1. Introduction
In 2019, Peru was the largest producer of gold in Latin America and the 8th largest producer of gold in the world [
1]. The Arequipa region in south-western Peru, accounts for 14.6% of gold production along with the highest share of mining employment. This region also produces the third highest output of gold from artisanal and small-scale operations in the country [
1]. Despite the economic importance of gold production, the environmental impacts of extraction operations on surface and groundwater quality and human health have become a primary concern in Peru [
2,
3,
4,
5,
6]. Growth in gold extraction has brought increased social conflicts, often centering on water supply and, most commonly, water contamination [
7,
8,
9]. This is particularly important in Arequipa, one of Peru’s most arid regions, barely receiving 75 mm of mean annual rainfall concentrated during summer months [
10]. Conflicts over the effects of gold extraction on water pollution has in some instances pitted mining against agriculture, another leading economic sector and the main water consumer [
9]. The combined strains of Arequipa’s arid climate and water demands pose challenges to mining sustainability due to ineffective or lacking water treatment, water reuse, resource recovery, and conflict resolution.
Recent extreme degradation of surface water quality in the Arequipa region, such as the cases of Tambo, Chili, Ocoña, and Coralaque rivers, particularly with regard to heavy metals contamination and cyanide spills [
11], have pointed to potential impacts from mining-related activities [
12]. Even with efforts from the Peruvian government to develop water quality regulations for human consumption and environmental quality standards for water [
13,
14], administrative mechanisms to enforce poor corporate environmental practices are needed [
15]. This has led to proactive measures aiming to develop sustainable mining practices at established mines and mineral processing facilities, such as the Cerro Verde copper mine, where access to water for mining expansion was granted in exchange for treating the municipal sewage from the city of Arequipa [
16].
Conventional mineral-processing facilities in the Arequipa region primarily use the cyanidation process for dissolution of gold-containing ores [
1]. However, after cyanide-gold complexes are removed (e.g., adsorption with activated carbon), the residual cyanide-rich effluents are commonly poorly treated prior to reuse or discharge. The mineral processing operations in this arid region typically send their cyanide containing effluents to large evaporation ponds to undergo sedimentation and passive exposure to ultraviolet (UV) irradiation before being reused onsite for additional batch processing. However, significant progress can be made to optimize chemically based treatment processes for centralized and decentralized operation facilities, which often differ in their water quality, types of available utilities, and onsite resources. Treatment processes such as advanced oxidation may be used process reuse, discharge, or nutrient recovery, depending on local needs and these processes can be further optimized to reduce chemical consumption.
Cyanide can be degraded using a variety of advanced oxidation processes (AOP). Hydrogen peroxide is routinely used for converting free and weak acid dissociable metal-cyanide complexes into the much less toxic cyanate [
17], according to the following reactions:
where Reaction (1) is optimal under alkaline conditions and the end-product, cyanate, can hydrolyze into ammonium (Reaction (2)), a process that can be faster under acidic conditions [
17,
18,
19]. When peroxide is combined with UV, two hydroxyl (OH) radicals are formed by homolytic cleavage of the central HO–OH bond [
20]. While two ·OH radicals are formed, only one is able to participate in the AOP because the second one is surrounded by water molecules in a solvent cage [
21]. Despite the one-to-one stoichiometric effectiveness of UV/H
2O
2, this process has been shown to be effective for treating cyanide-containing effluents [
22,
23] and is preferred over other AOPs, such as ozone, because peroxide is relatively inexpensive, water soluble, and simple to store and handle [
18]. UV irradiation in combination with hydrogen peroxide (H
2O
2) is also a particularly attractive treatment option [
24], as the Arequipa region receives high solar radiation ranging between 850 to 950 W/m
2 and a UV intensity of ~0.2 mW/cm
2, which may be used in place of UV lamps [
25]. Titanium dioxide (TiO
2) photocatalysts have also been used to accelerate degradation in cyanide containing effluents [
26,
27], although with varying degrees of success. The major end-products of cyanide destruction using TiO
2 are cyanate, nitrite, and nitrate [
26,
27], which feed the nitrogen cycle [
28]. Mechanistically, when TiO
2 is excited by UV light, an electron hole is created on CN
− and on water molecules, which generates ·OH radicals for further oxidative degradation [
29,
30]. However, due to the low quantum yield for ·OH radical generation, UV/TiO
2 alone is typically not considered an efficient AOP [
31].
The aim of this investigation was to evaluate the effectiveness of alternate UV sources (ambient UV versus a UV lamp), optimize the dosing requirements of peroxide at different cyanide concentrations, and evaluate the effectiveness of a TiO2 photocatalyst, to provide treatment guidelines for the various types of mineral processing facilities (centralized versus decentralized) in the Arequipa region. To accomplish this, we obtained water quality data from two mineral processing facilities with different site characteristics in the Arequipa region. The first processing plant, called La Quinta, operating near the town of Vitor was chosen for its remote location and geographic distance from centralized utilities, where water and chemicals are transported to the site. The second plant, called Cepromet Minera Porvenir S.A.C. (hereafter referred to as Cepromet), is in a suburb outside of Arequipa city and was chosen as a counterpart to La Quinta for its proximity to centralized utilities. La Quinta also processes ore from a single site, whereas Cepromet processes ore from a variety of locations, depending on demands. Both sites reuse their processed water but without further treatment or water purification, which may affect process efficiency and may contaminate local water resources if spilled. Three experiments were conducted with synthetic cyanide-containing wastewater of similar concentrations to those found at the two previously mentioned processing facilities: (1) UV only; (2) peroxide with ambient UV (0.2 mW/cm2); and (3) peroxide with 4.6 mW/cm2 UV intensity at total cyanide concentrations of ~1000 mg/L and ~100 mg/L, all to elucidate the effects of cyanide concentration on destruction rate. Additionally, a fourth experiment was performed treating real cyanide-containing wastewater from Cepromet using a low ratio of cyanide:peroxide (1:0.1 and 1:0.2) in combination with TiO2 in a compound parabolic concentrator (CPC) with an approximate intensity of 0.4 mW/cm2.
2. Materials and Methods
2.1. Site and Processing Characteristics
At the La Quinta mineral processing facility, ore transported from a local mine is ground to about 76 µm using a ball mill. Fresh water for the process is trucked in from the Vitor River and stored in an open lined pond prior to use. Approximately 4 kg CN/Mg of ore is used for each batch, which occurs in two 30 m3 reaction tanks in series. After cyanide reacts with ground ore for approximately 36 h, the water is pumped through granular activated carbon (GAC) supported on a mesh screen with 20 kg of GAC used per m3 of metal/cyanide-containing process water. This facility processes intrusive rocks of monzotonalite composition, belonging to the Punta Coles super unit of the Cordillera de La Costa. La Quinta has a daily treatment capacity of 25–30 Mg of gold ore, with the ore containing ~5–10 g of gold/Mg of ore. It is estimated that ~90% is recovered using this process (personal communication). After the gold–cyanide complexes have been adsorbed onto GAC, the waste effluent is pumped to a lined retention pond where sedimentation occurs. Clarified effluent is then pumped and stored in two ~15 m3 storage tanks prior to reuse.
At the Cepromet facilities, on the other hand, four tanks in series (total volume of 22 m3) are used in the cyanidation process and allowed to react for 3 days. Similar to La Quinta, 4 kg CN−/Mg of ore are also used for each batch. After the reaction is complete, 20 kg of GAC are used for every m3 of concentrate to retain the cyanide–gold complexes. The cyanide–gold slurry is then passed through a trommel used to separate GAC from the slurry. The waste effluent is then transferred to a lined retention pond that is covered by a large solar shade, where it sits for sedimentation and is thereafter transferred to a concrete storage basin to be reused for future batches without any additional treatment.
2.2. Sampling
Water used in the cyanidation process was collected from the waste tanks at each facility using a sampling tube (1/2” outside diameter), connected to a cordless drill-powered pump (Wayne Company, Harrison, OH, USA) and filtered through a 0.45 µm inline filter. Fifty ml of cyanide-containing water were collected for each sample analysis. For total cyanide, samples were field-adjusted to pH 12 using standard protocols [
17]. A hydrogen cyanide gas detector was used to warn against exposure (BW Technologies).
2.3. Sample Analysis
Total cyanide was measured using Hach Test N′ Tube (TNT) 862 pyridine barbituric acid method (Hach, Loveland, CO, USA). Dissolved organic carbon (DOC) was measured using a total organic carbon (TOC) TOC-L analyzer (Shimadzu, Columbia, MD, USA). Total nitrogen (TN) was evaluated simultaneously with TNM to run the ASTM D 8083 method by high-temperature catalytic combustion and chemiluminescent detection. Ammonia was measured using Hach TNTplus 831 by salicylate method (Hach, Loveland, CO, USA). For cyanide destruction end-product analysis involving the experiment with low/high cyanide concentrations, the end-point sample pH was lowered to 2.5 with 1% (v/v) sulfuric acid. Inorganic anions were measured using ion chromatography (Dionex 1500, Sunnyvale, CA, USA), with IonPac AS14 and guard columns. Alkalinity was measured using the titration 8203 method (model 16900, Hach, Loveland, CO, USA). Elemental analysis was carried out by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Avio-500, Perkin-Elmer, Fremont, CA, USA).
2.4. Experimental Design
2.4.1. Batch Experiments at Ambient UV Intensity
Oxidant experiments (hydrogen peroxide 30%
w/
w in H
2O; Sigma-Aldrich, St. Louis, MO, USA) with simulated natural UV radiation were accomplished using 100 mL volume batches in 1 L fused-quartz Erlenmeyer flasks (Technical Glass Products Inc, Painesville, OH, USA) on a NEST array (Lumenautix, LLC. Reno, NV, USA), following the procedure by Read et al. [
32]. Briefly, this array is comprised of multiple discrete solid-state emitters operating in two modes, VIS and UV, connected to a control unit. The output is regulated, and temperature compensated for constant DC current, which allows for precise control and stability of light output. Visible light emitting diodes (LEDs) are blue (
n = 3), red (
n = 3), and white (
n = 6), while the UV devices emit at 285 nm (
n = 3, UVB range). For this investigation, only the UV emitters with an irradiance of 0.2 mW/cm
2 were used.
2.4.2. Batch Experiments with Recirculation and Medium-Intensity UV Lamp
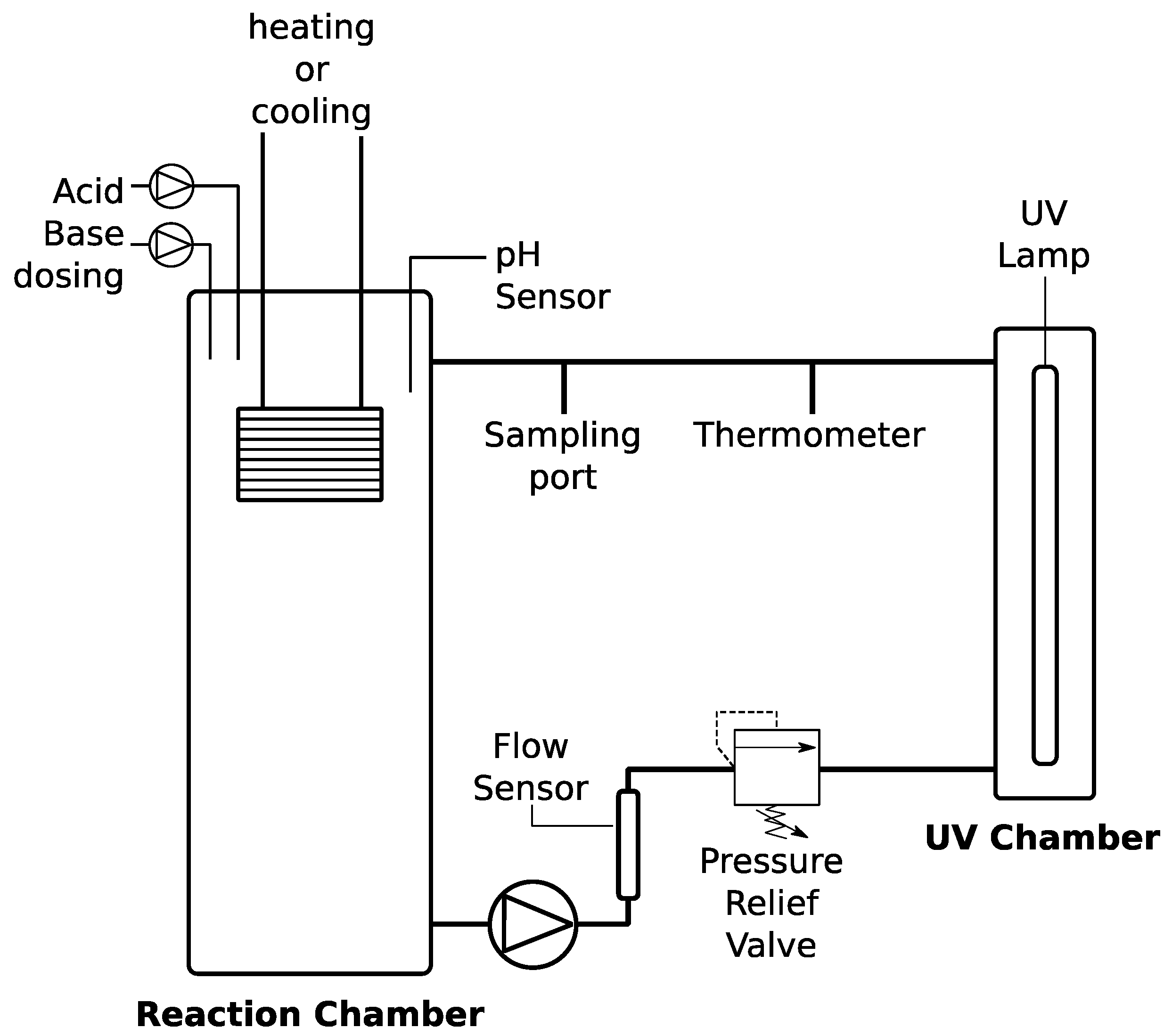
To test the effectiveness of an intensity UV source higher than the intensity of natural UV and the impact of hydrogen peroxide (30%
w/
w in H
2O; Sigma-Aldrich, St. Louis, MO, USA), a 3.3 L automated UV-based cyanide-destruction batch reactor was constructed (
Figure 1). A residential UV lamp was chosen because it is an off-the-shelf item that can be easily obtained in Peru by local vendors, in addition to the fact that it is powerful enough to activate hydroxyl radicals [
21]. The UV lamp emits at 254 nm with an energy density of 40 mJ/cm
2 measured by the manufacturer at a flow rate of 8.3 LPM (Lumenor, Guelph, ON, Canada) and a working volume of 1.2 L. The power density, which is independent of the flowrate, can be calculated based on the energy density measured at a given flowrate. For example, at a flowrate of 8.3 LPM and a lamp’s working volume of 1.2 L, the residence time in the device is 7.9 s. Dividing the reported energy density by the residence time yields a 4.6 mW/cm
2 energy density.
The system is also equipped with sensor ports for online pH monitoring and is temperature controlled (IsoTemp 3006, Fischer Scientific, Waltham, MA, USA) by a virtual interface (VI) programmed in LabView (hosted on the user’s laptop). The system is gas tight and was pressure tested prior to experimentation and is also equipped with pH control by dosing with 0.1 M sulfuric acid and 0.1 M sodium hydroxide. Oxidant dosing is also configured; however, manual dosing was used in oxidant-to-cyanide ratio experiments.
2.4.3. Batch Experiment with Compound Parabolic Concentrator
A compound parabolic concentrator (CPC) was used to direct solar radiation for field studies conducted with real cyanide effluent at the Cepromet mineral-processing facility. The CPC consists of a metal base that supports an array of three non-UV absorbing acrylic tubes (85 cm long and 4.36 cm inside diameter) that are superimposed on a metal support structure with an aluminum reflective surface. A 30° inclination plane was used according to the latitude of Arequipa during summer season (December–February). Available UV-B power density in January for southern Peru is around 0.2 mW/cm
2 [
33]. Based on the dimensions of the CPC (10 cm diameter), a concentration factor of 2 can be achieved, yielding an effective power density of 0.4 W/m
2. For each CPC, a 1 L volume was used during experimental testing. With this configuration, the effect of concentrated ambient UV was tested with peroxide (14.31%
w/
w in H
2O; Sigma-Aldrich, St. Louis, MO, USA) at two peroxide concentrations 1 mL and 2 mL peroxide/L of cyanide solution (1280 mg free CN
−/L; ratios 1:0.1 and 1:0.2, respectively) and a titanium dioxide photocatalyst at two catalyst concentrations: 50 mg/L and 500 mg/L TiO
2. The photocatalyst used in this study was a rutile titanium dioxide semiconductor with a 0.23 µm average particle size and was recovered after each experiment. Photocatalyst concentrations of 50 mg/L and 500 mg/L were used to prevent optical shielding that is known to occur at higher concentrations [
27]. Real cyanidation effluent from the Cepromet plant was used. The control for the experiment consisted of cyanidation effluent in the absence of TiO
2 to establish baseline conditions. An additional experiment was conducted to provide a central point among the three independent variables: irradiation time (synonymous with runtime), peroxide concentration, and TiO
2 concentration (mg TiO
2/L CN
− containing waste). These values were 130 min irradiation, 1.5 mL peroxide/L of cyanide solution (ratio 1:0.14), and 275 mg/L TiO
2. This served to create a 2
3 factorial design with 8 experimental tests and 3 replicates at the central point.
2.4.4. Data Analysis
Destruction rates were calculated as
r = ((
C0 −
C1)/(
t1 −
t0)) × volume, where
C0 and
C1 are the corresponding concentrations for each timepoint,
t0 is the starting timepoint when hydrogen peroxide was dosed, and
t1 is the subsequent point. Rate constants were estimated by adjusting the rate constant such that the sum of the squared error between modeled and measured data was maximized with a non-linear approach using Equation (3), where
C0 is the concentration at time
t0,
k is the rate constant, and
tn is the time at each sampling timepoint.
4. Discussion
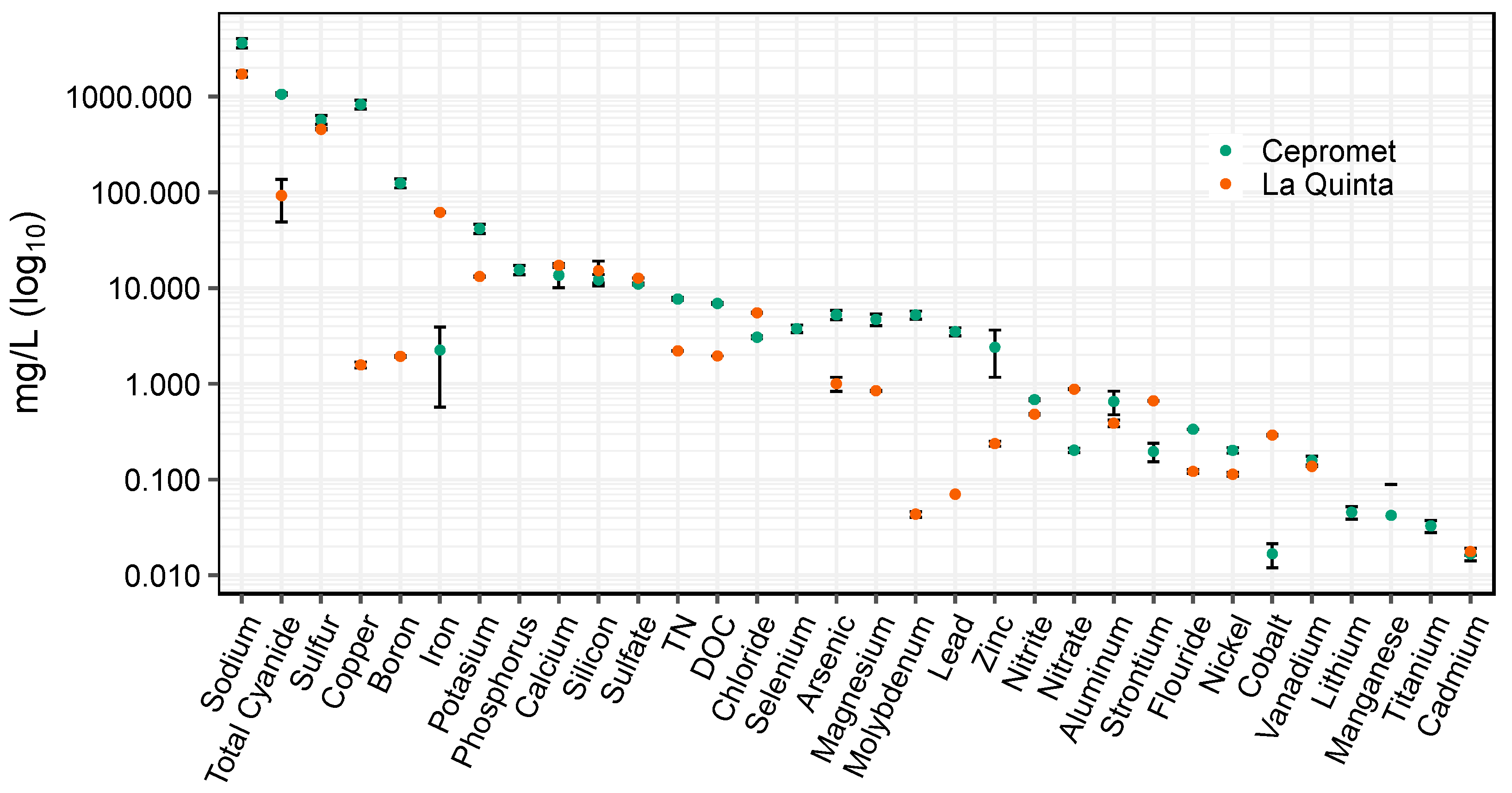
The aim of this investigation was to evaluate water treatment options for two geographically disparate mineral processing facilities in the Arequipa region of southern Peru: a remote site that processes ore from a single mine location (La Quinta), and a centralized facility that receives ore from various sources (Cepromet). First, we characterized the water quality of each site to determine viable treatment options (
Figure 2). Based on these results, which revealed an order of magnitude difference in cyanide concentrations, three treatment options were tested: (1) a 1:0.1 dose of peroxide in combination with low UV strength of intensity similar to natural sunlight (
Figure 3a); (2) a 1:0.1 dose of peroxide and a UV lamp (
Figure 3c), and a 1:1 dose of peroxide with a UV lamp (
Figure 4); and (3) CPC-concentrated UV with peroxide, in combination with a TiO
2 photocatalyst to accelerate degradation of high strength cyanide containing effluents using minimal peroxide (
Figure 5 and
Figure 6).
Peru is a mining epicenter with increasing concerns about the sustainability of mining-related activities and their effects on water resources and livelihoods [
6]. The environmental impacts pertaining to the lack of water treatment in Peruvian gold mining are additionally challenging because many artisanal and small-scale mining operations use mercury to extract gold from ore [
34]. Tailings from mineral processing with mercury are often sold to processing facilities (e.g., Cepromet) for further gold extraction using cyanide, leading to a treatment challenge, as mineral processing facilities may not only treat ore from different sites, but also often treat ore that has been previously treated with mercury. While mercury concentrations were not measured in this study, there is significant evidence for mercury use in surrounding regions [
5,
35] and in the Arequipa territory. Thus, the ore may have different mineralogical and chemical compositions, thus affecting post-cyanidation water quality. Furthermore, cyanide processing of mercury-contaminated tailings creates Hg–CN
− complexes that are highly toxic and bioavailable [
36]. When Cepromet’s water quality was characterized, it was found that it contained an order of magnitude higher cyanide concentration than the remote site (La Quinta), and had significantly higher concentrations of TN, TOC, copper, boron, fluoride, arsenic, selenium, and magnesium (see
Figure 2). Thus, even if all free and complexed cyanide could be destroyed or recovered, the water quality of both mineral processing plants is not suitable for surface water discharge. This is due to the elevated concentrations of analytes such as arsenic [
37], requiring further attenuation of contaminants using technologies such as nanofiltration [
38], engineered wetlands [
39], or other methods.
Advanced water treatment of cyanide-based mineral processing may be necessary under certain site conditions, such as dense clusters of processing activity that directly impact rivers [
36]. This investigation primarily focused on establishing baseline treatment guidelines such as testing the effectiveness of different UV sources, optimizing peroxide dose requirements, and calculating rate constants to evaluate the overall effectiveness of cyanide destruction at mineral processing facilities in the Arequipa region. Currently, both mineral processing facilities evaluated in this research re-use contaminated water with minimal treatment. For example, both sites store waste cyanidation effluents onsite, exposing them to natural sunlight for extended periods of time (days to weeks) and yet these waters still contain elevated concentrations of cyanide (
Figure 2). If cyanide can be effectively neutralized, other treatment processes may be performed to further elevate water quality. Because the Arequipa region has significant solar radiation [
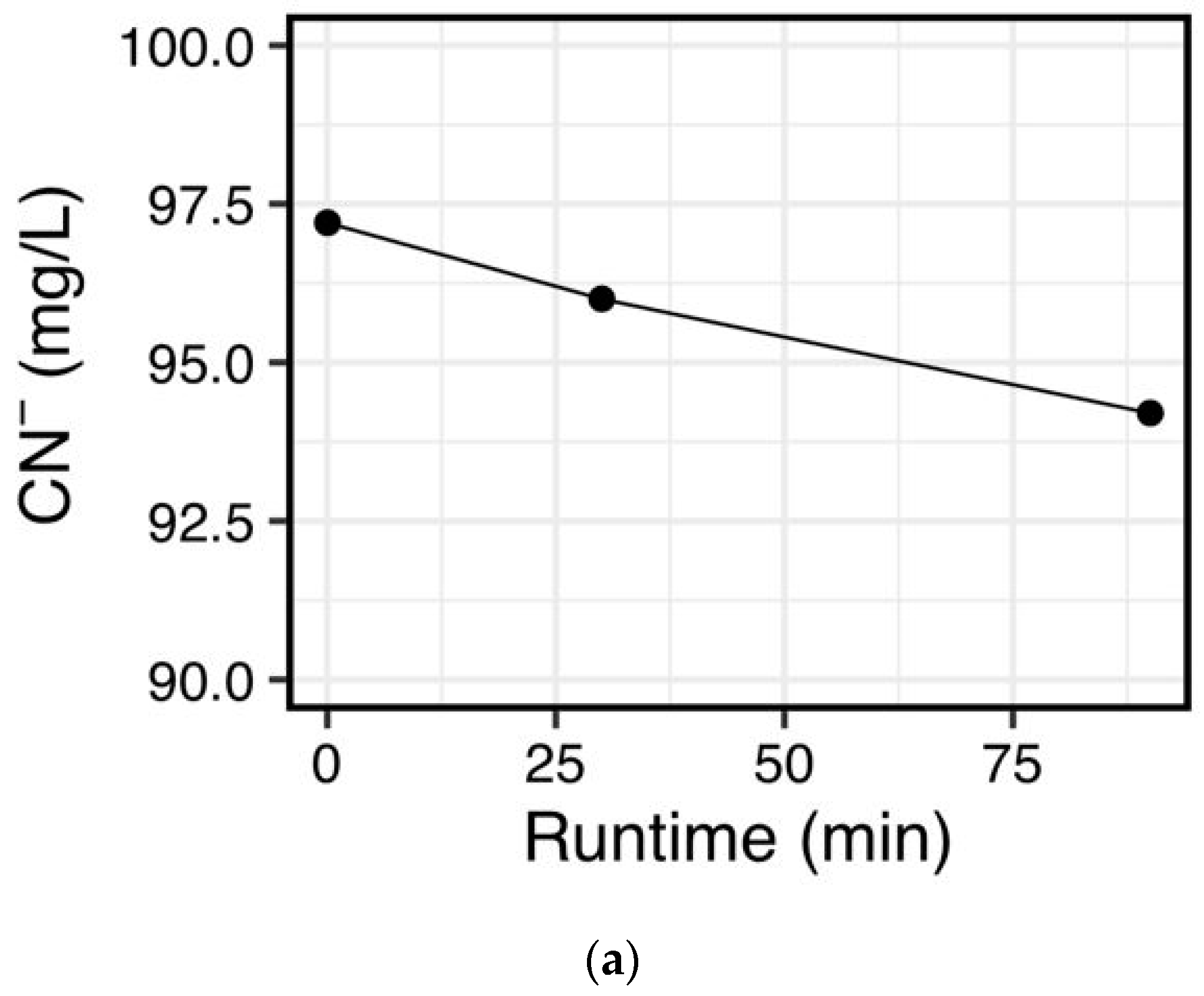
25], the first goal was to test the destruction of cyanide using ambient UV irradiation in combination with peroxide. However, little support for this type of treatment option was found (
Figure 3a) as a rapid destruction method for treating cyanide-containing effluents. This is in contrast to other work that reportedly used simulated natural UV intensities and found rapid degradation of free cyanide and thiocyanates [
40]. This result may be due to the UV wavelengths used in our study (285 nm), which fall in the UVB spectrum, whereas Mediavilla et al. [
40] used a UVC lamp, which emits at shorter wavelengths (254 nm). However, Mediavilla et al. [
40] also reported an irradiance of 26 mW/cm
2, which is two orders of magnitude higher to that natural UV irradiation (0.2 mW/cm
2). Nonetheless, no change was observed in this study in initial cyanide concentrations when residential-use UV lamp was used with an irradiance of 4.6 mW/cm
2 (
Figure 3b). This result clearly demonstrates that when UV alone is used, much stronger UV intensities than what a residential UV lamp can deliver are needed to rapidly degrade cyanide via photocatalytic oxidation. If rapid destruction is not a priority, then passive photocatalytic oxidation with peroxide may be a low-cost solution in processing plants that have downtime between each batch of ore. However, a variety of factors should be considered when attempting treatment in this manner, including sunlight intensity, turbidity, temperature, and depth of the water column. An additional factor for consideration is the presence of cyanide-containing compounds, such as thiocyanates and metal-cyanide complexes, which have slower degradation rates than free cyanide [
17,
40]. Furthermore, while cyanide-containing compounds, like thiocyanates, may be less toxic, their destruction and neutralization must be considered for any practical treatment option.
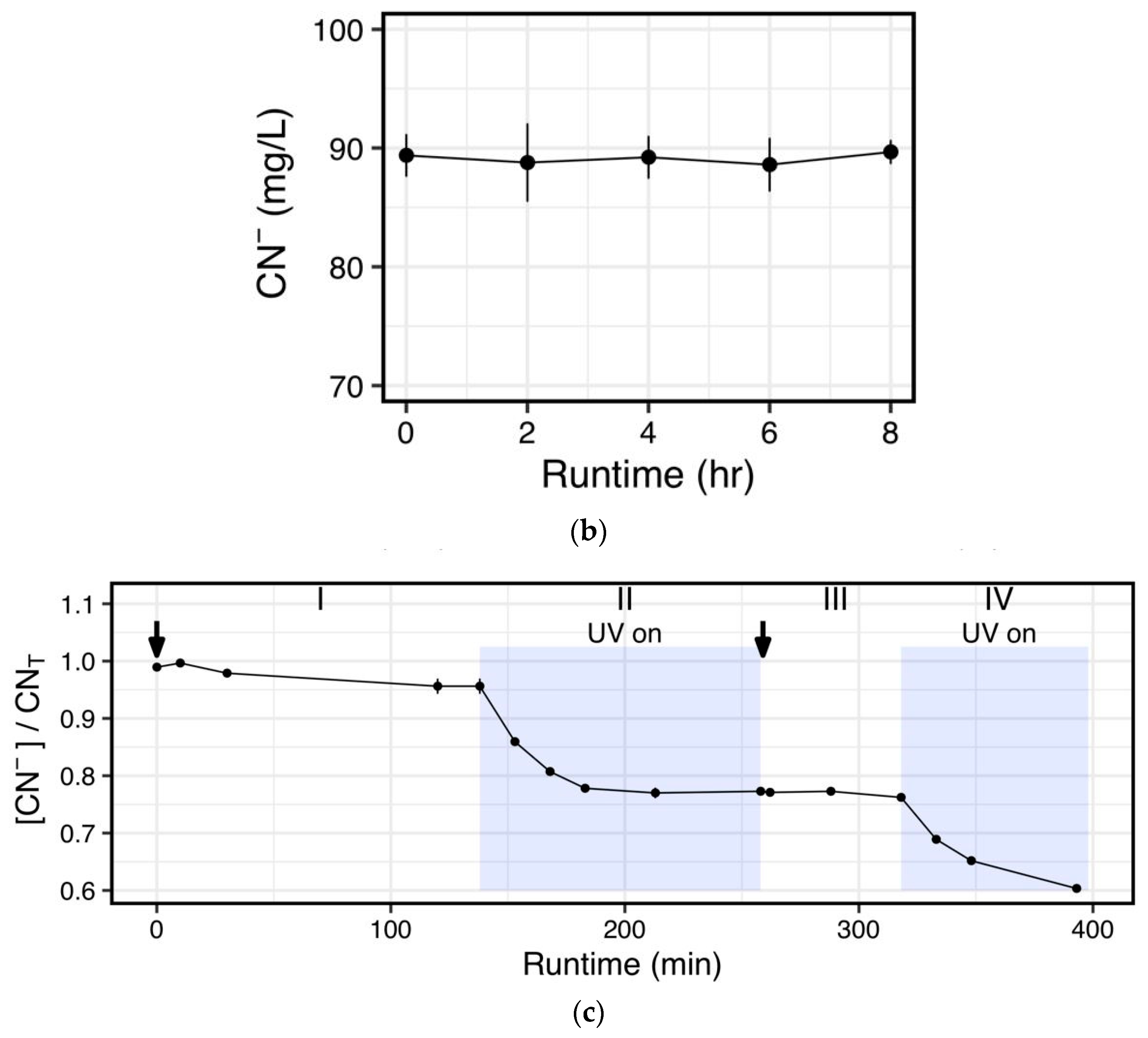
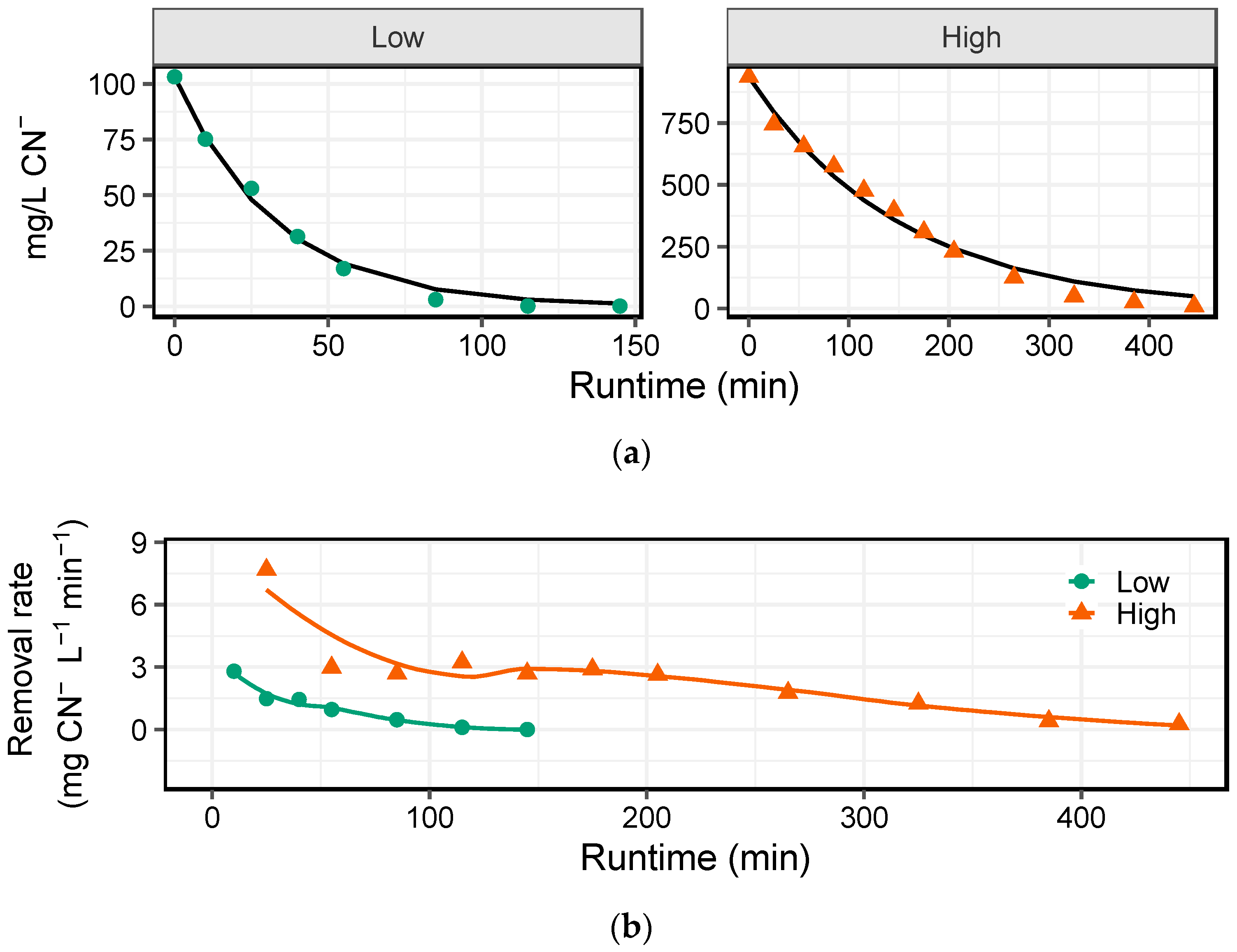
The time series analysis of the effects of peroxide alone and in combination with a UV lamp (
Figure 3c) clearly demonstrates the need for above ambient UV intensities to accelerate degradation of free cyanide. This result is not surprising, considering that UV breaks the central bond of peroxide to yield ·OH radicals [
20]. However, when high strength cyanide-containing effluents are encountered, the reaction time takes significantly longer (
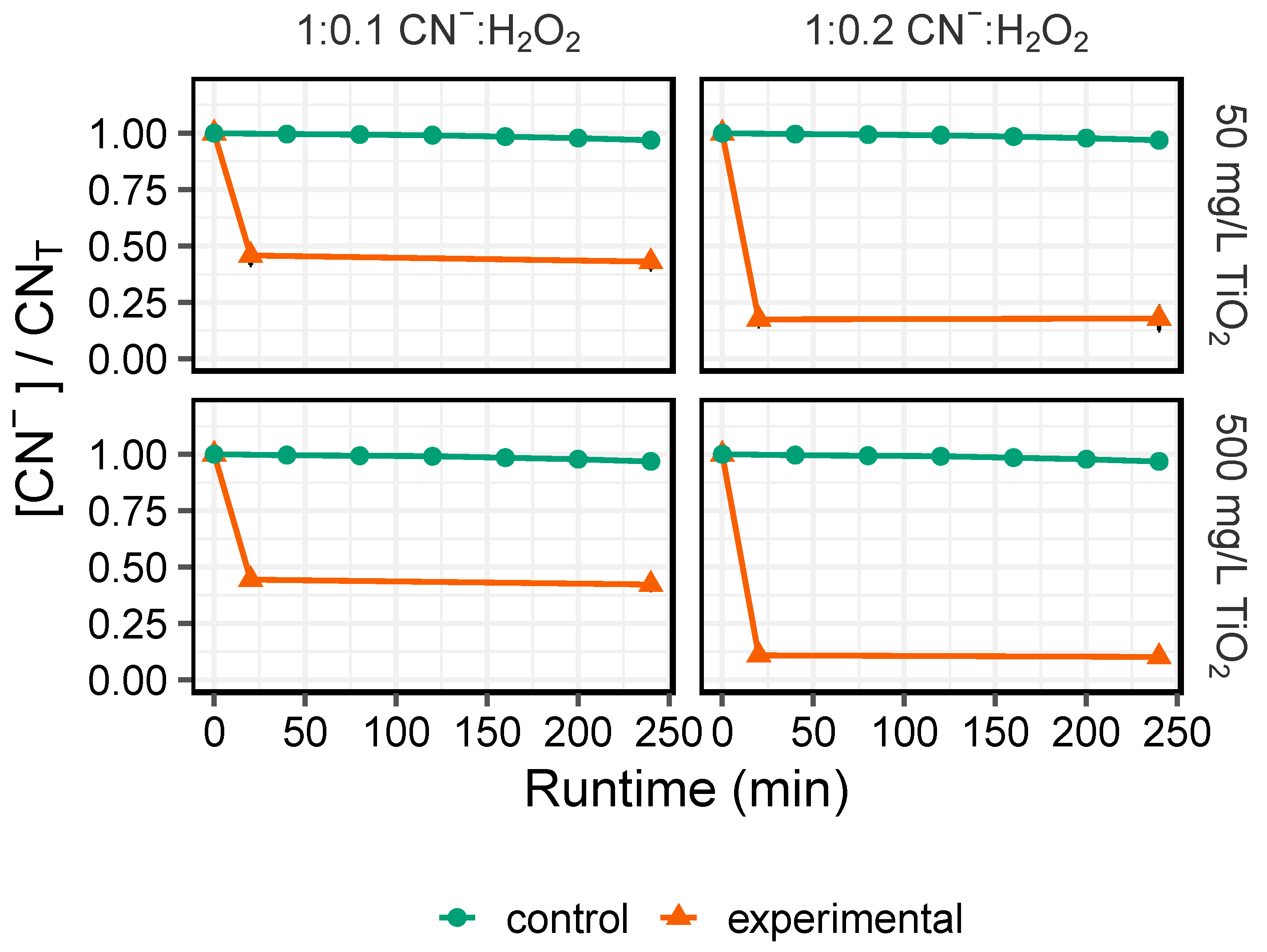
Figure 4). Thus, it is evident that under circumstances that require rapid turnover of process waters for cyanidation, a catalyst may be needed. When a TiO
2 photocatalyst was used in combination with low stoichiometric additions of cyanide to peroxide in a CPC, significantly higher degradation of high strength cyanide effluents (>1g/L CN
−) was observed within a 20 min period (82.1% ± 5.2% and 89.9% ± 1.9% at 50 mg/L and 500 mg/L TiO
2) (
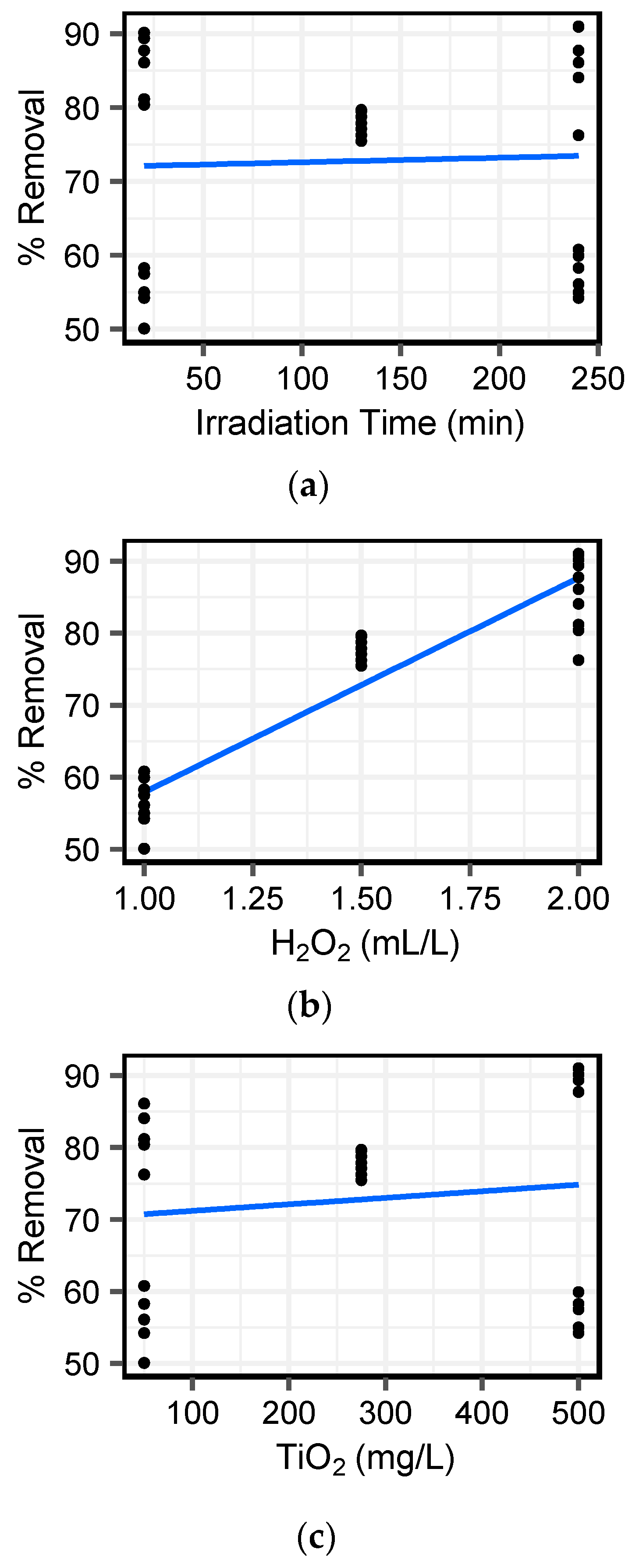
Figure 5), with destruction efficiencies significantly correlated with peroxide dose (
Figure 6). This result suggests that the UV–H
2O
2–TiO
2 combination is effective for rapidly degrading high strength cyanide effluents, using minimal chemical addition, and should be explored further at the pilot scale.
Titanium dioxide combined with UV has been postulated to not have a high enough quantum yield to generate sufficient ·OH radicals to enable efficient degradation [
31]. This is exemplified by Mohammadi-Moghamadam et al. [
26], which showed that the best removal efficiency obtained (using 300 mg/L CN
− starting concentration and 250 mg/L of substrate-stabilized TiO
2 particles) was ~38% destruction after 1 h and ~75% after 4 h. Other studies have also shown low degradation efficiencies: Kim et al. [
27] reached ~62% removal of 27 mg/L CN
− starting concentration using high intensity UV and 50 mg/L Degussa P25-type TiO
2 after 5 h. However, because rate constants were not reported in these studies, it is difficult to make direct comparisons with the results obtained in this investigation. Nonetheless, the reason why such rapid degradation with the combinations of UV, peroxide, and TiO
2 was observed in this study might be explained by the interaction of excited TiO
2 particles on water molecules that form the solvent cage around ·OH radicals [
21]. The excited TiO
2 particles might break the solvent cage around the ·OH radicals, thereby liberating the ·OH radical to react with cyanide molecules. However, this investigation specifically focused on the destruction of free cyanide. Future work should characterize these effects on total cyanide, which may contain significant weak acid dissociable (WAD) and strong acid dissociable (SAD) metal cyanide species. Lastly, a full characterization of transformation byproducts is needed for our two main treatment combinations (i.e., UV/H
2O
2 and UV/H
2O
2/TiO
2) in order to ascertain what downstream unit processes are needed to remove metals and nitrogen species (e.g., cyanate and ammonia).