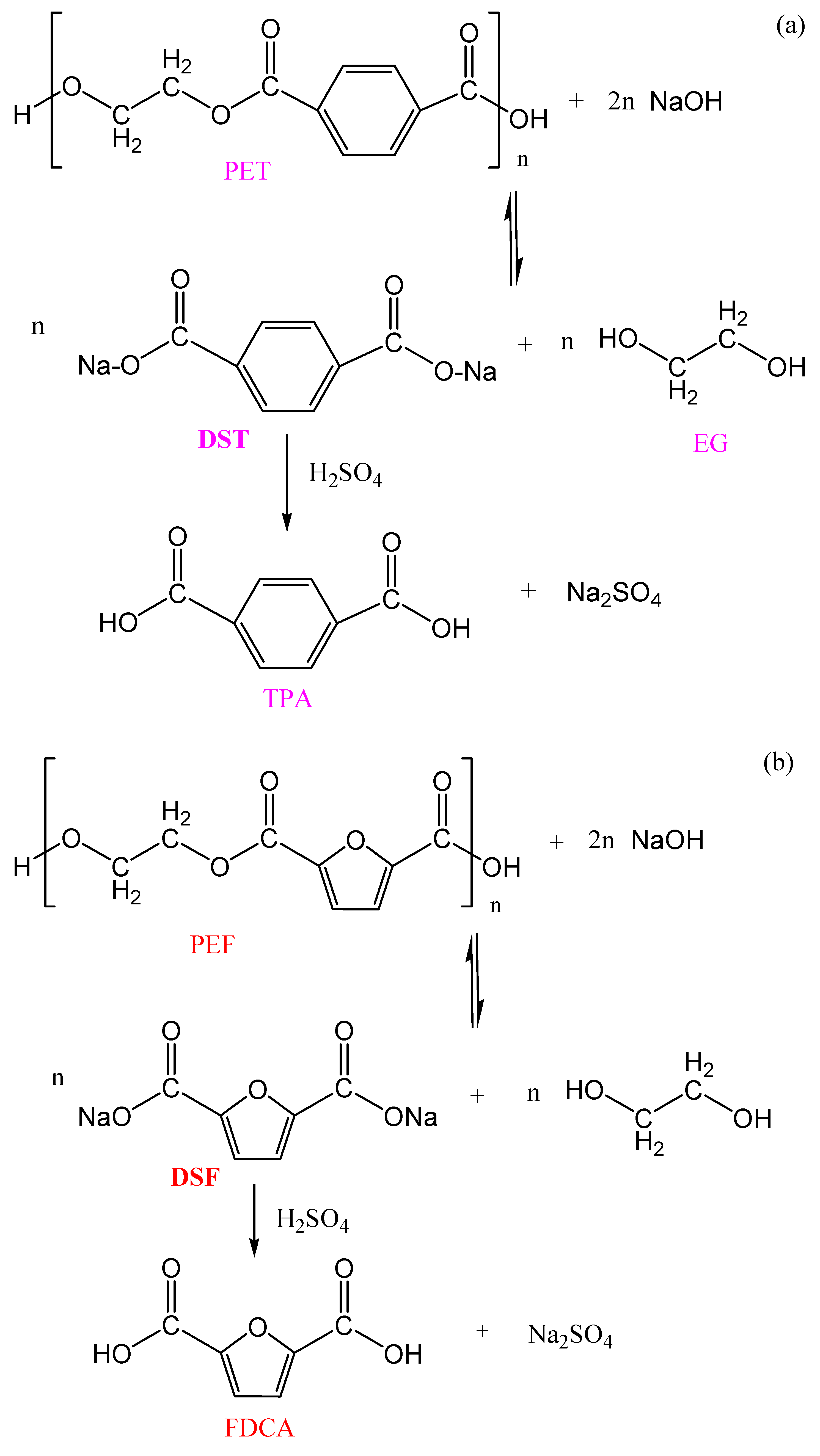
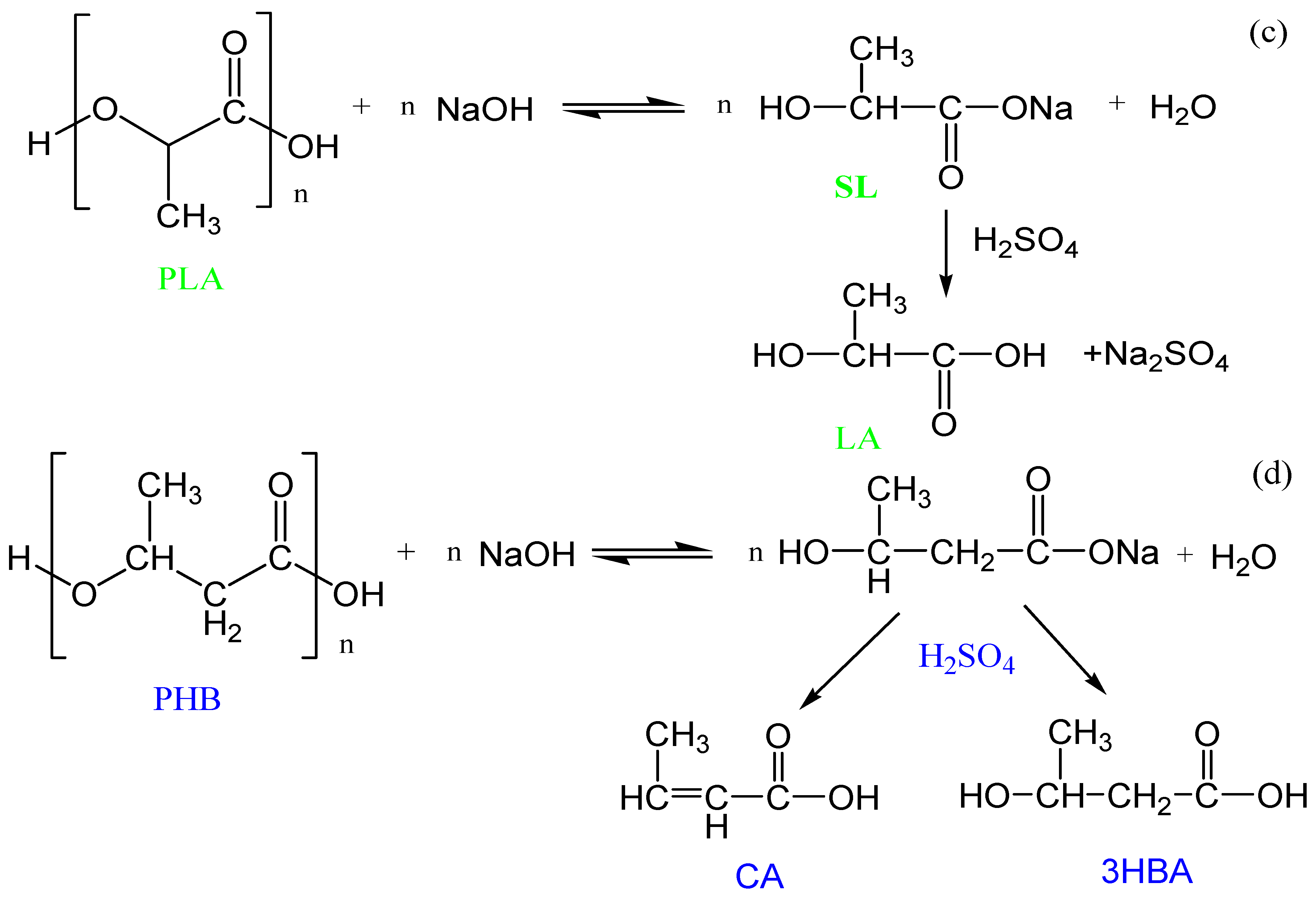
3.1.2. Alkaline Hydrolysis
3.1.3. Acid Hydrolysis
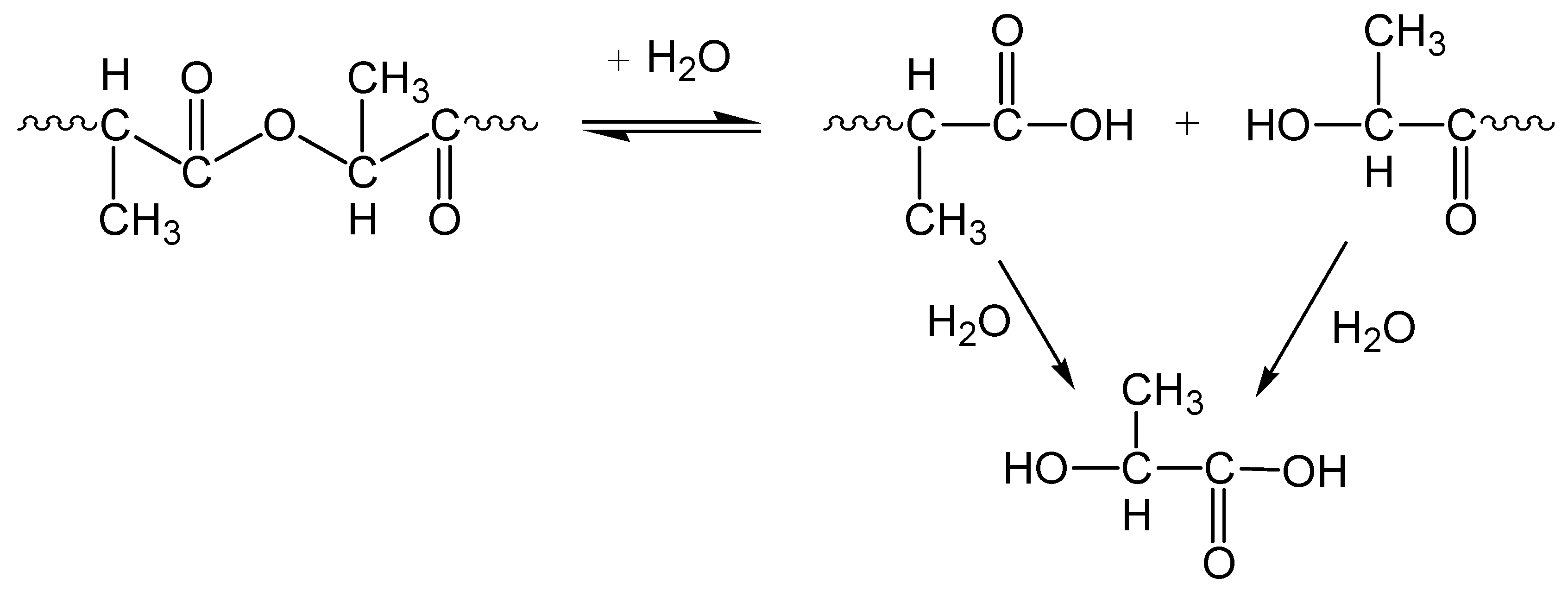
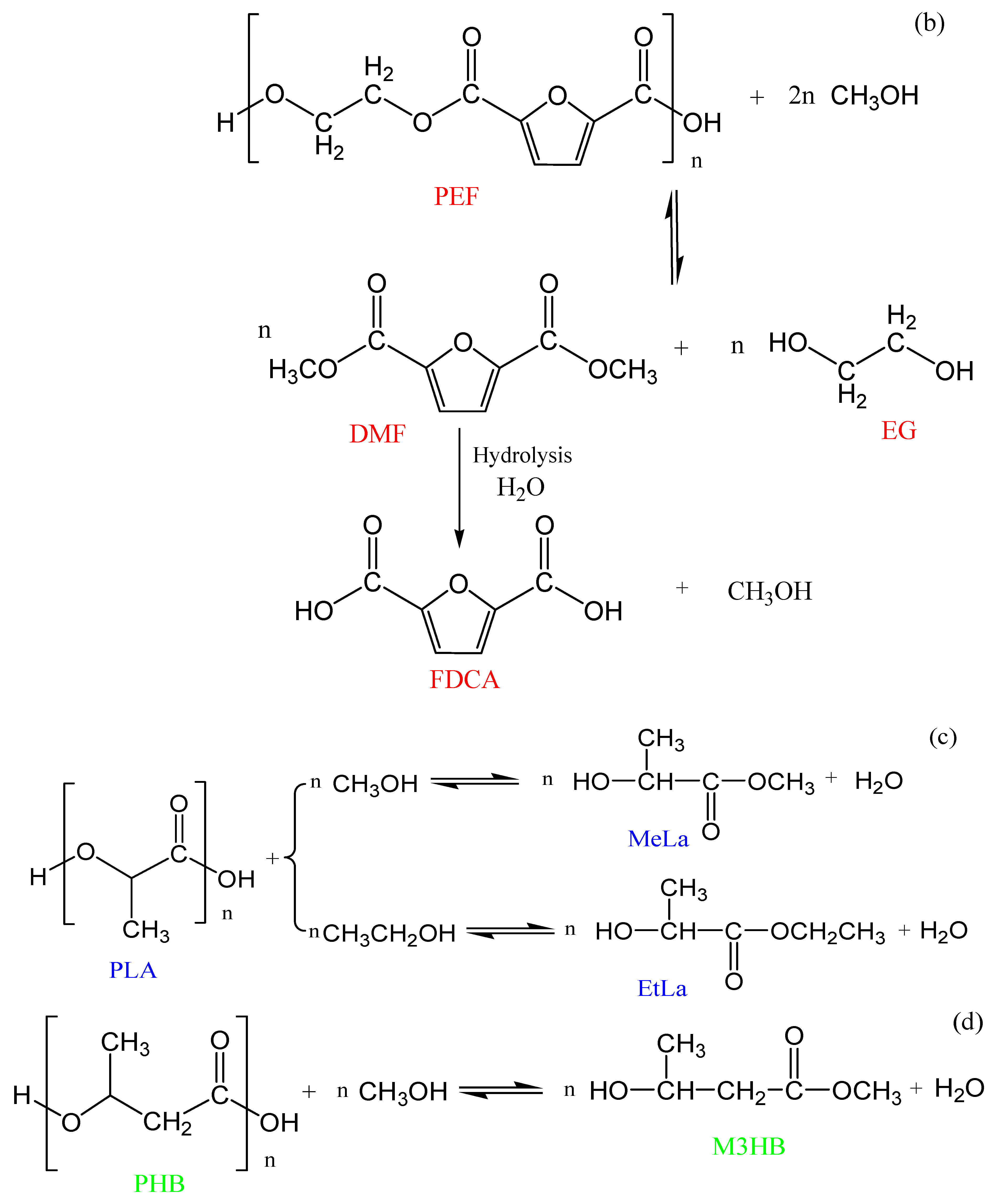
3.2. Chemolysis by Alcoholysis
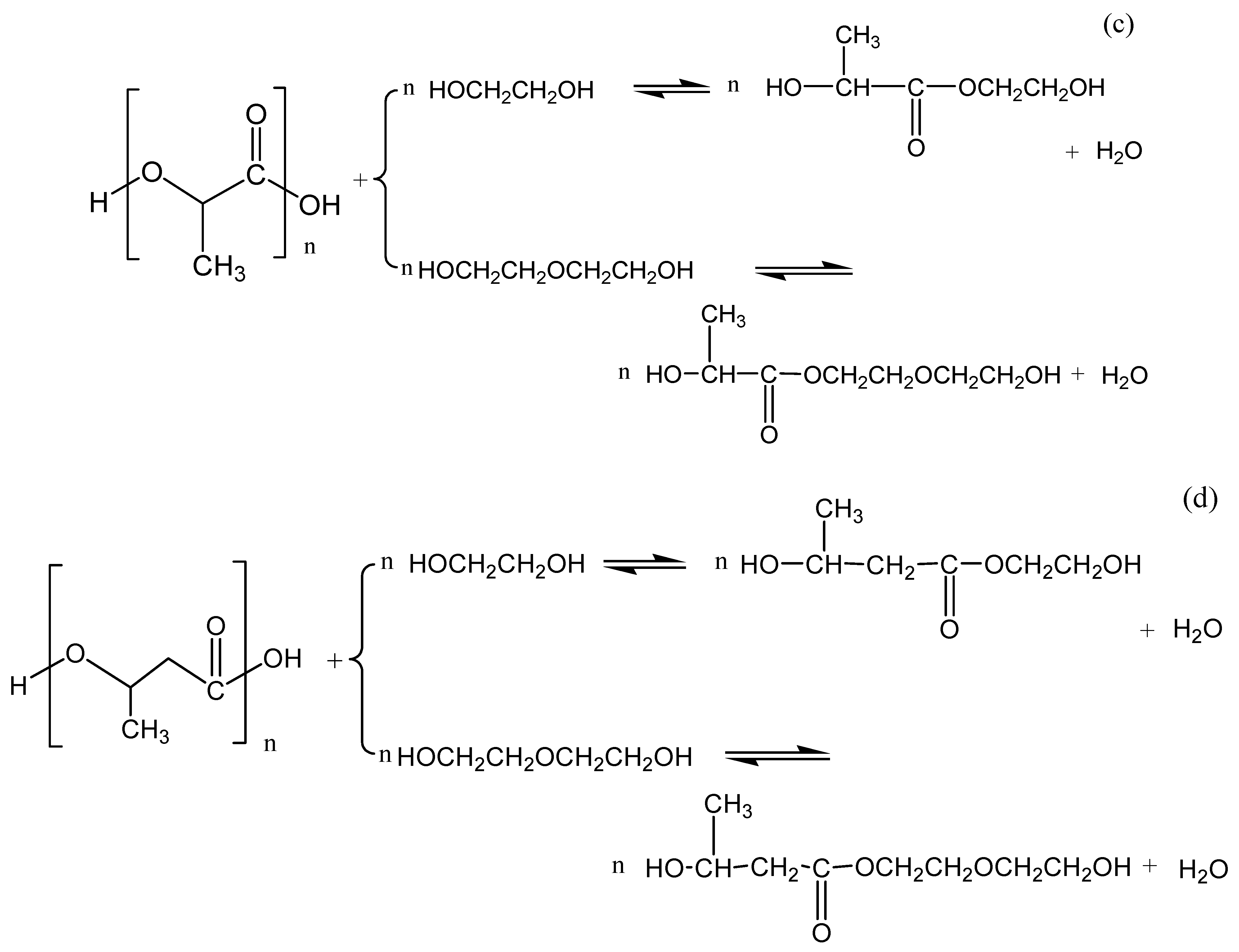
3.3. Chemolysis by Glycolysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Bioplastics, Global Production Capacities of Bioplastics 2017-22-Bioplastics Market Data 2019; Nova Institute: Berlin, Germany, 2020; Available online: https://docs.european-bioplastics.org/publications/ (accessed on 15 August 2021).
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics. 2016. Available online: https://www.newplasticseconomy.org/about/publications/report-2016 (accessed on 15 August 2021).
- PlasticsEurope. Plastics—The Facts. 2020. Available online: https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts- (accessed on 25 June 2021).
- European Bioplastics Fact Sheet: What Are Bioplastics? 2018. Available online: https://docs.european-bioplastics.org/publications/fs/EuBP_FS_What_are_bioplastics.pdf (accessed on 15 August 2021).
- The Global Commitment 2020 Progress Report; Ellen MacArthur Foundation: Cowes, UK, 2020.
- Albertsson, A.-C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Majgaonkar, P.; Hanich, R.; Malz, F.; Brüll, R. Chemical recycling of post-consumer PLA waste for sustainable production of ethyl lactate. Chem. Eng. J. 2021, 423, 129952. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60. [Google Scholar] [CrossRef]
- Alaerts, L.; Augustinus, M.; Van Acker, K. Impact of bio-based plastics on current recycling of plastics. Sustainability 2018, 10, 1487. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, A.C.; Collinson, S.R. The selective recycling of mixed plastic waste of polylactic acid and polyethylene terephthalate by control of process conditions. Eur. Polym. J. 2011, 47, 1970–1976. [Google Scholar] [CrossRef] [Green Version]
- Kasmi, N.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State polymerization of poly (Ethylene Furanoate) biobased Polyester, II: An efficient and facile method to synthesize high molecular weight polyester appropriate for food packaging applications. Polymers 2018, 10, 471. [Google Scholar] [CrossRef] [Green Version]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-state polymerization of poly (ethylene furanoate) biobased polyester, I: Effect of catalyst type on molecular weight increase. Polymers 2017, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Gabirondo, E.; Melendez-Rodriguez, B.; Arnal, C.; Lagaron, J.M.; de Ilarduya, A.M.; Sardon, H.; Torres-Giner, S. Organocatalyzed closed-loop chemical recycling of thermo-compressed films of poly(ethylene furanoate). Polym. Chem. 2021, 12, 1571–1580. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Feghali, E.; Tauk, L.; Ortiz, P.; Vanbroekhoven, K.; Eevers, W. Catalytic chemical recycling of biodegradable polyesters. Polym. Degrad. Stab. 2020, 179, 109241. [Google Scholar] [CrossRef]
- European Commission. Closing the Loop: An Ambitious EU Circular Economy Package. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%52015DC0614 (accessed on 15 August 2021).
- European Commission. A European Strategy for Plastics in a Circular Economy. 2018. Available online: https://ec.europa.eu/environment/circular-economy/pdf/plasticsstrategy-brochure.pdf (accessed on 15 August 2021).
- European Commission. Directive 2015/720 Amending Directive 94/62/EC as Regards Reducing the Consumption of Light-Weight Plastic Carrier Bags. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015L0720 (accessed on 15 August 2021).
- IHS Markit, Biodegradable Polymers—Chemical Economics Handbook. 2018. Available online: https://ihsmarkit.com/products/biodegradablepolymers-chemical-economics-handbook.html (accessed on 21 June 2021).
- PlasticsEurope. Plastics—The Facts. 2019. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 25 March 2021).
- Dimitris, S.; Achilias, L.A. Recent advances in the chemical recycling of polymers (PP, PS, LDPE, HDPE, PVC, PC, Nylon, PMMA). Mater. Recycl. Trends Perspect. 2012, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Aldas, M.; Pavon, C.; De La Rosa-Ramírez, H.; Ferri, J.M.; Bertomeu, D.; Samper, M.D.; López-Martínez, J. The impact of biodegradable plastics in the properties of recycled polyethylene terephthalate. J. Polym. Environ. 2021, 29, 2686–2700. [Google Scholar] [CrossRef]
- Niaounakis, M. Recycling of biopolymers—The patent perspective. Eur. Polym. J. 2019, 114, 464–475. [Google Scholar] [CrossRef]
- NatureWorks LLC. Using Near-Infrared Sorting to Recycle PLA Bottles. Available online: https://www.natureworksllc.com (accessed on 8 August 2021).
- La Mantia, F.P.; Botta, L.; Morreale, M.; Scaffaro, R. Effect of small amounts of poly(lactic acid) on the recycling of poly(ethylene terephthalate) bottles. Polym. Degrad. Stab. 2012, 97, 21–24. [Google Scholar] [CrossRef]
- McLauchlin, A.; Ghita, O.R. Studies on the thermal and mechanical behavior of PLA-PET blends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Gere, D.; Czigany, T. Future trends of pastic bottle recycling: Compatibilization of PET and PLA. Polym. Test. 2020, 81, 106160. [Google Scholar] [CrossRef]
- Åkesson, D.; Kuzhanthaivelu, G.; Bohlén, M. Effect of a small amount of thermoplastic starch blend on the mechanical recycling of conventional plastics. J. Polym. Environ. 2020, 29, 985–991. [Google Scholar] [CrossRef]
- Briassoulis, D.; Pikasi, A.; Hiskakis, M. Recirculation potential of post-consumer /industrial bio-based plastics through mechanical recycling—Techno-economic sustainability criteria and indicators. Polym. Degrad. Stab. 2020, 183, 109217. [Google Scholar] [CrossRef]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of bioplastics: Routes and benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Palma-Ramirez, D.; Dominguez-Crespo, M.A.; Del Angel-Lopez, D.; De La Fuente, D. Comparative assessment of miscibility and degradability on PET/PLA and PET/chitosan blends. Eur. Polym. J. 2014, 61, 285–299. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lσpez, J.; Ferrandiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- De La Rosa-Ramírez, H.; Aldas, M.; Ferri, J.M.; López-Martínez, J.; Samper, M.D. Modification of poly (lactic acid) through the incorporation of gum rosin and gum rosin derivative: Mechanical performance and hydrophobicity. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Material Data Center Datasheet Biomer® P226. Available online: https://www.materialdatacenter.com/ms/en/Biomer/Biomer/Biomer%c2%ae+P226/63ccd%2045d/ (accessed on 12 August 2021).
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, D.; Rayon, E.; Carbonell-Verdu, A.; Lopez-Martinez, J.; Balart, R. Improvement of the compatibility between poly(3-hydroxybutyrate) and poly(e-caprolactone) by reactive extrusion with dicumyl peroxide. Eur. Polym. J. 2017, 86, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; Lopez, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- European Parliament. Directive 2008/98/ec of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Official Journal of the European Union, 312. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0098&from=EN (accessed on 2 June 2021).
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; Van Harmelen, T.; De Wild, P.; Van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assess-ment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef]
- Schlegel, I. Deception by Numbers. Greenpeace. 2020. Available online: https://www.greenpeace.org/usa/wp-content/uploads/2020/09/GP_Deception-by-the-Numbers-3.pdf (accessed on 26 March 2021).
- Patel, D.; Moon, D.; Tangri, N.; Wilson, M. All Talk and No Recycling: An Investigation of the U.S. “Chemical Recycling” Industry. Global Alliance for Incinerator Alternatives. 2020. Available online: https://www.no-burn.org/chemical-recycling-us/ (accessed on 26 March 2021).
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Khoonkari, M.; Haghighi, A.H.; Sefidbakht, Y.; Shekoohi, K.; Ghaderian, A. Chemical recycling of PET wastes with different catalysts. Int. J. Polym. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of poly(ethylene terephthalate)—A review focusing on chemical methods. Express Polym. Lett. 2016, 10, 559–586. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S. Chemical Recycling of Poly(ethylene terephthalate). Macromol. Mater. Eng. 2007, 292, 128–146. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Achilias, D.S. Recycling of poly(ethylene terephthalate) waste through methanolic pyrolysis in a microwave reactor. J. Anal. Appl. Pyrolysis 2012, 98, 214–220. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. Pet waste management by chemical recycling: A review. J. Polym. Environ. 2008, 18, 8–25. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Nikles, D.E.; Farahat, M.S. New motivation for the depolymerization products derived from poly(ethylene terephthalate) (PET) waste: A review. Macromol. Mater. Eng. 2005, 290, 13–30. [Google Scholar] [CrossRef]
- Carta, D.; Cao, G.; D’Angeli, C. Chemical recycling of poly(ethylene terephthalate) (pet) by hydrolysis and glycolysis. Environ. Sci. Pollut. Res. 2003, 10, 390–394. [Google Scholar] [CrossRef]
- McKeown, P.; Jones, M.D. The chemical recycling of PLA: A review. Sustain. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Piemonte, V.; Gironi, F. Kinetics of hydrolytic degradation of PLA. J. Polym. Environ. 2012, 21, 313–318. [Google Scholar] [CrossRef]
- Piemonte, V.; Gironi, F. Lactic acid production by hydrolysis of PLA in aqueous solutions: An experimental and kinetic study. J. Polym. Environ. 2013, 21, 275–279. [Google Scholar] [CrossRef]
- Piemonte, V.; Sabatini, S.; Gironi, F. Chemical recycling of PLA: A great opportunity towards the sustainable development? J. Polym. Environ. 2013, 21, 640–647. [Google Scholar] [CrossRef]
- Cristina, A.M.; Sara, F.; Fausto, G.; Vincenzo, P.; Rocchina, S.; Claudio, V. Degradation of Post-consumer PLA: Hydrolysis of polymeric matrix and oligomers stabilization in aqueous phase. J. Polym. Environ. 2018, 26, 4396–4404. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Rubino, M.; Dolan, K.; Soto-Valdez, H.; Selke, S. Effect of nanoparticles on the hydrolytic degradation of PLA-nanocomposites by water-ethanol solutions. Polym. Degrad. Stab. 2017, 146, 287. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Kosmidis, V.A.; Achilias, D.S.; Karayannidis, G.P. Poly(ethylene terephthalate) recycling and recovery of pure terephthalic acid. Kinetics of a phase transfer catalyzed alkaline hydrolysis. Macromol. Mater. Eng. 2001, 286, 640–647. [Google Scholar] [CrossRef]
- Yagihashi, M.; Funazukuri, T. Recovery ofl-lactic acid from poly(l-lactic acid) under hydrothermal conditions of dilute aqueous sodium hydroxide solution. Ind. Eng. Chem. Res. 2010, 49, 1247–1251. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly(L-lactide). II. Hydrolysis in alkaline solution. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 59–66. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Kolokotsiou, L.; Vouvoudi, E.; Redhwi, H.H.; Al-Arfaj, A.A.; Achilias, D.S. Depolymerization of PLA by phase transfer catalysed alkaline hydrolysis in a microwave reactor. J. Polym. Environ. 2020, 28, 1664–1672. [Google Scholar] [CrossRef]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; Van Nostrum, C.F.; Kettenes-van den Bosch, J.J.; Hennink, W.E. New insights into the hydrolytic degradation of poly (lactic acid): Participation of the alcohol terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Van Nostrum, C.F.; Veldhuis, T.F.; Bos, G.W.; Hennink, W.E. Hydrolytic degradation of oligo(lactic acid): A kinetic and mechanistic study. Polymer 2004, 45, 6779–6787. [Google Scholar] [CrossRef]
- Yu, J.; Plackett, D.; Chen, L.X.L. Kinetics and mechanism of the monomeric products from abiotic hydrolysis of poly[(R)-3-hydroxybutyrate] under acidic and alkaline conditions. Polym. Degrad. Stab. 2005, 89, 289–299. [Google Scholar] [CrossRef]
- Saeki, T.; Tsukegi, T.; Tsuji, H.; Daimon, H.; Fujie, K. Hydrolytic degradation of poly[(R)-3-hydroxybutyric acid] in the melt. Polymer 2005, 46, 2157–2162. [Google Scholar] [CrossRef]
- Chen, L.X.; Yu, J. Abiotic Hydrolysis of Poly[(R)-3-hydroxybutyrate] in Acidic and Alkaline Media. Macromol. Symp. 2005, 224, 35–46. [Google Scholar] [CrossRef]
- Bonartsev, A.; Boskhomodgiev, A.P.; Iordanskii, A.L.; Bonartseva, G.; Rebrov, A.V.; Makhina, T.K.; Myshkina, V.L.; Yakovlev, S.A.; Filatova, E.A.; Ivanov, E.A.; et al. Hydrolytic degradation of poly(3-hydroxybutyrate), polylactide and their derivatives: Kinetics, crystallinity, and surface morphology. Mol. Cryst. Liq. Cryst. 2012, 556, 288–300. [Google Scholar] [CrossRef]
- Pellis, A.; Haernvall, K.; Pichler, C.M.; Ghazaryan, G.; Breinbauer, R.; Guebitz, G.M. Enzymatic hydrolysis of poly(ethylene furanoate). J. Biotechnol. 2016, 235, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rosenboom, J.G.; Hohl, D.K.; Fleckenstein, P.; Storti, G.; Morbidelli, M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018, 9, 2701–2707. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, S.; Codari, F.; Storti, G.; Morbidelli, M.; Moscatelli, D. Modeling the pH-dependent PLA oligomer degradation kinetics. Polym. Degrad. Stab. 2014, 110, 80–90. [Google Scholar] [CrossRef]
- Shih, C. Chain-end scission in acid catalyzed hydrolysis of poly (d,l-lactide) in solution. J. Control. Release 1995, 34, 9–15. [Google Scholar] [CrossRef]
- Codari, F.; Lazzari, S.; Soos, M.; Storti, G.; Morbidelli, M.; Moscatelli, D. Kinetics of the hydrolytic degradation of poly(lactic acid). Polym. Degrad. Stab. 2012, 97, 2460–2466. [Google Scholar] [CrossRef]
- Liu, M.; Guo, J.; Gu, Y.; Gao, J.; Liu, F. Versatile imidazole-anion-derived ionic liquids with unparalleled activity for alcoholysis of polyester wastes under mild and green conditions. ACS Sustain. Chem. Eng. 2018, 6, 15127–15134. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Liu, F.; Yu, S. Kinetics and mechanism of monomeric product from methanolysis of poly (3-hydroxybutyrate) catalyzed by acidic functionalized ionic liquids. Polym. Degrad. Stab. 2016, 130, 22–29. [Google Scholar] [CrossRef]
- Song, X.; Liu, F.; Wang, H.; Wang, C.; Yu, S.; Liu, S. Methanolysis of microbial polyester poly(3-hydroxybutyrate) catalyzed by Brønsted-Lewis acidic ionic liquids as a new method towards sustainable development. Polym. Degrad. Stab. 2018, 147, 215–221. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Wang, C.; Liu, F.; Yu, S.; Liu, S.; Song, Z. Chemical recycling of bio-based poly(3-hydroxybutyrate) wastes under methanolysis condition catalyzed by Fe-containing magnetic ionic liquid. J. Polym. Environ. 2019, 27, 862–870. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.H.; Lim, I.T.; Han, K.; Lee, S.Y. Preparation of alkyl (R)-(-)-3-hydroxybutyrate by acidic alcoholysis of poly-(R)-(-)-3-hydroxybutyrate. Enzym. Microb. Technol. 2000, 27, 33–36. [Google Scholar] [CrossRef]
- Fernández-Dacosta, C.; Posada, J.A.; Ramirez, A. Techno-economic and carbon footprint assessment of methyl crotonate and methyl acrylate production from waste water-based polyhydroxybutyrate (PHB). J. Clean. Prod. 2016, 137, 942–952. [Google Scholar] [CrossRef]
- de Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.J. Furandicarboxylic acid (FDCA), a versatile building block for a very in-teresting class of polyesters. ACS Symp. Ser. Am. Chem. Soc. 2012, 1105, 1–13. [Google Scholar]
- Mishra, S.; Goje, A.S. Kinetics of glycolysis of poly(ethylene terephthalate) waste powder at moderate pressure and temperature. J. Appl. Polym. Sci. 2002, 87, 1569–1573. [Google Scholar] [CrossRef]
- Xi, G.; Lu, M.; Sun, C. Study on depolymerization of waste polyethylene terephthalate into monomer of bis(2-hydroxyethyl terephthalate). Polym. Degrad. Stab. 2005, 87, 117–120. [Google Scholar] [CrossRef]
- Pardal, F.; Tersac, G. Kinetics of poly (ethylene terephthalate) glycolysis by diethylene glycol. Part II: Effect of temperature, catalyst and polymer morphology. Polym. Degrad. Stab. 2007, 92, 611–616. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the catalytic glycolysis of polyethylene terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef]
- Duque-Ingunza, I.; Lopez-Fonseca, R.; De Rivas, B.; Gutiérrez-Ortiz, J.I. Process optimization for catalytic glycolysis of post-consumer PET wastes. J. Chem. Technol. Biotechnol. 2013, 89, 97–103. [Google Scholar] [CrossRef]
- Al-Sabagh, A.; Yehia, F.; Eshaq, G.; Rabie, A.; ElMetwally, A. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Torpanyacharn, O.; Sukpuang, P.; Petchsuk, A.; Opaprakasit, P. Curable precursors derived from chemical recycling of poly(ethylene terephthalate) and polylactic acid and physical properties of their thermosetting (co)polyesters. Polym. Bull. 2017, 75, 395–414. [Google Scholar] [CrossRef]
- Sukpuang, P.; Opaprakasit, M.; Petchsuk, A.; Tangboriboonrat, P.; Sojikul, P.; Opaprakasit, P. Polylactic acid glycolysate as a cross-linker for epoxidized natural rubber. J. Elastomers Plast. 2014, 48, 105–121. [Google Scholar] [CrossRef]
- Tounthai, J.; Petchsuk, A.; Opaprakasit, P. Curable polyester precursors from polylactic acid glycolyzed products. Polym. Bull. 2013, 70, 2223–2238. [Google Scholar] [CrossRef]
- Nim, B.; Opaprakasit, M.; Petchsuk, A.; Opaprakasit, P. Microwave-assisted chemical recycling of polylactide (PLA) by alcoholysis with various diols. Polym. Degrad. Stab. 2020, 181, 109363. [Google Scholar] [CrossRef]
- Jehanno, C.; Flores, I.; Dove, A.P.; Müller, A.J.; Ruipérez, F.; Sardon, H. Organocatalysed depolymerisation of PET in a fully sustainable cycle using thermally stable protic ionic salt. Green Chem. 2018, 20, 1205–1212. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.N.; Redhwi, H.H.; Al-Arfaj, A.A.; Achilias, D.S. Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review. Sustainability 2021, 13, 10528. https://doi.org/10.3390/su131910528
Siddiqui MN, Redhwi HH, Al-Arfaj AA, Achilias DS. Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review. Sustainability. 2021; 13(19):10528. https://doi.org/10.3390/su131910528
Chicago/Turabian StyleSiddiqui, Mohammad Nahid, Halim Hamid Redhwi, Abdulrahman A. Al-Arfaj, and Dimitris S. Achilias. 2021. "Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review" Sustainability 13, no. 19: 10528. https://doi.org/10.3390/su131910528
APA StyleSiddiqui, M. N., Redhwi, H. H., Al-Arfaj, A. A., & Achilias, D. S. (2021). Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review. Sustainability, 13(19), 10528. https://doi.org/10.3390/su131910528








